
Comparative study of electrocardiogram-gated computed tomography (CT) and respiratory four-dimensional CT for radiotherapy in breast cancer patients
Introduction
Postoperative radiotherapy for breast cancer patients has been shown to be effective in reducing both recurrence and mortality (1). However, radiotherapy to the chest wall, especially to the left-sided breast cancer, increases the risk of heart disease (2). To further reduce the cardiac dose, deep-inspiration breath-hold (DIBH) has been implemented for left-sided breast cancer (3). However, implementing the DIBH technique requires additional equipment and patient coaching, resulting in a longer treatment duration and a greater need for patient compliance (4). Hence, motion analysis of the cardiac substructure (5) for left-sided breast cancer can aid in evaluating the risk of heart disease (2,6) when DIBH is not indicated. Furthermore, free-breathing respiratory gating is a promising alternative to reducing left anterior descending (LAD) artery dose (7). Therefore, evaluating the motion of the cardiac substructures is essential to identify the subset of patients that will benefit the most from gating technology.
Recently, researchers have explored the potential of the motion evaluation of cardiac substructures via electrocardiogram-gated computed tomography (ECG-CT) (5,8-10). Theoretically, ECG-CT can provide a more accurate depiction of the beating heart movement. However, the patient’s heart motion encompasses cardiac and respiratory pulsation. ECG-CT is a snapshot over a shorter period of a few cardiac cycles, omitting respiratory motion (5), which is not a routine clinical scenario in breast cancer radiotherapy. Furthermore, cardiac radiation ablation technology is becoming more prevalent in clinical practice (11). Respiratory-gated 4D CT is more commonly used (4,7,12-16) than ECG-CT to evaluate cardiac motion and cone beam computed tomography (CBCT) image registration in cardiac radiation ablation, cardiac-gated radiotherapy is too technically demanding and cannot be implemented at present.
Therefore, the objectives of this work were as follows: (I) to compare the cardiac substructure motion between ECG-CT and 4D CT; (II) to investigate the feasibility of 4D CT instead of ECG-CT for assessing cardiac substructure motion; (III) to evaluate the dosimetric benefit of the gating treatment in postoperative radiotherapy for left-sided breast cancer patients, to explore the feasibility of using a gating plan as an alternative to the DIBH plan; and (IV) to determine which cardiac substructure can be regarded as a reference for CBCT image registration in cardiac radiation ablation. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1579/rc).
Methods
Study population and image acquisition
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Ethics Committee of West China Hospital of Sichuan University approved this study (No. 2020-312). Informed consent was provided by all individual participants included in the study. From January 2021 to May 2024, 20 patients with left-sided breast cancer receiving postoperative radiotherapy (including 11 patients who underwent mastectomy and 9 patients who received breast-conserving surgery) underwent ECG-CT scanning and respiratory 4D CT scanning. Among these patients, ages ranged from 28 to 62 years, heights ranged from 146 to 169 cm, and weights ranged from 49 to 68 kg. ECG-CT images were acquired using a GE RevolutionTM CT (256 slices, with the ECG-CT package; GE Healthcare, Chicago, IL, USA) while the patients were breathing freely, with intravenous contrast administered (60 mL of iodine contrast followed by a mixture of 15 mL/15 mL of iodine and saline at a flow rate of 4.5 mL/s). ECG-CT data acquisition was triggered using a bolus-tracking program (SmartPrep, GE Healthcare), which was delayed 7.6 seconds after the CT value in the descending aorta (DAo) and was higher than the triggering threshold of 80 Hounsfield units (HU). The tube current was Smart-mA with a range from 400 to 680 mA, and the voltage was 100 kV. ECG-CT (0–90%, interval 10%) images were reconstructed according to ECG curves (thickness of 2.5 mm). The 4D CT scan mode commenced immediately after the patient completed the ECG-CT scan (thickness of 2.5 mm), and 4D images (0–90% phases, interval 10%) were reconstructed utilizing the breathing curve collected by the Varian Respiratory Gating for Scanners (RGSC).
Definition of cardiac substructures
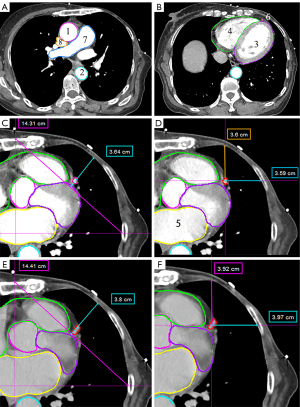
A 7-year experienced physicist and a 10-year experienced radiation oncologist delineated and reviewed the contours of cardiac substructures under ECG-CT and 4D CT images at each phase in the MIM system (MIM Software Inc., Cleveland, OH, USA), including ascending aorta (AAo), DAo, left ventricle + ventricular wall (LV_S), right atrium + right ventricle (RA + RV), left atrium (LA), LAD, pulmonary artery (PA), and superior vena cava (SVC) (see Figure 1).
Motion evaluation of cardiac substructures
The following metrics were defined and calculated to compare the motion of cardiac substructures obtained from the ECG-CT and 4D CT. Before comparison, the spinal bone registration between the ECG-CT and 4D CT was performed to eliminate setup errors.
- The absolute motion: the absolute difference from the centroid position in each phase to the average position of all ten phases in each direction.
Where D represents the motion direction, D = superior-inferior (SI), anterior-posterior (AP), right-left (RL). n stands for the nth phase, n =0, 1, ..., 9, CPD_a is the centroid position of the nth phase in D direction and CPD_a is the average of centroid positions for all ten phases. The three-dimensional (3D) coordinates of the centroid position of the cardiac substructure are obtained in the MIM system. Then, the mean absolute motion of all phases was compared between ECG-CT and 4D CT. Additionally, motion ranges were defined as the differences between the maximum and minimum centroid positional coordinates among all phases.
- The vector distance: the vector distance to the average position of all phases.
Where k is the number of phases, a is the average of all phases, and CP is the same as in Eq. [1].
- The Dice similarity coefficient (DSC) and Hausdorff distance (HD) (17): similarity evaluation between the internal target volume (ITV) of ECG-CT and 4D CT was performed by fusing all phases on the average density projection for similarity comparison; DSC and HD were calculated in the MIM system.
- The volume increment ratios:
Where GTVK is the gross tumor volume (GTV) in the Kth phase. ITVall is the volume of a single substructure fused with the contour expansion of ten temporal phases.
- Centroid position difference: calculate the spatial distance of the coordinate system of the fusion and expansion structure contour in ten phases of 4D CT and ECG-CT.
The higher DSC, smaller HD, and small volume increment ratio suggest that the two structures are more similar and have smaller position displacements when searching for which cardiac substructures can serve as matching structures for cardiac radiation ablation.
Left breast cancer program design
For left-sided breast cancer patients, the target volume is generated by combining the clinical target volume (CTV) delineated in the three gating phases. The treatment regimen for coplanar volumetric-modulated arc therapy (VMAT) consists of two partial arcs: clockwise (CW) with gantry angles ranging from 290° to 140°±10°, and counterclockwise (CCW) with gantry angles ranging from 140° to 290°±10°. The specific angles should be adjusted according to the patient’s situation. The prescription dose delivered to planning target volume (PTV) was 5,000 cGy (200 cGy per fraction ×25). The dose limitations for organs at risk were as follows: stomach mean dose (Dmean) <4,000 cGy, lung right V5 <20%, breast right Dmean <500 cGy, LAD Dmean <2,500 cGy, heart V5 (volume receiving 5 Gy) <40%, heart Dmean <600 cGy, lung left V5 <50%, lung left Dmean <1,400 cGy, lung left V2 <25%, humerus head left maximum dose (Dmax) <4,000 cGy, V30 <35%, cord planning organ at risk volume (PRV) Dmax <2,500 cGy, cord Dmax <2,000 cGy.
The quantitative evaluation of dosimetric advantages in the gating plan based on 4D CT
To select the optimal phases for the gating plan, as well as to compare the capability of chest wall motion description between 4D CT and ECG-CT, the distance between LAD and chest wall in RL and AP directions, chest wall motion in AP directions, and diaphragm motion in SI direction were measured and compared between 4D CT and ECG-CT at each phase. Among all phases, we calculated the maximum distance between LAD and chest wall in the RL and AP directions. The distance between LAD and chest wall in RL and AP directions was measured at the plane 1 and 2 (15 and 30 mm below the coronary artery outlet, respectively), as shown in Figure 1D,1F. Vertical lines from the midline and horizontal lines from the midaxillary line were drawn for the chest wall motion in the AP direction. The two lines intersect with the chest wall were connected as tangent lines. Then, we measured the distance from the chest wall perpendicular to the tangent line at planes 1, 2, and 3 (located at the heart base) (7). It is defined as the chest wall motion in the AP direction.
Due to the superior motion description of the chest wall and diaphragm and the inherent gating capability of 4D CT, it was selected for the gating plan rather than ECG-CT in this study. In the 4D CT scan mode, between 0% and 90% of the structure delineated by the imaging is projected onto the average intensity projection (AIP) (18) image, fused into a new structure, and subsequently utilized for developing the radiotherapy plan. Second, AIP images (19) reconstructed from 4D CT are widely adopted as reference images for CBCT registration. According to these literature reports and actual clinical application, AIP images from 4D CT were used for treatment planning. Therefore, based on the distance between the LAD and the chest wall in RL and AP directions, three optimal phases where the LAD is farthest from the chest wall were selected to design the gating plan. To demonstrate the dosimetric advantage of the gating plan, we transferred the contours of LAD delineated under AIP images (LADAIP) to the gating plan for dose evaluation. Then, we compared the LAD and LADAIP doses in the gating plan and the LAD and heart doses in the AIP and gating plan.
Results
For the 20 patients, as illustrated in Figure 2 and Table 1, we observed the maximum absolute motion and motion range for the LAD. There were no statistically significant differences in the SI and RL directions of the LAD’s mean absolute motion and motion ranges between ECG-CT and 4D CT. A statistically significant difference was observed in the AP direction. The motion range of LAD measured in 4D CT and ECG-CT was 8.52±4.35 mm (range, 2.57–17.57 mm) vs. 6.46±2.58 mm (range, 2.63–11.71 mm) in the AP direction. In the SI direction, except for LAD, there were statistically significant differences in the mean absolute motion measured between ECG-CT and 4D CT for other substructures. The LAD had maximum vector distances of 3.83±2.68 and 3.39±2.06 mm measured by 4D CT and ECG-CT, respectively. The heart had smaller vector distances of 1.59±0.86 and 0.80±0.36 mm, and the DAo had the smallest vector distances of 1.43±1.03 and 0.61±0.52 mm. The vector distances of the LAD, LV_S, and SVC were not statistically significant, whereas other substructural vector differences were statistically significant. The heart had minimum mean absolute motion in the AP direction (0.26±0.22 mm) and minor motion in SI (1.37±0.89 mm) and RL (0.57±0.41mm) directions in 4D CT.
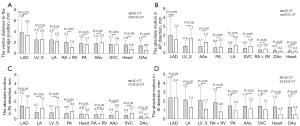
Table 1
| Substructures | SI | RL | AP | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECG-CT | 4D CT | P value | ECG-CT | 4D CT | P value | ECG-CT | 4D CT | P value | ||||||||||||
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |||||||||
| AAo (mm) | 3.97±1.42 | 1.32–7.20 | 5.71±1.65 | 2.62–9.96 | <0.01 | 2.68±0.62 | 1.94–3.95 | 1.61±0.59 | 0.62–2.63 | <0.01* | 4.02±0.81 | 2.33–5.53 | 2.66±0.60 | 1.75–3.78 | <0.01* | |||||
| DAo (mm) | 2.01±1.41 | 0.49–5.55 | 5.04±1.93 | 2.74–9.94 | <0.01 | 0.57±0.17 | 0.24–0.95 | 0.89±0.46 | 0.32–1.84 | 0.01 | 0.69±0.28 | 0.13–1.30 | 1.12±0.38 | 0.53–1.98 | <0.01 | |||||
| LA (mm) | 4.73±1.11 | 2.63–6.54 | 8.23±1.58 | 4.88–10.99 | <0.01 | 4.47±1.38 | 1.66–7.43 | 3.02±1.00 | 1.23–5.13 | <0.01* | 3.36±0.92 | 1.39–5.07 | 2.21±0.43 | 1.44–3.16 | <0.01* | |||||
| LAD (mm) | 8.99±2.78 | 4.69–17.18 | 10.64±3.09 | 5.83–18.32 | 0.10 | 5.04±2.29 | 2.19–13.04 | 5.94±2.44 | 2.34–11.11 | 0.24 | 6.46±2.58 | 2.63–11.71 | 8.52±4.35 | 2.57–17.57 | 0.03 | |||||
| LV_S (mm) | 4.11±1.23 | 2.04–7.27 | 8.05±2.35 | 3.23–12.87 | <0.01 | 4.34±1.00 | 1.90–5.76 | 2.76±0.85 | 1.00–4.54 | <0.01* | 5.30±1.52 | 2.59–8.65 | 3.11±1.03 | 1.25–5.32 | <0.01* | |||||
| PA (mm) | 3.27±0.94 | 1.89–4.74 | 5.44±1.22 | 3.16–7.84 | <0.01 | 3.02±0.85 | 1.75–4.83 | 2.45±0.76 | 1.11–3.97 | 0.03* | 2.61±0.77 | 1.51–4.31 | 2.47±1.03 | 1.09–5.86 | 0.65 | |||||
| RA + RV (mm) | 3.17±0.98 | 2.03–5.29 | 6.82±1.75 | 3.09–10.17 | <0.01 | 1.80±0.63 | 0.56–3.02 | 1.77±0.67 | 0.81–3.27 | 0.88 | 1.67±0.60 | 0.80–2.93 | 1.37±0.44 | 0.57–2.36 | 0.06 | |||||
| SVC (mm) | 4.03±1.19 | 1.58–5.67 | 5.35±1.60 | 2.81–8.71 | <0.01 | 2.09±0.67 | 0.98–3.79 | 1.50±0.58 | 0.55–2.50 | <0.01* | 2.63±0.68 | 1.60–3.65 | 2.09±0.63 | 1.13–3.17 | <0.01* | |||||
| Heart (mm) | 1.76±0.63 | 0.85–2.90 | 4.85±1.35 | 0.85–6.95 | <0.01 | 1.69±0.55 | 0.58–2.92 | 2.07±0.69 | 0.97–3.14 | 0.02 | 0.62±0.25 | 0.27–1.17 | 0.94±0.46 | 0.29–2.04 | 0.02 | |||||
* represents the motion of cardiac substructures based on ECG-CT is significantly bigger than that of 4D CT. 4D CT, four-dimensional computed tomography; AAo, ascending aorta; AP, anterior-posterior; DAo, descending aorta; ECG-CT, electrocardiogram-gated computed tomography; LA, left atrium; LAD, left anterior descending artery; LV_S, left ventricle + ventricular wall; PA, pulmonary artery; RA + RV, right atrium + right ventricle; RL, right-left; SI, superior-inferior; SVC, superior vena cava; SD, standard deviation.
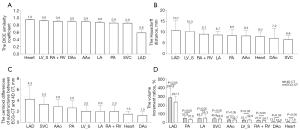
When comparing the structure delineation based on ECG-CT and 4D CT (see Figure 3), the higher DSC coefficients were observed for the heart (0.95), LV_S (0.92), RA + RV (0.91), and DAo (0.90), whereas the lower was for LAD (0.59). The heart (7.96 mm), DAo (7.21 mm), and SVC (6.64 mm) had smaller HD, whereas the largest was for LAD (10.67 mm). The centroid difference of cardiac substructures measured by ECG-CT and 4D CT were the most pronounced for the LAD (4.25±2.46 mm), with the minor difference observed for the DAo (1.26±0.68), followed by the heart (1.52±0.52 mm) and RA + RV (1.97±1.06 mm). For the volume increment ratio, LAD had the maximum value with 237.69%±64.90% for ECG-CT and 286.84%±105.22% for 4D CT, whereas the minimum was for the heart, with 7.75%±2.85% for ECG-CT and 11.71%±2.99% for 4D CT. DAo showed a smaller volume increment ratio of 9.97%±4.64% for ECG-CT and 13.14%±4.95% for 4D CT. The results suggest that RA + RV, DAo, and heart can serve as reference structures for cardiac radiation ablation matching.
The maximum distance between LAD and chest wall in RL and AP directions, the chest wall motion in the AP direction, and the diaphragmatic motion in the SI direction (see Table 2) measured based on 4D CT were significantly larger than those of ECG-CT. The mean diaphragmatic motion in the SI direction was 4.83±3.38 and 17.82±3.87 mm for ECG-CT and 4D CT, respectively. The AP direction motion of the chest wall was minimal in both ECG-CT and 4D CT, with 0.22±0.38/1.56±1.90 mm, 0.25±0.41/1.17±0.83 mm, and 0.10±0.30/1.16±0.77 mm in planes 1, 2, and 3, respectively.
Table 2
| Patients | Plane 1 | Plane 2 | Plane 3 | Diaphragm (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAD AP (mm) | LAD RL (mm) | Chest AP (mm) | LAD AP (mm) | LAD RL (mm) | Chest AP (mm) | Chest AP (mm) | ECG-CT | 4D CT | |||||||||||||||
| ECG-CT | 4D CT | ECG-CT | 4D CT | ECG-CT | 4D CT | ECG-CT | 4D CT | ECG-CT | 4D CT | ECG-CT | 4D CT | ECG-CT | 4D CT | ||||||||||
| No. 1 | 5.40 | 8.80 | 8.70 | 11.10 | 0.00 | 9.00 | 1.80 | 1.60 | 2.40 | 2.00 | 0.00 | 0.80 | 0.00 | 1.70 | 6.60 | 22.90 | |||||||
| No. 2 | 9.90 | 13.70 | 10.50 | 15.10 | 0.50 | 1.40 | 2.90 | 4.20 | 9.40 | 7.10 | 0.80 | 1.50 | 0.00 | 1.90 | 10.30 | 18.90 | |||||||
| No. 3 | 4.70 | 8.10 | 2.70 | 4.80 | 0.00 | 1.40 | 2.90 | 3.00 | 3.20 | 4.10 | 0.00 | 0.90 | 0.00 | 1.50 | 4.90 | 16.30 | |||||||
| No. 4 | 15.80 | 28.80 | 13.30 | 28.70 | 1.50 | 2.50 | 7.90 | 11.50 | 8.10 | 16.10 | 1.30 | 1.80 | 0.00 | 1.00 | 3.00 | 16.60 | |||||||
| No. 5 | 8.80 | 11.80 | 7.90 | 14.00 | 0.80 | 2.30 | 3.10 | 5.00 | 12.00 | 8.80 | 1.20 | 2.40 | 0.00 | 2.00 | 4.00 | 11.90 | |||||||
| No. 6 | 2.60 | 6.00 | 2.70 | 4.10 | 0.00 | 0.70 | 1.30 | 3.00 | 5.40 | 5.00 | 0.00 | 0.60 | 0.00 | 1.00 | 2.60 | 15.40 | |||||||
| No. 7 | 11.20 | 2.70 | 10.70 | 7.00 | 0.00 | 1.10 | 4.50 | 8.40 | 6.00 | 10.20 | 0.00 | 1.00 | 0.00 | 1.00 | 3.30 | 13.70 | |||||||
| No. 8 | 4.50 | 5.00 | 6.50 | 9.10 | 0.00 | 1.70 | 3.90 | 6.10 | 4.80 | 9.60 | 0.00 | 1.50 | 0.00 | 1.10 | 1.70 | 23.50 | |||||||
| No. 9 | 6.00 | 10.10 | 5.20 | 7.80 | 0.00 | 0.00 | 2.70 | 3.30 | 5.00 | 4.90 | 0.00 | 0.00 | 0.00 | 0.00 | 11.00 | 18.20 | |||||||
| No. 10 | 11.00 | 23.10 | 8.80 | 19.90 | 0.00 | 1.00 | 1.60 | 5.20 | 4.30 | 7.40 | 0.00 | 3.60 | 0.00 | 1.00 | 9.20 | 17.90 | |||||||
| No. 11 | 4.80 | 25.20 | 3.50 | 16.80 | 0.00 | 1.90 | 2.10 | 5.00 | 3.70 | 7.80 | 0.00 | 1.20 | 0.00 | 0.80 | 1.00 | 22.80 | |||||||
| No. 12 | 10.50 | 12.20 | 13.10 | 20.90 | 0.00 | 2.00 | 4.10 | 5.60 | 3.70 | 8.60 | 0.50 | 1.80 | 0.00 | 0.70 | 9.50 | 18.20 | |||||||
| No. 13 | 7.30 | 11.70 | 8.20 | 12.00 | 0.00 | 2.20 | 2.00 | 6.00 | 1.40 | 7.60 | 0.00 | 1.60 | 1.30 | 2.60 | 1.00 | 16.00 | |||||||
| No. 14 | 10.00 | 8.90 | 10.00 | 7.70 | 0.40 | 0.70 | 5.20 | 5.30 | 7.10 | 6.80 | 0.00 | 0.50 | 0.30 | 0.90 | 1.80 | 10.50 | |||||||
| No. 15 | 6.90 | 11.20 | 13.60 | 15.50 | 0.30 | 0.50 | 3.70 | 6.70 | 2.60 | 5.30 | 0.50 | 0.50 | 0.00 | 0.40 | 10.00 | 17.00 | |||||||
| No. 16 | 2.90 | 4.00 | 5.60 | 6.40 | 0.30 | 0.30 | 1.00 | 1.20 | 6.30 | 2.30 | 0.40 | 0.60 | 0.00 | 0.70 | 4.70 | 17.20 | |||||||
| No. 17 | 6.50 | 14.60 | 8.50 | 17.90 | 0.00 | 0.30 | 2.60 | 7.00 | 3.50 | 7.50 | 0.00 | 0.10 | 0.00 | 0.00 | 2.40 | 13.00 | |||||||
| No. 18 | 7.80 | 18.10 | 5.30 | 9.60 | 0.30 | 0.60 | 2.00 | 6.00 | 4.30 | 4.60 | 0.00 | 0.90 | 0.00 | 3.00 | 4.50 | 21.10 | |||||||
| No. 19 | 10.30 | 15.50 | 5.70 | 14.10 | 0.00 | 1.00 | 2.20 | 5.40 | 3.50 | 13.70 | 0.00 | 0.80 | 0.00 | 0.80 | 1.60 | 22.60 | |||||||
| No. 20 | 5.50 | 7.70 | 4.20 | 6.80 | 0.30 | 0.50 | 1.50 | 2.00 | 3.50 | 5.20 | 0.30 | 1.30 | 0.30 | 1.00 | 3.40 | 22.70 | |||||||
| Mean ± SD | 7.62±3.30 | 12.36±6.99 | 7.74±3.42 | 12.47±6.30 | 0.22±0.38 | 1.56±1.90 | 2.95±1.62 | 5.08±2.42 | 5.01±2.57 | 7.23±3.45 | 0.25±0.41 | 1.17±0.83 | 0.10±0.30 | 1.16±0.77 | 4.83±3.38 | 17.82±3.87 | |||||||
| P value | <0.01 | <0.01 | 0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 | |||||||||||||||
4D CT, four-dimensional computed tomography; AP, anterior-posterior; ECG-CT, electrocardiogram-gated computed tomography; LAD, left anterior descending artery; RL, right-left; SD, standard deviation.
For the dose comparison between the gating and AIP plans (see Table 3), there were no statistically significant differences in the average dose of LAD, the heart and V500 cGy. In contrast, the LAD volume based on gating plans was significantly smaller than those of AIP images (3.07±1.09 vs. 5.71±1.53 cc, P<0.01). The average dose for LADAIP in the gating plan was markedly lower than that of the LAD in the AIP plan (1,685.85±355.99 vs. 1,844.05±394.43 cGy, P<0.01) and gating plan (1,685.85±355.99 vs. 1,827.30±411.13 cGy, P<0.01).
Table 3
| Patient | Phases (%) | LAD volume (cc) | LAD mean dose (cGy) | Heart mean dose (cGy) | Heart V5 (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gating¶ | AIP§ | Gating¶ | AIP§ | LADAIP† in gating plan |
Gating¶ | AIP§ | Gating¶ | AIP§ | |||||
| No. 1 | 80–0 | 2.62 | 3.95 | 1,741.00 | 1,750.00 | 1,631.00 | 537.00 | 484.00 | 21.00 | 17.50 | |||
| No. 2 | 90–10 | 2.55 | 4.96 | 1,922.00 | 1,929.00 | 1,501.00 | 417.00 | 435.00 | 13.40 | 20.50 | |||
| No. 3 | 0–20 | 2.86 | 6.76 | 1,930.00 | 1,968.00 | 1,891.00 | 509.00 | 519.00 | 20.63 | 20.61 | |||
| No. 4 | 90–10 | 1.71 | 3.65 | 1,977.00 | 1,934.00 | 1,837.00 | 592.00 | 607.00 | 23.50 | 23.55 | |||
| No. 5 | 90–10 | 3.17 | 5.29 | 1,676.00 | 1,804.00 | 1,526.00 | 450.00 | 457.00 | 13.40 | 13.50 | |||
| No. 6 | 0–20 | 3.19 | 5.29 | 1,799.00 | 1,904.00 | 1,878.00 | 519.00 | 520.00 | 23.06 | 21.90 | |||
| No. 7 | 0–20 | 3.25 | 5.94 | 1,903.00 | 1,858.00 | 1,762.00 | 587.00 | 598.00 | 32.00 | 33.00 | |||
| No. 8 | 90–10 | 2.03 | 4.16 | 1,897.00 | 1,821.00 | 1,763.00 | 524.00 | 507.00 | 21.00 | 19.50 | |||
| No. 9 | 0–20 | 2.43 | 6.01 | 2,494.00 | 2,423.00 | 2,133.00 | 536.00 | 551.00 | 21.50 | 20.50 | |||
| No. 10 | 0–20 | 2.74 | 5.29 | 1,968.00 | 1,991.00 | 1,808.00 | 445.00 | 470.00 | 18.50 | 20.50 | |||
| No. 11 | 90–20 | 2.44 | 3.34 | 1,643.00 | 1,630.00 | 1,561.00 | 324.00 | 359.00 | 15.18 | 15.00 | |||
| No. 12 | 0–20 | 3.81 | 7.78 | 1,812.00 | 1,828.00 | 1,551.00 | 540.00 | 561.00 | 31.00 | 28.00 | |||
| No. 13 | 0–20 | 1.91 | 4.66 | 1,926.00 | 1,918.00 | 1,601.00 | 486.00 | 480.00 | 20.00 | 19.00 | |||
| No. 14 | 90–10 | 2.79 | 5.28 | 1,770.00 | 1,733.00 | 1,610.00 | 422.00 | 432.00 | 14.00 | 14.50 | |||
| No. 15 | 90–10 | 4.60 | 8.09 | 2,481.00 | 2,461.00 | 2,313.00 | 549.00 | 515.00 | 21.23 | 15.50 | |||
| No. 16 | 80–20 | 6.31 | 7.58 | 1,642.00 | 1,731.00 | 1,647.00 | 541.00 | 561.00 | 22.00 | 22.50 | |||
| No. 17 | 80–0 | 2.81 | 5.70 | 2,429.00 | 2,469.00 | 2,115.00 | 587.00 | 612.00 | 32.00 | 28.00 | |||
| No. 18 | 0–20 | 4.77 | 8.83 | 917.00 | 923.00 | 836.00 | 302.00 | 293.00 | 9.50 | 8.50 | |||
| No. 19 | 0–20 | 2.78 | 6.97 | 1,772.00 | 1,887.00 | 1,865.00 | 522.00 | 519.00 | 19.00 | 18.50 | |||
| No. 20 | 90–10 | 2.69 | 4.71 | 847.00 | 919.00 | 888.00 | 277.00 | 325.00 | 8.00 | 12.00 | |||
| Mean ± SD | – | 3.07±1.09 | 5.71±1.53 | 1,827.30±411.13 | 1,844.05±394.43 | 1,685.85±355.99 | 483.30±93.61 | 490.25±88.48 | 20.00±6.67 | 19.63±5.81 | |||
| P value | – | <0.01 | 0.23 | <0.01* | 0.20 | 0.56 | |||||||
¶, the gating plan is based on the gating phases’ image; §, the AIP plan is based on the AIP image from 4D CT; †, the contours of LAD delineated under AIP images were transferred to the gating plan for dose evaluation; *, t-test comparison between LADAIP dose and LAD dose in the gating plan, as well as between LADAIP dose in the gating plan and LAD dose in the AIP plan. 4D CT, four-dimensional computed tomography; AIP, average intensity projection; LAD, left anterior descending; SD, standard deviation.
Discussion
Qi et al. reported that heart-induced motion in LAD and the max heart depth (MHD) varied up to 9 and 11 mm, respectively (7). Tan et al. demonstrated that the average motion of the coronary arteries was 2.8–5.9, 3.5–6.6, and 3.8–5.3 mm in the AP, LR, and SI directions, respectively (20). Li et al. reported that the motion of the caudal portion of the LAD was 4.6±2.4 (1.2–13.2) mm (AP direction), 6.4±3.4 (2.4–17.0) mm (RL direction), and 7.7±3.0 (3.4–15.5) mm (SI direction) (8). Ouyang et al. reported that the maximum motion of the LAD related to its average position was 5.45 mm (5).
However, studies conducted by Ouyang et al. (5), Li et al. (8), and Su et al. (9) all performed ECG-CT scans under breath-holding, which eliminated the influence of respiratory motion and were inconsistent with clinical treatment scenarios. Therefore, the LAD motion in these studies is smaller than ours [the maximum motion ranges of LAD obtained from 4D CT and ECG-CT were 18.32 mm (SI), 11.11 mm (RL), and 17.57 mm (AP) vs. 17.18 mm (SI), 13.04 mm (RL), and 11.71 mm (AP), respectively; see Table 1]. Meanwhile, Qi et al. (7) and Vasquez Osorio et al. (21) utilized 4D CT to analyze the cardiac motion without a direct comparison with ECG-CT. Our results revealed that absolute motion and motion range evaluation in the SI direction based on 4D CT were larger than those of ECG-CT for all cardiac substructures except LAD. This difference was primarily attributed to the predominance of respiratory motion in the SI direction. ECG-CT scans eliminate the effect of respiratory motion, so the motion range evaluated by the ECG-CT in the SI direction is smaller than that of the 4D CT. The contrasting results of LAD may mainly be attributed to the longer length of LAD in the SI direction, resulting in respiratory motion-induced changes being overshadowed. According to these findings, we can conclude that 4D CT is a better way to evaluate the motion of cardiac substructures than ECG-CT in the SI direction.
Meanwhile, in the RL and AP directions, the motion of substructures such as LA, LV_S, AAo, SVC, and PA assessed by ECG-CT was larger than that of 4D CT. We speculate that cardiac pulsation mainly affects these substructure motions, and respiratory motion has a marginal impact. Therefore, 4D CT may not effectively reflect these motions. For RA + RV, there were no differences in absolute motion and motion range assessed by ECG-CT and 4D CT. Although there were differences between DAo and the heart in these two directions, the magnitudes of motion were small. Regarding LAD, the motion assessed by 4D CT in the AP direction was greater than that assessed by ECG-CT, whereas there was no statistically significant difference in the RL direction. We speculate that this may be because the AP direction is mainly influenced by respiratory motion, whereas the RL direction is primarily influenced by cardiac contraction. Prusator et al. (22) reported that cardiac motion alone, as captured by ECG-CT, was similar to the combined cardiac and respiratory motion obtained from 4D CT due to using abdominal compression to reduce respiratory motion. Based on the above results, we believe that compared to 4D CT, ECG-CT has advantages in assessing the motion of structures for AAo, LA, LV_S, and SVC in the AP and RL directions and PA in the RL direction.
In our study, the heart, LV_S, RA + RV, and DAo demonstrated higher DSC coefficients, indicating greater contour similarity between the two scanning modes. Small centroid differences were observed for RA + RV (1.97 mm), heart (1.52 mm), and DAo (1.26 mm). This was smaller than the results reported by Qi et al. [the heart’s maximum centroid movement ranged from 0.8 to 7.7 mm (14)]. The low cardiac volume increment ratio further suggests negligible differences in overall cardiac contour volumes between modalities, demonstrating high consistency. In addition, some studies have utilized ECG-gated magnetic resonance imaging (MRI) to investigate the motion or volume change of cardiac substructures at different phases (23,24). The study by Yan et al. calculated the cardiac volume changes between the end-systole and end-diastole phases based on MRI, with a mean volume difference of 99.5 cm3 (approximately a 14.38% change). The differences compared to our study may be due to variations in the calculation methods and the populations enrolled (25). The heart (7.96 mm), DAo (7.21 mm), and SVC (6.64 mm) had smaller HD, indicating that these three substructures have better shape similarity between 4D CT and ECG-CT. Thus, our study evaluated these parameters to help image registration and PTV margin calculation in cardiac ablation treatment.
To ensure treatment accuracy in cardiac ablation (11), AIP images (19) reconstructed from 4D CT are widely adopted as reference images for CBCT registration. The heart’s movement is influenced by respiratory motion and cardiac pulsation. 4D CT captures multiple complete respiratory cycles, thereby accurately representing respiratory motion. In contrast, ECG-CT provides higher temporal resolution, effectively capturing cardiac systolic and diastolic motion while ignoring the respiratory motion. If the results of DSC, HD, centroid distance, and volume increment ratio for cardiac substructures obtained from 4D CT and ECG-CT are comparable, this suggests minimal differences in motion characterization between the two imaging modalities. Given that the SVC is poorly visualized on CBCT, RA + RV, DAo, and heart contours may serve as reliable landmarks for aligning planning AIP images, thereby ensuring treatment accuracy in cardiac ablation procedures. Meanwhile, there are discrepancies in the volume and centroid of the target area in AIP images compared to those based on reconstructions from ten phases combined (representing the entire range of respiratory motion). Therefore, we calculated the centroid differences of the heart based on the AIP image and the combined image of all ten phases. The centroid differences of the heart were −0.73±0.86, 1.07±0.53, and −0.20±0.39 mm in the SI, RL, and AP directions, respectively.
It is worth noting that cardiac radiation ablation is primarily used for treating ventricular tachycardia, with the most common target located in the left ventricle (LV). To accurately define the target of cardiac radiation ablation, the American Heart Association (AHA) has standardized the LV into 17 segments (26). Additionally, Poon et al. (27) quantified the motion for 17 different LV segments. However, their study used MRI for analysis, and patients underwent MRI during breath-hold. Based on the data from Poon et al. (27), segments 5 (basal inferolateral) and 6 show the largest epicardial and endocardial motion amplitudes, approaching 10 mm. When using the 0.45× (28) formula to calculate the ITV margin, these two segments require an ITV margin close to 5 mm. Considering setup errors in different radiation departments and the centroid differences of the heart based on the AIP image and the combined image of all ten phases, an additional 3–5 mm PTV margin is needed, making the total margin exceed 10 mm (27).
According to the maximum distance between LAD and chest wall in the RL and AP directions, as well as in the AP direction of chest wall motion, 4D CT is more suitable for a gating plan. Meanwhile, the cardiac gating treatment (22,29) is currently not available for clinical use. El-Sherif et al. (13) suggested using 4D CT to estimate the dose for the LAD. In our study, we selected three phases with the farthest RL and AP distances between LAD and the chest wall for a gating plan in 4D CT and compared it with the AIP plan. The volume of LAD in the gating plan was significantly smaller than that of the AIP plan, and there were no statistically significant differences in LAD mean dose, heart mean dose, and heart V5 between the AIP plan and gating plan. We transferred the delineated contour of the LAD from AIP to the gating plan and re-evaluated the dose to the LAD and heart. The results showed that All patients had smaller doses of LAD in the gating plan than in the AIP plan, as shown in Table 3. Meanwhile, we noticed that some patients exhibited small motion ranges of LAD (2.57 and 2.34 mm, see Table 1) and a small distance between the LAD and the chest wall in the AP and RL directions (1.2 and 2.0 mm, respectively, at plane 2; see Table 2). However, the doses of LAD in these patients in the gating plan were still smaller than those in the AIP plan. Furthermore, our study employed three phases for the gating plan, which appears more clinically feasible compared to the single-phase approach used by Qi et al. (7). These results suggested that a gating plan based on three phases is a valid way to reduce LAD dose regardless of LAD motion in the RL and AP directions for left-sided breast cancer patients if DIBH is unavailable.
This study focuses on a dosimetric comparison of inspiratory phase gating therapy, which has not been implemented in clinical practice. The selected inspiratory phase for gating therapy results in a treatment duration that is at least 3.3 times longer than that under free breathing (FB) conditions. Therefore, the extended treatment duration may introduce uncertainties in treatment accuracy, posing potential challenges for clinical application. Based on our experience and findings from relevant literature (30), respiratory training can ensure a more predictable and consistent breathing pattern, which may help mitigate the uncertainties associated with prolonged treatment durations, thereby improving treatment accuracy and feasibility.
The motion of the chest wall was minimal (1.56±1.90, 1.17±0.83, and 1.16±0.77 mm in plane 1, plane 2, and plane 3, respectively), consistent with a previous study result (31). Simultaneously, Qi et al. found that the maximum centroid movement of the ipsilateral breast ranged from 1.1 to 3.9 mm (14). Negligible chest movement was observed between FB exhale and FB inhale (median: 0.1 cm, range: 0.2 cm) (32). Bedi et al. (12) pointed out that as the motion of the whole breast was generally small throughout the respiratory cycle, the radiation treatment plan based on 3D CT scans reflected the dose distributions in 4D CT-derived datasets well. Therefore, we proposed that for patients with right-sided breast cancer, who have minimal chest wall respiratory motion, and for whom there is no need to consider the radiation dose to the heart, 3D free-breathing helical CT (3D FH-CT) can be an alternative to 4D CT for simulation. For patients with left-sided breast cancer, we recommended the use of 4D CT scans to evaluate the motion of the LAD in the AP and RL directions. This assessment can aid in determining whether a gating plan should be implemented to minimize the radiation dose to the LAD. Future studies should focus on developing an automatic deep-learning method for the segmentation of cardiac substructures (33) and evaluating the clinical efficiency of the gating treatment.
Conclusions
4D CT is a superior method to ECG-CT for evaluating the motion of cardiac substructures in the SI direction. The evaluation of motion for AAo, LA, LV_S, and SVC in RL and AP directions and PA in RL direction based on ECG-CT is better than that of 4D CT. 4D CT-based gating provides a viable alternative to reducing the LAD dose when DIBH is unavailable. Furthermore, the heart, DAo, and RA + RV can be employed as reference structures for cardiac radiation ablation.
Acknowledgments
We appreciate the support provided by GE Corporation for the ECG-CT scanning technology.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1579/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1579/coif). All authors report the funding from the 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2021HXFH029); and the Science and Technology Support Program of Sichuan Province, China (No. 2021YFQ0065). GE Corporation has provided support for ECG-CT scanning technology free of charge. M.G. is a current employee of GE Healthcare Co. Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2020-312) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, Wang Y, Wang Z, Darby S. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Taylor C, McGale P, Brønnum D, Correa C, Cutter D, Duane FK, Gigante B, Jensen MB, Lorenzen E, Rahimi K, Wang Z, Darby SC, Hall P, Ewertz M. Cardiac Structure Injury After Radiotherapy for Breast Cancer: Cross-Sectional Study With Individual Patient Data. J Clin Oncol 2018;36:2288-96. [Crossref] [PubMed]
- Ball HJ, Santanam L, Senan S, Tanyi JA, van Herk M, Keall PJ. Results from the AAPM Task Group 324 respiratory motion management in radiation oncology survey. J Appl Clin Med Phys 2022;23:e13810. [Crossref] [PubMed]
- Chau OW, Fakir H, Lock M, Dinniwell R, Perera F, Erickson A, Gaede S. Dosimetric Planning Comparison for Left-Sided Breast Cancer Radiotherapy: The Clinical Feasibility of Four-Dimensional-Computed Tomography-Based Treatment Planning Optimization. Cureus 2022;14:e24777. [Crossref] [PubMed]
- Ouyang Z, Schoenhagen P, Wazni O, Tchou P, Saliba WI, Suh JH, Xia P. Analysis of cardiac motion without respiratory motion for cardiac stereotactic body radiation therapy. J Appl Clin Med Phys 2020;21:48-55. [Crossref] [PubMed]
- Naimi Z, Moujahed R, Neji H, Yahyaoui J, Hamdoun A, Bohli M, Kochbati L. Cardiac substructures exposure in left-sided breast cancer radiotherapy: Is the mean heart dose a reliable predictor of cardiac toxicity? Cancer Radiother 2021;25:229-36. [Crossref] [PubMed]
- Qi XS, Hu A, Wang K, Newman F, Crosby M, Hu B, White J, Li XA. Respiration induced heart motion and indications of gated delivery for left-sided breast irradiation. Int J Radiat Oncol Biol Phys 2012;82:1605-11. [Crossref] [PubMed]
- Li Q, Tong Y, Yin Y, Cheng P, Gong G. Definition of the margin of major coronary artery bifurcations during radiotherapy with electrocardiograph-gated 4D-CT. Phys Med 2018;49:90-4. [Crossref] [PubMed]
- Su M, Gong G, Qiu X, Tong Y, Li Q, Yin Y. Study on the Effect of 4D-CT Special Reconstruction Images for Evaluation of the Cardiac Structure Dose in Radiotherapy for Breast Cancer. Front Oncol 2020;10:433. [Crossref] [PubMed]
- Tong Y, Yin Y, Cheng P, Gong G. Impact of deformable image registration on dose accumulation applied electrocardiograph-gated 4DCT in the heart and left ventricular myocardium during esophageal cancer radiotherapy. Radiat Oncol 2018;13:145. [Crossref] [PubMed]
- Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW, Hallahan D, Rudy Y, Robinson CG. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med 2017;377:2325-36. [Crossref] [PubMed]
- Bedi C, Kron T, Willis D, Hubbard P, Milner A, Chua B. Comparison of radiotherapy treatment plans for left-sided breast cancer patients based on three- and four-dimensional computed tomography imaging. Clin Oncol (R Coll Radiol) 2011;23:601-7. [Crossref] [PubMed]
- El-Sherif O, Yu E, Xhaferllari I, Gaede S. Assessment of Intrafraction Breathing Motion on Left Anterior Descending Artery Dose During Left-Sided Breast Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;95:1075-82. [Crossref] [PubMed]
- Qi XS, White J, Rabinovitch R, Merrell K, Sood A, Bauer A, Wilson JF, Miften M, Li XA. Respiratory organ motion and dosimetric impact on breast and nodal irradiation. Int J Radiat Oncol Biol Phys 2010;78:609-17. [Crossref] [PubMed]
- Yan Y, Lu Z, Liu Z, Luo W, Shao S, Tan L, Ma X, Liu J, Drokow EK, Ren J. Dosimetric comparison between three- and four-dimensional computerised tomography radiotherapy for breast cancer. Oncol Lett 2019;18:1800-14. [Crossref] [PubMed]
- Harms J, Schreibmann E, Mccall NS, Lloyd MS, Higgins KA, Castillo R. Cardiac motion and its dosimetric impact during radioablation for refractory ventricular tachycardia. J Appl Clin Med Phys 2023;24:e13925. [Crossref] [PubMed]
- Walls GM, Giacometti V, Apte A, Thor M, McCann C, Hanna GG, O'Connor J, Deasy JO, Hounsell AR, Butterworth KT, Cole AJ, Jain S, McGarry CK. Validation of an established deep learning auto-segmentation tool for cardiac substructures in 4D radiotherapy planning scans. Phys Imaging Radiat Oncol 2022;23:118-26. [Crossref] [PubMed]
- Omidi A, Weiss E, Wilson JS, Rosu-Bubulac M. Effects of respiratory and cardiac motion on estimating radiation dose to the left ventricle during radiotherapy for lung cancer. J Appl Clin Med Phys 2023;24:e13855. [Crossref] [PubMed]
- Shirai K, Nishiyama K, Katsuda T, Teshima T, Ueda Y, Miyazaki M, Tsujii K. Phantom and clinical study of differences in cone beam computed tomographic registration when aligned to maximum and average intensity projection. Int J Radiat Oncol Biol Phys 2014;88:189-94. [Crossref] [PubMed]
- Tan W, Xu L, Wang X, Qiu D, Han G, Hu D. Estimation of the displacement of cardiac substructures and the motion of the coronary arteries using electrocardiographic gating. Onco Targets Ther 2013;6:1325-32. [Crossref] [PubMed]
- Vasquez Osorio EM, McCallum H, Bedair A, Faivre-Finn C, Haughey A, van Herk M, Iqbal MS, McWilliam A, Price G, Byrne J, Cobben D. Protecting the Heart: A Practical Approach to Account for the Full Extent of Heart Motion in Radiation Therapy Planning. Int J Radiat Oncol Biol Phys 2020;108:1082-90. [Crossref] [PubMed]
- Prusator MT, Samson P, Cammin J, Robinson C, Cuculich P, Knutson NC, Goddu SM, Moore K, Hugo GD. Evaluation of Motion Compensation Methods for Noninvasive Cardiac Radioablation of Ventricular Tachycardia. Int J Radiat Oncol Biol Phys 2021;111:1023-32. [Crossref] [PubMed]
- Zhuang X, Shen J. Multi-scale patch and multi-modality atlases for whole heart segmentation of MRI. Med Image Anal 2016;31:77-87. [Crossref] [PubMed]
- Bai W, Shi W, O'Regan DP, Tong T, Wang H, Jamil-Copley S, Peters NS, Rueckert D. A probabilistic patch-based label fusion model for multi-atlas segmentation with registration refinement: application to cardiac MR images. IEEE Trans Med Imaging 2013;32:1302-15. [Crossref] [PubMed]
- Yan R, Chu FI, Gao Y, Yu V, Yoon S, Elashoff D, Lee P, Hu P, Yang Y. Dosimetric impact from cardiac motion to heart substructures in thoracic cancer patients treated with a magnetic resonance guided radiotherapy system. Phys Imaging Radiat Oncol 2021;17:8-12. [Crossref] [PubMed]
- Morris E, Chin R, Wu T, Smith C, Nejad-Davarani S, Cao M. ASSET: Auto-Segmentation of the Seventeen SEgments for Ventricular Tachycardia Ablation in Radiation Therapy. Cancers (Basel) 2023.
- Poon J, Thompson RB, Deyell MW, Schellenberg D, Clark H, Reinsberg S, Thomas S. Analysis of left ventricle regional myocardial motion for cardiac radioablation: Left ventricular motion analysis. J Appl Clin Med Phys 2024;25:e14333. [Crossref] [PubMed]
- van Herk M, Witte M, van der Geer J, Schneider C, Lebesque JV. Biologic and physical fractionation effects of random geometric errors. Int J Radiat Oncol Biol Phys 2003;57:1460-71. [Crossref] [PubMed]
- Reis CQM, Robar JL. Evaluation of the feasibility of cardiac gating for SBRT of ventricular tachycardia based on real-time ECG signal acquisition. J Appl Clin Med Phys 2023;24:e13814. [Crossref] [PubMed]
- Kini VR, Vedam SS, Keall PJ, Patil S, Chen C, Mohan R. Patient training in respiratory-gated radiotherapy. Med Dosim 2003;28:7-11. [Crossref] [PubMed]
- Shen K, Xiong J, Wang Z, Wang W, Li W, Zhou J, Deng Z, Li B, Zhong R. Design of a new breast vacuum bag to reduce the global and local setup errors and to reduce PTV margin in post-mastectomy radiation therapy. J Radiat Res 2020;61:985-92. [Crossref] [PubMed]
- Habatsch M, Schneider M, Requardt M, Doussin S. Movement assessment of breast and organ-at-risks using free-breathing, self-gating 4D magnetic resonance imaging workflow for breast cancer radiation therapy. Phys Imaging Radiat Oncol 2022;22:111-4. [Crossref] [PubMed]
- van Velzen SGM, Bruns S, Wolterink JM, Leiner T, Viergever MA, Verkooijen HM, Išgum I. AI-Based Quantification of Planned Radiation Therapy Dose to Cardiac Structures and Coronary Arteries in Patients With Breast Cancer. Int J Radiat Oncol Biol Phys 2022;112:611-20. [Crossref] [PubMed]


