
Neural mechanisms underlying cognitive changes in individuals with alcohol use disorder: insights from resting-state functional magnetic resonance imaging
Introduction
Alcohol use disorder (AUD), which is characterized by compulsive heavy drinking and a loss of control over alcohol consumption, is one of the most prevalent mental disorders worldwide. According to a 2024 World Health Organization report, in 2019, 209 million people (3.7% of the adult world population) worldwide were living with alcohol dependence, and approximately 2.6 million deaths were caused by alcohol consumption (1). Long-term heavy alcohol consumption can damage brain structure and function, resulting in changes in cognitive states such as mood, behavior, attention, memory, language, and coordination (2), leading to medical, mental, and behavior-related problems and risks (3). Understanding changes in brain function in AUD patients, and analyzing the correlation between these changes and cognitive status could provide insights into the neural mechanisms underlying cognitive changes in AUD patients.
Some scholars have studied resting-state functional magnetic resonance imaging (RS-fMRI) in AUD patients, but have only examined changes in brain function indicators such as amplitude of low-frequency fluctuation (ALFF) in AUD patients, and have not conducted correlation studies of cognitive changes (4,5). Studies on functional connectivity (FC) are relatively common. A number of FC-based studies have found that the multimodal networks, including the default mode network (DMN), central executive network, and prominence network, are changed and reconstructed in AUD patients, and the correlation between the reduction in gray matter volume in some brain areas and the strength of FC and the score of the Montreal Cognitive Assessment (MoCA) has been analyzed (6-9). The thalamus and pallidum have been used as seed points for FC analysis (10,11); however, these seed points were determined before the experiments were conducted based on previous experience and hypothesis. In this study, the FC seed points were the brain regions with differences in spontaneous brain activity in the AUD group compared with the healthy control (HC) group as identified by ALFF, and thus have a stronger practical basis. With the development of brain functional imaging and the deepening of research, the joint study of multiple analysis methods has been employed. Accordingly, this study employed a combined ALFF and seed-based FC analysis to comprehensively characterize brain functional alterations in AUD patients across multiple dimensions to elucidate the neural mechanisms underlying cognitive impairment in this population. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2052/rc).
Methods
Patients
The study volunteers were recruited from Guizhou Province, China, using posters and social media. Participants were included in the study if they met the following inclusion criteria: (I) were aged 18–75 years; (II) were right-handed; (III) had no history of neuropsychiatric disorders other than AUD; (IV) had no history of craniocerebral trauma, surgery, tumor, inflammation, cerebrovascular accident, or other diseases; and (V) had no dependence on or had no history of drug and poison use other than nicotine and alcohol. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had contraindications to magnetic resonance imaging (MRI) examination; (II) had a history of neurological or psychiatric disorders, or family history of neurological or psychiatric disorders other than AUD; (III) had previously suffered from a brain injury or loss of consciousness; (IV) failed to complete the cognitive scale assessment; and/or (V) showed the presence of T2-weighted imaging (T2WI)/fluid attenuated inversion recovery (FLAIR) brain-white-matter high-signal (Fazekas, grade 3). Only individuals with AUD who consumed Chinese baijiu (alcohol content ~53%) were included in the study. The AUD diagnosis was made by an attending physician from the Department of Neurology using the Chinese Translation of the Alcohol Use Disorder Identification Test (AUDIT-Chinese), which consists of 10 items related to drinking volume and frequency. Individuals with an AUDIT-Chinese score ≥7 were included in the AUD group, while those with a score <7 were included in the control group (12). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and approved by the Medical Ethics Committee of the Affiliated Hospital of Guizhou Medical University (No. 2024-235). All the participants provided informed consent before undergoing examination.
Cognitive assessment
All the participants completed the Montreal Cognitive Assessment (MoCA) within 1 hour before undergoing the MRI scan (13). The cognitive assessment included the following subtests: visuospatial and executive function (5 points), naming (3 points), attention (6 points), language (3 points), abstraction (2 points), delayed recall (5 points), and orientation (6 points). Each subtest assesses a different aspect of cognition. The total score was the sum of all the subtest scores, and the maximum possible total score was 30 points.
Image acquisition
The data were acquired using a Philips Ingenia 3.0T magnetic resonance scanner (Amsterdam, Netherlands). We performed RS-fMRI and high-definition T1-weighted imaging (T1WI) for the data analysis, and conventional T2WI to exclude brain diseases. During scanning, the participants were positioned supine with a foam pad between their head and the coil to minimize head motion artifacts, and ear plugs were used to reduce noise. While undergoing Blood Oxygen Level Dependent (BOLD) scanning, the participants were instructed to keep their eyes closed, avoid complex thinking, and refrain from sleeping. Additionally, the participants were prohibited from consuming alcoholic beverages 24 hours before the cognitive assessment and MRI scan to exclude the effects of acute alcohol exposure.
The RS-fMRI images were acquired using the Field Echo-Echo Planar Imaging sequence. The scanning parameters were as follows: time of repetition (TR) =2,000 ms, time of echo (TE) =35 ms, flip angle (FA) =90°, field of view (FOV) =230 mm × 230 mm, matrix =256×256, voxel size =2.4 mm × 2.4 mm × 4.0 mm, and scanning duration =10 minutes and 20 seconds. A total of 180 time phases were acquired, with 40 slices per phase.
The T1WI scanning parameters were as follows: TR =6.6 ms, TE =3 ms, FA =8°, FOV =240 mm × 240 mm, matrix =256×256, voxel size =1 mm × 1 mm × 1 mm. The scanning duration was 5 minutes and 30 seconds, and a total of 173 slices were acquired.
Study on RS-fMRI
Pre-processing of RS-fMRI data
The RS-fMRI data pre-processing was performed using the Data Processing Assistant for RS-fMRI Advanced Edition (DPARSFA) toolkit (https://rfmri.org/DPARSF) on the Matrix Laboratory (MATLAB) R2022a platform (https://www.mathworks.com/products/matlab.html). The pre-processing steps were as follows: (I) conversion of data from Digital Imaging and Communications in Medicine (DICOM) to Neuroimaging Informatics Technology Initiative (NIfTI) format; (II) removal of the first 10 time points; (III) correction of time slices; (IV) application of the Friston 24 parameter model for head motion correction, with exclusion of participants exhibiting head motion >3 mm or rotation >3°; (V) use of New Segment and Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) technology to register high-definition 3D-T1WI images with low-definition BOLD images, followed by standardization to the Montreal Neurological Institute (MNI) coordinate system and resampling to 3 mm × 3 mm × 3 mm voxels; (VI) Removal of linear and quadratic trends; (VII) exclusion of global signal, white matter, and cerebrospinal fluid as covariates to minimize noise interference; and (VIII) application of band-pass filtering (0.01–0.1 Hz).
Calculation and difference statistics of RS-fMRI indicators
The RS-fMRI index was calculated as follows:
- The Anatomical Automatic Labeling (AAL2) template was used to analyze the amplitude of low-frequency fluctuations (ALFF) brain maps of each participant on the preprocessed images. To enhance data normality, the AAL2 template was used to classify brain regions. The ALFF map was transformed into the z-score transformation amplitude of low-frequency fluctuation (zALFF) map by Fischer transformation, and each zALFF brain map was smoothed by a 6-mm full-width half-Gaussian kernel. The Statistical Parametric Mapping 12 (SPM12) toolkit (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) was used to perform the statistical analysis of the zALFF and z-score transformation functional connectivity (zFC) maps. In the specify 2nd-level module, an independent sample t-test was used to establish a general linear model with age, gender, and years of education as the covariables, and the possible differences of these factors on resting brain function signals were regressed to calculate the differences between the RS-fMRI indicators in the AUD and HC groups. The results are reported using P<0.001, with clusters greater than the minimum range threshold of 20 contiguous voxels, which corresponds to a cluster-level AlphaSim correction of P<0.05 (https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf).
- zALFF mapping was performed using the Viewer module in the Data Processing and Analysis of Brain Imaging (DPABI) toolkit (https://rfmri.org/DPABI). Brain regions with statistically significant were used to generate regions of interest (ROIs), which were used as the seed points for the FC analysis. The Pearson correlation coefficient was used to calculate the time synchronization of the BOLD signals between the seed points and whole-brain voxels, and the FC brain maps were obtained. Similarly, the FC brain map was converted to the zFC brain map by Fischer transformation, and then smoothed, and the smoothed nucleus size was consistent with the zALFF brain map. The statistical calculation procedure used to determine the FC value difference between the AUD group and HC group was the same as that for the ALFF value.
Correlation analysis
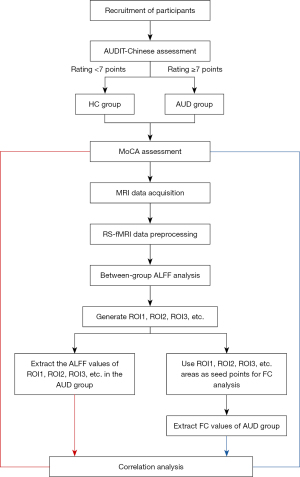
We performed correlation analyses between the RS-fMRI indicators and MoCA scores in the AUD group. First, the DPABI toolkit was used to generate the ROIs for the clusters with statistically significant in the ALFF and FC brain maps. The Utilities-ROI Signal Extractor module of DPABI toolkit was used to extract the ALFF value and FC value of each unsmoothed ROI in the AUD group. Finally, SPSS software (https://www.ibm.com/spss) was used to conduct a correlation analysis with the MoCA scores. A Pearson correlation analysis was conducted to analyze the correlation between the MoCA total score and ALFF/FC value. However, the sub-score data for visual space and executive function, naming, attention, language, abstraction, delayed recall, orientation, etc., did not conform to a normal distribution. Thus, a Spearman rank correlation analysis was conducted to examine the correlation between these sub-scores and the ALFF/FC values. The specific experimental process is shown in Figure 1.
Results
Clinical characteristics
This study included 30 AUD participants and 29 HC participants. There was no statistical difference between the AUD and HC groups in terms of age (t=1.410, P>0.05) and years of education (t=−1.534, P>0.05). However, there was a significant difference between the AUD and HC groups in terms of gender composition (χ2=9.247, P<0.05). To account for these differences, age, gender, and years of education were included as covariates in the general linear model for the statistical analysis of the RS-fMRI data. In terms of the cognitive assessment, the AUD group had lower scores than the HC group in terms of the total MoCA score (t=−2.434, P=0.019), visuospatial/executive function (Z=2.531, P=0.011), attention (Z=2.143, P=0.032), and orientation (Z=2.277, P=0.023) (Table 1).
Table 1
| Variables | AUD group (n=30) | HC group (n=29) | P |
|---|---|---|---|
| Demographic characteristics | |||
| Sex (male/female) | 24/6 | 17/12 | 0.002a* |
| Age (years) | 40.8±14.6 | 36.1±10.6 | 0.162b |
| Education (years) | 10.9±3.5 | 12.4±4.2 | 0.132b |
| AUDIT-Chinese | 16.9±5.1 | 0.6±1.1 | <0.001b* |
| Cognitive assessment | |||
| MoCA total score | 23.3±4.0 | 25.3±2.1 | 0.019b* |
| Visuospatial/executive | 2 (0–5) | 3 (1–5) | 0.011c* |
| Attention | 6 (4–6) | 6 (5–6) | 0.032c* |
| Orientation | 6 (4–6) | 6 (5–6) | 0.023c* |
| Language | 2 (1–3) | 3 (1–3) | 0.174c |
| Naming | 3 (0–3) | 3 (0–3) | 0.943c |
| Abstraction | 2 (0–2) | 1 (1–2) | 0.846c |
| Delayed recall | 3 (0–5) | 3 (1–5) | 0.735c |
Categorical variables are presented as n. Continuous variables are reported as the mean ± standard deviation. Ranked data are expressed as the median (range). a, Chi-square test; b, two-sample t-test; c, Mann-Whitney U test. *, statistically significant difference. AUD, alcohol use disorder; AUDIT-Chinese, Chinese Translation of Alcohol Use Disorder Identification Test; HC, healthy control; MoCA, Montreal Cognitive Assessment (Beijing Edition).
RS-fMRI analysis
Inter-group ALFF difference
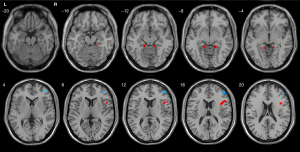
Compared with the HC group, the AUD group exhibited increased ALFF in the right inferior frontal gyrus, right parahippocampal gyrus, and left parahippocampal gyrus, but decreased ALFF in the right middle frontal gyrus (two-sample t-test, cluster-level AlphaSim correction; Table 2 and Figure 2).
Table 2
| Condition | Region | Clusters | MNI coordinate | t | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| AUD > HC | Right inferior frontal gyrus | 43 | 48 | 15 | 15 | 4.81 |
| Right parahippocampal gyrus | 23 | 18 | −39 | −9 | 5.40 | |
| Left parahippocampal gyrus | 21 | −15 | −39 | −9 | 4.95 | |
| HC > AUD | Right middle frontal gyrus | 35 | 48 | 4 | 18 | 4.15 |
The statistical method used was the two-sample t-test (age, gender, and years of education were included as covariates in a general linear model). The results are reported using Cluster-Level AlphaSim Correction (voxel size =3 mm × 3 mm × 3 mm). ALFF, amplitude of low-frequency fluctuation; AUD, alcohol use disorder; HC, healthy control; MNI, Montreal Neurological Institute.
Correlation analysis
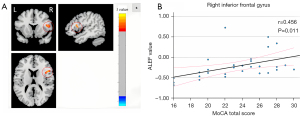
A Pearson correlation analysis was conducted to examine the relationship between the ALFF values and the total MoCA score in brain areas with statistically significant differences in the AUD group. The results indicated a positive correlation between the ALFF values and the total MoCA score in the right inferior frontal gyrus (r=0.456, P<0.05; Figure 3). However, no significant correlation was found between the ALFF values and the total MoCA score in the bilateral parahippocampal gyrus and right middle frontal gyrus.
FC analysis based on seed points
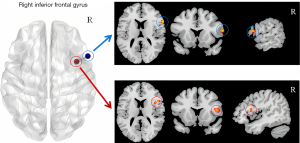
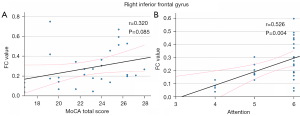
Clusters with statistically significant differences in ALFF in the right inferior frontal gyrus, bilateral parahippocampal gyrus, and right middle frontal gyrus were used as the seed points for the FC analysis with whole-brain voxels, and a statistical analysis was performed. The correlation analysis of the FC values and MoCA scores for the brain areas that differed significantly between the groups revealed enhanced FC between the internal and external areas of the opercular part of the right inferior frontal gyrus in the AUD group (Figure 4). A positive correlation was found between the FC values and the attention score in these areas (r=0.534, P<0.05). However, no significant correlation was found between the FC values between regions and the total MoCA score (Figure 5A). To account for cognitive changes related to age (14), a partial correlation analysis controlling for age was conducted, and the correlation remained significant (r=0.526, P<0.05, Figure 5B).
Discussion
Based on the BOLD signal, RS-fMRI detects deoxygenated hemoglobin in cerebral blood flow to reflect neural activities, making it a key method for studying brain function changes in various diseases (15). ALFF can reflect the BOLD signal intensity of spontaneous activity in brain regions (16). This study employed both ALFF and FC analysis methods. ALFF was first used to identify brain areas with significant differences in spontaneous neural activities between the AUD and HC groups. These areas were then used as seed points for the FC analysis, providing a multi-layered and multi-angular perspective on brain function changes in individuals with AUD. The study found significant differences in the ALFF values in the right inferior frontal gyrus, bilateral parahippocampal gyrus, and right middle frontal gyrus in the AUD group compared to the HC group. This indicates abnormalities in spontaneous neural activities in multiple brain regions of individuals with AUD (16). Additionally, the correlation analysis of the MoCA scores revealed a positive correlation between the ALFF values and MoCA scores in the right inferior frontal gyrus. More importantly, we found, for the first time, enhanced FC (i.e., a stronger FC self-interaction) between the lateral and medial regions of the right inferior frontal gyrus, and a positive correlation with attention scores. This finding suggests that this stronger FC self-interaction may play a role within a single brain region in cognitive function, and thus provides a new perspective on the neural mechanisms of cognitive changes in AUD patients.
Compared with the HC group, the AUD group exhibited increased ALFF in the right inferior frontal gyrus under resting-state conditions, and this ALFF value was positively correlated with the total MoCA score. The right inferior frontal gyrus is a core component of the ventral attention network (VAN), which is predominantly a right-hemisphere network (17,18). Many studies have indicated that cognitive functions in the right and left inferior frontal gyri are not entirely symmetric; the right inferior frontal gyrus is closely associated with cognitive control, attention, and mental disorders, while the left inferior frontal gyrus is primarily involved in linguistic functions (19-25). Yoon et al. suggested that the right hemisphere is more sensitive to alcohol damage than the left hemisphere (26). In this study, there were neither significant functional abnormalities nor notable linguistic function score changes in the left inferior frontal gyrus of individuals with AUD. Therefore, we hypothesize that the changes in cognitive functions such as attention in individuals with AUD may be related to damage within the VAN network loop, and that the right inferior frontal gyrus is a key node in this loop.
In this study, we discovered for the first time that there was self-interactive enhancement of FC in the right inferior frontal gyrus of individuals with AUD (Figure 4). Further, we found a positive correlation between the enhanced inter-area FC value and the decreased attention score in the AUD group. According to the hyperconnectivity hypothesis, damage to the nervous system often results in paradoxical hyperconnectivity, where FC between network areas are enhanced (27). Hillary et al. suggested that hyperconnectivity may re-establish network communication by increasing connections through the most central areas with the highest metabolic efficiency (i.e., the hubs) (27). The right inferior frontal gyrus, a core component of the VAN, coordinates with the dorsal attention network (DAN) to form the human brain’s attention system (17). Additionally, some nodes in both the VAN and DAN are associated with visuospatial/executive functions (28). In this study, individuals with AUD exhibited significantly decreased attention and visuospatial/executive function scores, alongside self-interactive enhancement of FC in the right inferior frontal gyrus. Thus, the hyperconnectivity hypothesis may help explain the observed changes in brain functions in individuals with AUD.
This study also found a significantly increased ALFF value in the bilateral parahippocampal gyrus of individuals with AUD, and a significantly decreased ALFF value in the right middle frontal gyrus. These brain areas are part of the DMN. Our findings align with those of Zheng et al. on changes in brain function due to acute alcohol exposure, suggesting a similar neural basis for chronic alcohol damage and acute alcohol impact (5). Chronic alcoholism is associated with decreased local blood flow and a trend of volume atrophy in the parahippocampal gyrus (29). The parahippocampal gyrus is crucial for memory function (30). The alcohol-related cognitive load increases dorsolateral prefrontal cortex activation (31). Despite increased ALFF values in the bilateral parahippocampal gyrus of individuals with AUD in this study, no significant clinical memory disorders were found; however, the enhanced spontaneous neural activity in this region might partially compensate for decreased local blood flow, thereby playing a compensatory role in memory function.
This study had some limitations. First, we did not classify or control for the severity of cognitive impairment in AUD patients during the recruitment process. Although there was a statistically significant difference between the AUD group and the HC group in terms of the cognitive scores, the absolute value of the difference was small, which might have resulted in an inability to reflect the ALFF and FC changes changes involved in the patients with severe cognitive impairment. Second, in our RS-fMRI research, we did not use important research methods such as regional homogeneity analysis and graph theory-based FC analysis. Previous scholars have used these methods to explore changes in brain function in relapsed alcohol-dependent patients (32,33), indicating that these methods have strong applicability. Third, the factors of age, gender, and years of education were regressed as covariates in the statistical modeling to eliminate interference. Additionally, we found that at P=0.05, the FC between the medial area of the right inferior frontal gyrus and the lateral area of the inferior frontal gyrus was enhanced, and there were also changes in the FC between the medial area and a few other brain regions. However, it could not be corrected by multiple comparisons. Therefore, we excluded these results as false positive results and did not discuss them. In future experiments, we will combine MRI and analysis methods with more parameters to explore the neural functional alterations in AUD patients.
Conclusions
This study found notable changes in ALFF values in individuals with AUD in the resting state, specifically in the right inferior frontal gyrus, bilateral parahippocampal gyrus, and right middle frontal gyrus. Increased ALFF in the right inferior frontal gyrus was positively correlated with cognitive scores. Notably, for the first time, the self-interactive enhancement of FC was observed in the right inferior frontal gyrus of individuals with AUD, which was also correlated with the attention score. These findings suggest that the right inferior frontal gyrus may be a critical node affected by cognitive changes in individuals with AUD, with the self-interactive enhancement of FC potentially serving as a compensatory mechanism for cognitive impairment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2052/rc
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2052/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Medical Ethics Committee of the Affiliated Hospital of Guizhou Medical University (No. 2024-235). All participants provided informed consent before undergoing examination.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Global status report on alcohol and health and treatment of substance use disorders. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO.
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet 2019;394:781-92. [Crossref] [PubMed]
- Edelman EJ, Fiellin DA. In the Clinic. Alcohol Use. Ann Intern Med 2016;164:ITC1-16. [Crossref] [PubMed]
- Dai X, Yu J, Gao L, Zhang J, Li Y, Du B, Huang X, Zhang H. Cortical thickness and intrinsic activity changes in middle-aged men with alcohol use disorder. Alcohol 2023;106:15-21. [Crossref] [PubMed]
- Zheng H, Kong L, Chen L, Zhang H, Zheng W. Acute effects of alcohol on the human brain: a resting-state FMRI study. Biomed Res Int 2015;2015:947529. [Crossref] [PubMed]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, Mayer AR, Hutchison KE. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res 2014;38:2445-53. [Crossref] [PubMed]
- Wang Y, Zhao Y, Nie H, Liu C, Chen J. Disrupted Brain Network Efficiency and Decreased Functional Connectivity in Multi-sensory Modality Regions in Male Patients With Alcohol Use Disorder. Front Hum Neurosci 2018;12:513. [Crossref] [PubMed]
- Suk JW, Hwang S, Cheong C. Functional and Structural Alteration of Default Mode, Executive Control, and Salience Networks in Alcohol Use Disorder. Front Psychiatry 2021;12:742228. [Crossref] [PubMed]
- Canessa N, Basso G, Manera M, Poggi P, Gianelli C. Functional Coherence in Intrinsic Frontal Executive Networks Predicts Cognitive Impairments in Alcohol Use Disorder. Elife 2021;10:e61679. [Crossref] [PubMed]
- Zhornitsky S, Ide JS, Wang W, Chao HH, Zhang S, Hu S, Krystal JH, Li CR. Problem Drinking, Alcohol Expectancy, and Thalamic Resting-State Functional Connectivity in Nondependent Adult Drinkers. Brain Connect 2018;8:487-502. [Crossref] [PubMed]
- Fede SJ, Abrahao KP, Cortes CR, Grodin EN, Schwandt ML, George DT, Diazgranados N, Ramchandani VA, Lovinger DM, Momenan R. Alcohol effects on globus pallidus connectivity: Role of impulsivity and binge drinking. PLoS One 2020;15:e0224906. [Crossref] [PubMed]
- Li Q, Babor TF, Hao W, Chen X. The Chinese translations of Alcohol Use Disorders Identification Test (AUDIT) in China: a systematic review. Alcohol Alcohol 2011;46:416-23. [Crossref] [PubMed]
- Zhuang L, Yang Y, Gao J. Cognitive assessment tools for mild cognitive impairment screening. J Neurol 2021;268:1615-22. [Crossref] [PubMed]
- Kim BJ, Oh SH. Age-related changes in cognition and speech perception. Korean J Audiol 2013;17:54-8. [Crossref] [PubMed]
- Lang S, Duncan N, Northoff G. Resting-state functional magnetic resonance imaging: review of neurosurgical applications. Neurosurgery 2014;74:453-64; discussion 464-5. [Crossref] [PubMed]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008;172:137-41. [Crossref] [PubMed]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 2014;20:150-9. [Crossref] [PubMed]
- Farrant K, Uddin LQ. Asymmetric development of dorsal and ventral attention networks in the human brain. Dev Cogn Neurosci 2015;12:165-74. [Crossref] [PubMed]
- Grieder M, Soravia LM, Tschuemperlin RM, Batschelet HM, Federspiel A, Schwab S, Morishima Y, Moggi F, Stein M. Right Inferior Frontal Activation During Alcohol-Specific Inhibition Increases With Craving and Predicts Drinking Outcome in Alcohol Use Disorder. Front Psychiatry 2022;13:909992. [Crossref] [PubMed]
- Schaum M, Pinzuti E, Sebastian A, Lieb K, Fries P, Mobascher A, Jung P, Wibral M, Tüscher O. Right inferior frontal gyrus implements motor inhibitory control via beta-band oscillations in humans. Elife 2021;10:e61679. [Crossref] [PubMed]
- Steward T, Das P, Malhi GS, Bryant RA, Felmingham KL. Dysfunctional coupling of the parahippocampal cortex and inferior frontal gyrus during memory suppression in posttraumatic stress disorder. Eur Neuropsychopharmacol 2020;41:146-51. [Crossref] [PubMed]
- Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol 2011;2:330. [Crossref] [PubMed]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 2010;50:1313-9. [Crossref] [PubMed]
- Devine MJ, Bentley P, Jones B, Hotton G, Greenwood RJ, Jenkins IH, Joyce EM, Malhotra PA. The role of the right inferior frontal gyrus in the pathogenesis of post-stroke psychosis. J Neurol 2014;261:600-3. [Crossref] [PubMed]
- Yoshioka A, Tanabe HC, Nakagawa E, Sumiya M, Koike T, Sadato N. The Role of the Left Inferior Frontal Gyrus in Introspection during Verbal Communication. Brain Sci 2023;13:111. [Crossref] [PubMed]
- Yoon HW, Chung JY, Oh JH, Min HK, Kim DJ, Cheon Y, Joe KH, Kim YB, Cho ZH. Differential activation of face memory encoding tasks in alcohol-dependent patients compared to healthy subjects: an fMRI study. Neurosci Lett 2009;450:311-6. [Crossref] [PubMed]
- Hillary FG, Grafman JH. Injured Brains and Adaptive Networks: The Benefits and Costs of Hyperconnectivity. Trends Cogn Sci 2017;21:385-401. [Crossref] [PubMed]
- Ahrens MM, Veniero D, Freund IM, Harvey M, Thut G. Both dorsal and ventral attention network nodes are implicated in exogenously driven visuospatial anticipation. Cortex 2019;117:168-81. [Crossref] [PubMed]
- van der Burght CL, Goucha T, Friederici AD, Kreitewolf J, Hartwigsen G. Intonation guides sentence processing in the left inferior frontal gyrus. Cortex 2019;117:122-34. [Crossref] [PubMed]
- Lee WH, Frangou S. Linking functional connectivity and dynamic properties of resting-state networks. Sci Rep 2017;7:16610. [Crossref] [PubMed]
- Li G, Cao Y, Yang C, Li X, Yang Y, Yang L, Hao D, Li CR. Sex differences in dorsolateral prefrontal cortical and superior colliculus activities support the impact of alcohol use severity and sleep deficiency on two-back memory. Quant Imaging Med Surg 2024;14:4972-86. [Crossref] [PubMed]
- Deng R, Yang X, Meng YJ, Tao YJ, Wang HY, Li XJ, et al. Data-driven study on resting-state functional magnetic resonance imaging during early abstinence of alcohol dependence in male patients and its predictive value for relapse. BMC Psychiatry 2022;22:143. [Crossref] [PubMed]
- Böhmer J, Reinhardt P, Garbusow M, Marxen M, Smolka MN, Zimmermann US, Heinz A, Bzdok D, Friedel E, Kruschwitz JD, Walter H. Aberrant functional brain network organization is associated with relapse during 1-year follow-up in alcohol-dependent patients. Addict Biol 2023;28:e13339. [Crossref] [PubMed]


