
A scoring system predicts the adverse outcomes for cesarean scar pregnancy treated with transvaginal hysterotomy
Introduction
Cesarean scar pregnancy (CSP) occurs when the gestational sac (GS) implants at a previous surgical scar (1,2). In recent years, the incidence of CSP has been on the rise, attributable to the increasing global cesarean section rate and the advancement of diagnostic technology. It is estimated that the incidence of CSP after a cesarean section ranges from approximately 1:1,800 to 1:2,000 pregnancies (3). As the pregnancy advances, CSP is a dangerous disease; the invasion of trophoblast cells into the myometrium can readily lead to uterine rupture and severe bleeding (4). Therefore, scholars advocate for the prompt termination of pregnancy upon a CSP diagnosis (5,6). Unfortunately, there are currently no well-established guidelines for CSP. management.
The guiding principle of CSP treatment is to safeguard the health of pregnant woman while preserving their fertility as much as possible. Transvaginal sonography have served as a key diagnostic tool for CSP (7). Suction evacuation, lesion excision, and intrauterine balloon catheter placement demonstrate high efficacy (>90% success rate) in resolving CSP while preserving fertility (8). Among these, lesion excision is extensively employed in clinical practice (6). It includes resection of the lesion along with scar repair via laparoscopy, laparotomy, or transvaginal approaches. In severe instances, hysterectomy may even be considered. Transvaginal hysterotomy offers notable advantages in terms of safety, efficacy, and patient outcomes, making it an important option for specific CSP cases.
Firstly, transvaginal surgery is a minimally invasive procedure that accesses to the lesion through the natural vaginal canal, avoiding abdominal incisions or pneumoperitoneum. Compared to laparoscopic or abdominal surgery, it causes less trauma and enables a quicker recovery (9-11). Secondly, it allows for precise lesion removal and effective hemostasis (12,13). With direct visualization and with an open surgical field, transvaginal surgery can accurately and completely excise CSP tissue. In contrast, uterine curettage often struggles to remove invasive tissue, and medical treatments may be ineffective for cases with deep invasion or rich vascularization. Moreover, the clear surgical field facilitates rapid hemostasis through suturing, ligation, or electrocautery, which is more effective than laparoscopic techniques. This is especially vital for CSP cases with abundant blood flow. Finally, it can optimize the repair of uterine scar. Transvaginal approach can simultaneously address the lower uterine segment and scar area, repair scar, optimize uterine anatomy, improve scar healing, and reduce the risks associated with future pregnancies.
In recent years, transvaginal hysterotomy has been proposed as a first-line treatment for CSP (4,14-22). A systematic review indicated a high success rate (99.2%) and a low hysterectomy rate (0.85%), underscoring its efficacy and safety (6). Our center has been utilizing this technique (4) and has achieved favorable outcomes in terms of both safety and efficacy (17). However, as with all surgical procedures, complications may still occur. Therefore, reliable prediction models to screen suitable candidates and enhance treatment outcomes. Although there have been explorations of uterine evacuation scoring systems (23-25), many of these are limited to specific cases or lack formal validation, and currently, there is no comprehensive model tailored for transvaginal hysterotomy.
Twenty-eight experts in obstetrics and gynecology ultrasonography reached a consensus and developed standardized sonographic evaluations based on relevant ultrasound features (26). These evaluations clarified the types of CSP according to their location and other features that might impact treatment outcomes. We integrated these consensus features to develop a prediction model and a simplified scoring system to screen suitable candidates for transvaginal hysterotomy. This approach aims to provide more individualized treatment and improve the prognosis for CSP patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1337/rc).
Methods
Design and data sources
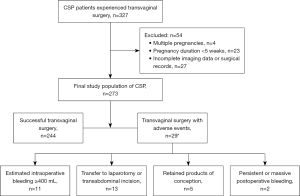
This retrospective observational study was conducted in the First Affiliated Hospital of Sun Yat-sen University in China from January 2009 to February 2023. Patients diagnosed with CSP between 5+ to 17+ weeks of gestation and treated with transvaginal hysterotomy were enrolled in the study. CSP was diagnosed via transvaginal ultrasound based on the following criteria (4): the uterine cavity and cervical canal were empty, and a gestational mass was located in the anterior part of the isthmic portion of the uterus, accompanied by a diminished myometrial layer between the bladder and the sac. All diagnoses were ultimately confirmed through surgical intervention, and routine histopathological examinations were conducted on the surgical specimens after the operations. Adverse events were defined as meeting any of the following criteria: (I) intraoperative bleeding ≥400 mL; (II) transfer to laparotomy or transabdominal incision; (III) persistent or massive postoperative bleeding; (IV) pregnancy residue. The exclusion criteria included patients who did not undergo transvaginal hysterotomy, those with multiple pregnancies, or those with incomplete data (including ultrasound images and surgical details). This study was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University (approval [2023] No. 863; approval date: 12/11/2023) and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Given the retrospective nature of the study, the requirement for patients’ informed consent was waived.
Procedure of transvaginal surgery
Patients were placed in the lithotomy position with an emptied bladder under general or combined spinal-epidural anesthesia. An incision was made at the anterior cervicovaginal junction. Subsequently, the bladder was bluntly dissected away from the cervix and the anterior portion of the lower segment until the anterior peritoneal reflection was clearly identified. Adrenaline was injected at the cervicovaginal junction to aid in hydro-dissection and attain hemostasis. The CSP was identified as a “purple bulge” and excised via a transverse incision. Myometrial and vaginal defects were then closed using 2-0 absorbable sutures. At our center, all transvaginal hysterotomy for treating CSP were performed by experienced physicians who had served as attending physicians for at least 5 years.
Data collection and analysis
Demographic data of patients was collected, encompassing maternal age, obstetric history (including previous miscarriage and cesarean delivery), gestational age determined by the last menstrual period, vaginal bleeding, abdominal pain, and preoperative serum levels of β-human chorionic gonadotropin (β-hCG) levels. Any preoperative interventions the patient had undergone, such as medication, uterine curettage and systemic methotrexate injection, were also recorded.
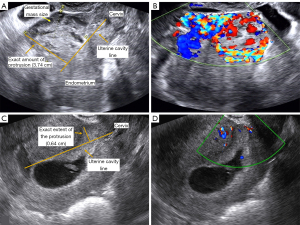
The ultrasound characteristics of lesion were collected by reviewing the reporting system and the images in accordance with relevant consensus (26). These features included GS and embryonic cardiac activity, size and location of the gestational mass, residual myometrial thickness (RMT), exact extent of the protrusion (gestational mass beyond the uterine cavity line), and vascularity. The location of the gestational mass can be classified into three types (26): Type 1, mostly of gestational mass protrudes towards the uterine cavity; Type 2, mostly of gestational mass is embedded in the myometrium; and Type 3, gestational mass is partially located outside the outer contour of the cervix or the uterus (Figure 1).
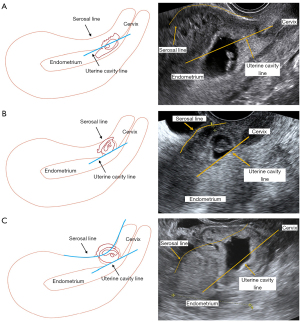
The measurement of the extent of gestational mass protrusion beyond the uterine cavity line followed a standardized protocol. A sagittal plane image of the uterus was selected, clearly presenting the endometrium and cervix, defining the interface between the basal layer of the endometrium and the anterior wall of the myometrium as the uterine cavity line, and using it as a reference point. Then, the protrusion distance of the gestational tissue beyond this line was measured and the thickness of the RMT was recorded on the same plane. This method adheres to international consensus recommendations and can objectively quantify the localization of gestational mass, thereby reducing variability in clinical application. The measurements were accomplished using Free ImageJ Fiji software (Johannes Schindelin, Albert Cardona, Mark https://imagej.net/Fiji/Downloads), version 1.2, without the use of any specific plugin. Inter- and intra-observer reliability of the paired measurements were evaluated using a 2-way mixed effects model intraclass correlation coefficient (ICC) and a Bland-Altman analysis.
Peri-gestational mass blood supply was assessed using the Alder semi-quantitative grading method. This method is widely recognized for its simple and reliable assessment of vascularization based on color Doppler ultrasound signals. Blood flow was divided into four grades (27): I = no discernible blood flow; II = minimal blood flow present; III = moderate blood flow with limited but noticeable vascular distribution within the lesion; IV = high blood flow, characterized by evenly distributed dense, reticular, or arborized vascular patterns with uniform distribution.
In this study, two physicians (M.L. & J.Z.) with over 10 years of diagnostic experience independently evaluated the ultrasound images to ensure accuracy and reliability. Discrepancies in evaluation were resolved through consensus discussions, minimizing subjectivity and ensuring consistency.
We compared the characteristics of two groups of patients with and without adverse events, and identified the factors linked to adverse events through univariate analysis. Based on the Akaike information criterion, the significant factors were included in a multivariable logistic regression analysis using the forward stepwise selection. The final model was simplified to generate a scoring system and calculate the risk probability according to the “The Framingham Study risk score system” (28).
Statistical analysis
Continuous variables were presented as the mean ± standard deviation for normal distributions, or as the median (interquartile range) for non-normal distributions. Missing data were handled with single imputation. Statistical tests included Student’s t-test or Mann-Whitney U test for continuous variables and Chi-squared or Fisher’s exact test for categorical variables. The scoring system was developed based on logistic regression, following the “The Framingham Study risk score system” described by Sullivan et al. (28), and was validated via 5-fold cross-validation (29). Model performance was assessed using the Hosmer-Lemeshow test and the receiver operating characteristic (ROC) curves. Accuracy, sensitivity, and specificity were also assessed. To eliminate the potential confounding effects of preoperative intervention, we carried out a tiered analysis. In the multivariable adjustment, the intervention measures were divided into three groups: no intervention, other interventions (such as curettage, medication), and any use of the uterine artery embolization (UAE)/methotrexate (MTX). Covariate adjustment was performed for clinical and ultrasound characteristics. Sensitivity analysis was only conducted on cases without UAE/MTX exposure and the multiplicative interaction terms between different intervention types were evaluated through interaction testing.
All statistical analyses were performed using open-source Python libraries, such as NumPy (https://numpy.org), with significance set at a two-sided P value <0.05.
Results
A total of 504 women were diagnosed with CSP. Among them, 327 (64.8%) underwent transvaginal hysterotomy, but comprehensive information was available for 273 (83.5%) of them, who formed the study group. Among these 273 patients, 244 (89.4%) had successful hysterotomy without any complications, while 29 (10.6%) experienced at least one complication. The median intraoperative blood loss in the adverse events group was 200 mL (range, 10 to 2,500 mL), which was significantly higher than the 30 mL (range, 0–300 mL) in the non-complication group (P<0.001). Among 29 patients experienced complications, with two cases involving concurrent occurrences of two complications, resulting in a total of 31 adverse events (Figure 2): 11 (35.4%) had intraoperative blood loss exceeding 400 mL, 13 (41.9%) needed to convert to laparotomy or transabdominal incision as a remedial measure, 5 (16.1%) had residual pregnancy products, and 2 (6.5%) required blood transfusion due to persistent postoperative hemorrhage. To avoid double-counting, complication rates were calculated on a per-patient basis (29 cases).
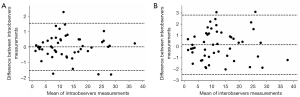
Measurement of the exact extent of protrusion of the gestational mass had high repeatability. The inter- and intra-observer correlations were 0.98 and 0.99, respectively. Bland-Altman analysis revealed a minimal bias of 0.01 (95% limits of agreement, −1.53 to 1.54) for intra-observer measurement variation and 0.16 (95% limits of agreement, −2.45 to 2.78) for inter-observer measurement variation in determining the exact extent of protrusion (Figure 3).
As shown in Table 1, patients with a higher history of miscarriage (P=0.021), a longer pregnancy duration (P<0.001), a larger gestational mass size (P<0.001), and a more prominent gestational mass (P<0.001) were more prone to experiencing adverse events. In addition, there were significant differences between the two groups regarding the presence of a GS (P=0.005), the location type of the gestational mass (P<0.001), and the level of blood flow (P<0.001).
Table 1
| Characteristic | Group with adverse event (n=29) | Group without adverse event (n=244) | P values |
|---|---|---|---|
| Maternal characteristic | |||
| Maternal age (years) | 34.07±4.54 | 33.80±4.78 | 0.776 |
| Previous miscarriage, n (%) | 0.021 | ||
| 0 | 3 (10.3) | 53 (21.7) | |
| 1 | 13 (44.8) | 71 (29.1) | |
| 2 | 6 (20.7) | 57 (23.4) | |
| ≥3 | 7 (24.1) | 63 (25.8) | |
| Previous cesarean delivery, n (%) | 0.626 | ||
| 1 | 20 (69.0) | 167 (68.4) | |
| 2 | 8 (27.6) | 74 (30.3) | |
| ≥3 | 1 (3.4) | 3 (1.2) | |
| Bleeding, n (%) | 0.381 | ||
| 0 | 10 (34.5) | 61 (25.0) | |
| 1 | 19 (65.5) | 183 (75.0) | |
| Pain, n (%) | 0.278 | ||
| 0 | 24 (82.8) | 174 (71.3) | |
| 1 | 5 (17.2) | 70 (28.7) | |
| Preoperative interventions, n (%) | 0.068 | ||
| 0 | 20 (69.0) | 206 (84.4) | |
| 1 | 9 (31.0) | 38 (15.6) | |
| Gestational age (weeks) | 9 [7–10] | 7 [6–8] | <0.001 |
| Serum β-hCG level before surgery (IU/L) | 23,581.00 [9,622.00–93,532.00] |
36,476.00 [11,413.50–68,719.75] |
0.729 |
| Intraoperative blood loss (mL) | 200 [50–500] | 30 [20–50] | <0.001 |
| Ultrasound characteristic | |||
| Presence of a GS, n (%) | <0.001 | ||
| Presence | 18 (62.1) | 216 (88.5) | |
| Absence | 11 (37.9) | 28 (11.5) | |
| Fetal cardiac activity at diagnosis, n (%) | 0.342 | ||
| Presence | 18 (62.1) | 124 (50.8) | |
| Absence | 11 (37.9) | 120 (49.2) | |
| Gestational mass size (cm) | 4.39±2.13 | 3.15±1.51 | 0.005 |
| Localization types, n (%) | <0.001 | ||
| Type 1 | 5 (17.2) | 119 (48.8) | |
| Type 2 | 16 (55.2) | 114 (46.7) | |
| Type 3 | 8 (27.6) | 11 (4.5) | |
| RMT (cm) | 0.19 [0.16–0.28] | 0.22 [0.15–0.32] | 0.263 |
| Exact extent of protrusion (cm) | 2.07 [1.26–2.84] | 0.99 [0.61–1.57] | <0.001 |
| Vascularity supplying, n (%) | <0.001 | ||
| No detectable blood flow | 0 | 7 (2.9) | |
| Minimal blood flow | 5 (17.2) | 61 (25.0) | |
| Moderate blood flow | 8 (27.6) | 134 (54.9) | |
| High blood flow | 16 (55.2) | 42 (17.2) | |
Data are presented as mean ± standard deviation, median [interquartile range] or number (percentage). β-HCG, β-human chorionic gonadotropin; CSP, cesarean scar pregnancy; GS, gestation sac; RMT, residual myometrial thickness.
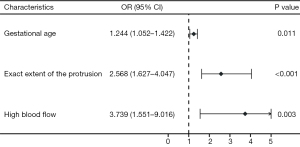
Multivariable regression analysis revealed that gestational age (OR =1.244, 95% CI: 1.052–1.422, P=0.011), exact extent of protrusion (OR =2.568, 95% CI: 1.627–4.047, P<0.001), and high blood flow (OR =3.739, 95% CI: 1.551–9.016, P=0.003) were independent predictive factors incorporated into the final prediction model (Figure 4). This prediction regression model provided an estimated probability of adverse outcome in CSP patients undergoing transvaginal hysterotomy. This probability was equal to: where z equals to: −5.84+0.94×(exact extent of protrusion)+0.22×(gestational age)+1.32×(high blood flow).
As shown in Figure 5A, we verified the performance of the prediction model via stratified 5-fold cross-validation. The findings indicated that the model accurately classified 90.1% (95% CI: 86.4–93.8%) of patients, boasting a sensitivity of 83.3% (95% CI: 72.8–93.8%) and a specificity of 85.7% (95% CI: 78.7–92.7%). The mean AUC of the final model was 0.828 (95% CI: 0.769–0.887). The model had an acceptable goodness of fit according to the Hosmer-Lemeshow test (P=0.223).
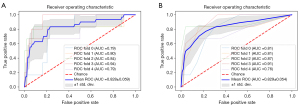
The scores assigned to each category of risk factors were calculated and are presented in Table 2. Table 3 shows the scoring system derived from the mathematical models, along with the estimated probabilities corresponding to each total point. The scoring system exhibited good discrimination with mean AUC: 0.829 (95% CI: 0.775–0.883) after stratified 5-fold cross-validation (Figure 5B) and good calibration (Hosmer-Lemeshow test, P=0.707). It correctly classified 89.8% (95% CI: 83.5–96.1%) of the subjects, with a sensitivity of 83.3% (95% CI: 72.8–93.8%) and a specificity of 80.8% (95% CI: 69.9–91.7%).
Table 2
| Variable | Reference value (Wij) | βi | βi (Wij − WiREF) | Points |
|---|---|---|---|---|
| Gestational age (weeks) | 0.218 | |||
| 5–8 | 6.5= W1REF | 0.000 | 0 | |
| 9–12 | 10.5 | 0.872 | 1 | |
| 13–16 | 14.5 | 1.744 | 2 | |
| >16 | 16.7 | 2.224 | 3 | |
| Exact extent of the protrusion (cm) | 0.943 | |||
| 0.00–0.99 | 0.50= W2REF | 0.000 | 0 | |
| 1.00–1.99 | 1.50 | 0.943 | 1 | |
| 2.00–2.99 | 2.50 | 1.886 | 2 | |
| >3.00 | 3.73 | 3.017 | 3 | |
| High blood flow | 1.319 | |||
| No | 0= W3REF | 0.000 | 0 | |
| Yes | 1 | 1.3189 | 2 |
Points (rounded to the nearest integer) corresponding to each variable value are added to obtain an individual’ score. Points = βi (Wij−WiREF)/B, B=4*0.218. CSP, cesarean scar pregnancy.
Table 3
| Score | Estimated probability |
|---|---|
| 0 | 0.019 |
| 1 | 0.044 |
| 2 | 0.100 |
| 3 | 0.210 |
| 4 | 0.389 |
| 5 | 0.603 |
| 6 | 0.784 |
| 7 | 0.897 |
| 8 | 0.954 |
Equation of the risk prediction probability value for each score: , where is equal to: . CSP, cesarean scar pregnancy.
In a clinical setting, we demonstrated the application of the established prediction model and scoring system through two cases (Figure 6): Case 1 was a CSP patient with a gestational age of 7+ weeks. The exact extent of protrusion of the pregnancy mass was 3.74 cm, and the blood flow was high. According to the scoring system, the patient; total score was 5 points [gestational age of 7+ weeks (0 point) + exact protrusion of 3.74 cm (3 points) + high blood flow (2 points)], and the estimated probability of adverse events occurring during transvaginal hysterotomy was 60.9%, which was similar to the 60.3% computed by the prediction model. In reality, the patient underwent transvaginal hysterotomy with an estimated intraoperative bleeding of 400 mL. Case 2 was a CSP patient with 6+ weeks. The exact extent of protrusion at the pregnancy mass was 0.64 cm. The patient had a minimal blood flow. According to the score sheet, the patient’s total score was 0 [gestational age of 6 weeks (0 point) + exact protrusion of 0.64 cm (0 point) + minimal blood flow (0 point)], and the estimated probability of adverse events occurring during transvaginal hysterotomy was 2.0%, which was similar to the 1.9% computed by the prediction model. In real practice, the patient had a successful transvaginal hysterotomy, without any complication, and the amount of estimated bleeding was 50 mL. With this simple scoring system, physicians can intuitively predict the outcomes of transvaginal hysterotomy without the need for complex calculations and formulate appropriate treatment plans for patients.
Subgroup analysis of preoperative interventions
After adjusting the lesion parameters, no significant correlation was found between preoperative intervention and the primary outcome [other interventions: adjusted odds ratio (aOR) =0.779, 95% CI: 0.135–4.509; any UAE/MTX use: aOR =0.346, 95% CI: 0.025–4.707]. The results of the sensitivity analyses, which excluded cases involving UAE/MTX, were consistent with these findings (OR =0.465, 95% CI: 0.070–3.086). No significant interaction was detected among different intervention types (P>0.05). The full results are detailed in Tables S1-S3.
Discussion
Our study introduced a simple scoring system designed to predict adverse outcomes in CSP patients undergoing transvaginal hysterotomy. This scoring system offered a probability prediction comparable to that of the prediction model. It demonstrated strong discriminatory ability and was user-friendly. Moreover, the scores could directly reflect the likelihood of surgical complications. The scoring sheet clearly showcases the correlation between the risk probability and the measurable, observable characteristics, which greatly facilitates its integration into clinical decision-making.
The management of CSP remains a complex issue. Currently, there are numerous approaches for treating CSP, including systemic or local injection of methotrexate, as well as surgical methods, such as uterine curettage, laparoscopy or laparotomy, and transvaginal hysterotomy to remove the lesions. Previous studies have identified risk factors for treatment failure in CSP (25,30-36). However, most of these have focused on traditional approaches like uterine curettage, with relatively little research on transvaginal methods. Transvaginal hysterectomy has the advantages of directly accessing the lesion site through the natural cavity and is effective in managing complex CSP cases characterized by deep invasion, rich vascularization, or larger GSs.
In this study, we developed a scoring system for CSP patients undergoing transvaginal hysterotomy. The development was based on the TRIPOD guidelines and the Framingham Study risk score system (29). The strength of this scoring system lies in: firstly, it is based on a large cohort of 273 patients. To our knowledge, this is currently the largest cohort of CSP patients undergoing transvaginal hysterotomy treatment. Previously, the median sample size of such studies was only 25 (ranging from 1 to 90) (4,14-21,37,38). The large sample size reduces the risk of overfitting and poor reproducibility. Secondly, detailed ultrasound characteristics were collected and defined strictly according to the consensus, making the system more comprehensive. Notably, the system incorporates a unique clinical feature: the extent to which the gestational mass protrudes from the uterine cavity line. This feature reflects the depth of lesion invasion, directly indicates the complexity of the surgery and postoperative scar repair, and has not been systematically included in existing curettage-based models. Thirdly, the performance of the system was evaluated through stratified 5-fold cross-validation and the Hosmer-Lemeshow test. These evaluations demonstrated its excellent discriminatory and calibration capabilities, ensuring the robustness and reliability of the model.
Compared to existing uterine curettage models, our model highlights the distinct characteristics and advantages of transvaginal surgery. The models proposed by Fang et al.’s (23) and Sun et al.’s (24) are primarily applicable to early-stage pregnancies (<9 weeks) with shallow lesions and minimal vascularity. However, their applicability is limited when dealing with complex cases involving deep invasion or rich vascularization. Although Wang et al.’s model (25) covers a broader range of gestational age, it lacks formal validation, and its reliability is questionable. Our study centers on transvaginal surgery, offering a specialized tool to enhance prediction accuracy and establishing a novel framework for CSP risk assessment.
Among various treatment methods, transvaginal or laparoscopic approaches to resection of CSP were the preferred approach recommended by the Society for Maternal-Fetal Medicine (SMFM) (39). Wang et al. also noted that transvaginal approaches outperformed transabdominal surgery (14). Published case series have demonstrated that the incidence of complications in transvaginal hysterotomy is relatively low (4,15,17,37). Additionally, a small randomized controlled trial (16) has further confirmed its feasibility when compared to UAE and hysteroscopic removal with chemotherapy. In our study, we discovered notable disparities between patients who experienced complications and those who did not. These differences were evident in maternal age, obstetric history, gestational age, mass size, preoperative β-hCG levels, and the presence of positive cardiac activity. Our findings are consistent with the results of previous research on CSP (25,30-35,40,41). Notably, while other treatments encounter substantial challenges when dealing with mixed-mass CSP (15,30,42), our results showed no significant difference in outcomes between mixed-mass CSP and GS CSP when transvaginal hysterotomy was employed. This finding implies that transvaginal hysterotomy may be effective in treating mixed-mass CSP, but further studies are required to validate its efficacy.
Through multivariable regression analysis, only gestational age, exact extent of protrusion, and high blood flow were identified as independent factors and incorporated into the final prediction model. From the perspective of this surgical approach, this is reasonable. As emphasized by international consensus, accurately measuring the exact extent of protrusion beyond the “uterine cavity line” is critical (26). When performed in a standardized and reproducible manner (as demonstrated by the ICC and Bland-Altman plot), this measurement can objectively assess the gestational mass location and minimize physician subjectivity. Future investigations can utilize 3D volume imaging to obtain detailed anatomical information for CSP localization (43), as it is more accurate than 2D ultrasonography, which has limitations in detecting trophoblast invasion (44).
This study indicated that the vascularity of lesions significantly impacted the prognosis of CSP, with an OR of 3.739 (95% CI: 1.551–9.016, P=0.003). Richer vascularity indicates more severe villous proliferation and invasion, increasing the difficulty of lesion removal. Consequentlly, this can result in an extended surgical duration, more substantial blood loss, and a heightened likelihood of villous remnants. Jurkovic et al. also pointed out that increased blood loss in CSP cases was closely linked to lesion vascularity (45), suggesting that the color Doppler index (CDI) can be used to evaluate peri-trophoblastic perfusion through Doppler imaging (46). Multiple studies are consistent with our study, confirming that higher CDI scores increase the risk of surgical treatment failure (31,32).
Limitations
This study does have several limitations. Firstly, the small number of adverse events limited the analysis of factors associated with specific types of outcomes. Secondly, the study employed a retrospective design, which might introduce biases. Since it relied on past medical records, the quality and integrity of the data could vary. Additionally, retrospective studies are unable to establish causal relationships, and there may be selection bias because the patients included may not fully represent the broader target population. Thirdly, even though the model demonstrated good performance through internal validation, it was based on a single-center dataset. Given the differences in patient characteristics, surgical expertise, and institutional practices among different institutions, its generalizability was limited. Therefore, external validation is necessary. Lastly, although the stratified analysis indicated that the model was robust against confounding factors related to preoperative interventions, the sample size of UAE was only 3. This highlights the need to validate the research findings in a larger prospective cohort.
To address these issues, we propose the following measures: firstly, we are currently collecting and expanding the CSP case dataset. As the number of cases and complications increases, future research can conduct more reliable stratified analyses of various complications, offering stronger evidence for improving the diagnosis and management of CSP. Secondly, we plan to expand the sample size and conduct multicenter prospective studies to validate the model’s applicability in diverse clinical settings and further optimize its performance. Finally, we aim to validate the model in different populations to enhance its robustness and generalizability. Moreover, our model is expected to be utilized for prognosis prediction of UAE. By tracking the longitudinal score trajectories of patients, this tool can perform risk stratification and screen candidates for preoperative UAE. However, this hypothesis needs to be prospectively validated through dedicated clinical studies.
Conclusions
In conclusion, the prediction model developed in this study is capable of effectively identifying high-risk populations for adverse events among CSP undergoing transvaginal hysterotomy. The scoring system developed based on this model offers a convenient instrument for clinicians to make treatment decisions. However, the prediction model and the risk-scoring system still necessitate further assessment through multicenter prospective randomized controlled trials. Despite these areas for improvement, this study has achieved remarkable progress in the management of CSP patients, and holds the promise of enhancing the prognosis of this patient population.
Acknowledgments
The authors would like to extend their sincere thanks to professors Gu Jing from the Department of Statistics, Sun Yat-sen University for the guidance in statistical methods.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1337/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1337/coif). H.X. reports that this study was supported by the National Natural Science Foundation of China (No. 82171938). Q.Z. reports that this study was supported by the National Natural Science Foundation of China (No. 82202156). M.L. reports that this study was supported by the China Sonographer Technology New Star Program (No. KJX2019004). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University (approval [2023] No. 863; approval date: 12/11/2023) and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Given the retrospective nature of the study, the requirement for patients’ informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, Monteaugudo A, Buca D, Forlani F, Familiari A, Scambia G, Acharya G, D'Antonio F. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:169-75. [Crossref] [PubMed]
- Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG 2007;114:253-63. [Crossref] [PubMed]
- Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220-7. [Crossref] [PubMed]
- He M, Chen MH, Xie HZ, Yao SZ, Zhu B, Feng LP, Wu YP. Transvaginal removal of ectopic pregnancy tissue and repair of uterine defect for caesarean scar pregnancy. BJOG 2011;118:1136-9. [Crossref] [PubMed]
- Gonzalez N, Tulandi T. Cesarean Scar Pregnancy: A Systematic Review. J Minim Invasive Gynecol 2017;24:731-8. [Crossref] [PubMed]
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril 2016;105:958-67. [Crossref] [PubMed]
- Mao Y, Peng Y, Zheng M, Xiao J, Gong F, Li X, Ouyang Y. First-trimester ultrasound diagnosis and risk factor analysis of cesarean scar pregnancy after in vitro fertilization-embryo transfer. Quant Imaging Med Surg 2024;14:5028-39. [Crossref] [PubMed]
- Kaelin Agten A, Jurkovic D, Timor-Tritsch I, Jones N, Johnson S, Monteagudo A, Huirne J, Fleisher J, Maymon R, Herrera T, Prefumo F, Contag S, Cordoba M, Manegold-Brauer GCSP Collaborative Network. First-trimester cesarean scar pregnancy: a comparative analysis of treatment options from the international registry. Am J Obstet Gynecol 2024;230:669.e1-669.e19. [Crossref] [PubMed]
- OuYang ZB, Li HW, Quan S. The first-line approach for cesarean scar pregnancy: The most adopted being not the best. Taiwan J Obstet Gynecol 2016;55:761-2. [Crossref] [PubMed]
- Maheux-Lacroix S, Li F, Bujold E, Nesbitt-Hawes E, Deans R, Abbott J. Cesarean Scar Pregnancies: A Systematic Review of Treatment Options. J Minim Invasive Gynecol 2017;24:915-25. [Crossref] [PubMed]
- Kanat-Pektas M, Bodur S, Dundar O, Bakır VL. Systematic review: What is the best first-line approach for cesarean section ectopic pregnancy? Taiwan J Obstet Gynecol 2016;55:263-9. [Crossref] [PubMed]
- Sharma S, Imoh-Ita F. Surgical management of caesarean scar pregnancy. J Obstet Gynaecol 2005;25:525-6. [Crossref] [PubMed]
- Philippe HJ, Karanouh S, Rozenberg P, Dien DT, Nisand I. Transvaginal surgery for uterine scar dehiscence. Eur J Obstet Gynecol Reprod Biol 1997;73:135-8. [Crossref] [PubMed]
- Wang DB, Chen YH, Zhang ZF, Chen P, Liu KR, Li Y, Fu L. Evaluation of the transvaginal resection of low-segment cesarean scar ectopic pregnancies. Fertil Steril 2014;101:602-6. [Crossref] [PubMed]
- Huanxiao Z, Shuqin C, Hongye J, Hongzhe X, Gang N, Chengkang X, Xiaoming G, Shuzhong Y. Transvaginal hysterotomy for cesarean scar pregnancy in 40 consecutive cases. Gynecol Surg 2015;12:45-51. [Crossref] [PubMed]
- Le A, Shan L, Xiao T, Zhuo R, Xiong H, Wang Z. Transvaginal surgical treatment of cesarean scar ectopic pregnancy. Arch Gynecol Obstet 2013;287:791-6. [Crossref] [PubMed]
- Li JB, Kong LZ, Fan L, Fu J, Chen SQ, Yao SZ. Transvaginal surgical management of cesarean scar pregnancy: analysis of 49 cases from one tertiary care center. Eur J Obstet Gynecol Reprod Biol 2014;182:102-6. [Crossref] [PubMed]
- Chen YQ, Liu HS, Li WX, Deng C, Hu XW, Kuang PJ. Efficacy of transvaginal debridement and repair surgery for cesarean scar pregnancy: a cohort study compared with uterine artery embolism. Int J Clin Exp Med 2015;8:21187-93.
- Zhang H, Shi J, Yang Y, Liang Y, Gao X, Wang J, Liu H, Wu B, Zhao J. Transvaginal Surgical Management of Cesarean Scar Pregnancy II (CSP-II): An Analysis of 25 Cases. Med Sci Monit 2015;21:3320-6. [Crossref] [PubMed]
- Li YY, Yin ZY, Li S, Xu H, Zhang XP, Cheng H, Du L, Zhou XY, Zhang B. Comparison of transvaginal surgery and methotrexate/mifepristone-combined transcervical resection in the treatment of cesarean scar pregnancy. Eur Rev Med Pharmacol Sci 2017;21:2957-63.
- Chen H, Zhou J, Wang H, Tan W, Yao M, Wang X. The Treatment of Cesarean Scar Pregnancy with Uterine Artery Embolization and Curettage as Compared to Transvaginal Hysterotomy. Eur J Obstet Gynecol Reprod Biol 2017;214:44-9. [Crossref] [PubMed]
- Fu P, Sun H, Zhang L, Liu R. Efficacy and safety of treatment modalities for cesarean scar pregnancy: a systematic review and network meta-analysis. Am J Obstet Gynecol MFM 2024;6:101328. [Crossref] [PubMed]
- Fang Q, Sun L, Tang Y, Qian C, Yao X. Quantitative risk assessment to guide the treatment of cesarean scar pregnancy. Int J Gynaecol Obstet 2017;139:78-83. [Crossref] [PubMed]
- Sun QL, Luo L, Gao CY, Yan P, Yang Y, Chen ZQ. Scoring system for the prediction of the successful treatment modality in women with cesarean scar pregnancy. Int J Gynaecol Obstet 2019;146:289-95. [Crossref] [PubMed]
- Wang Q, Ma H, Peng H, He L, Bian C, Zhao X. Risk factors for intra-operative haemorrhage and bleeding risk scoring system for caesarean scar pregnancy: a case-control study. Eur J Obstet Gynecol Reprod Biol 2015;195:141-5. [Crossref] [PubMed]
- Jordans IPM, Verberkt C, De Leeuw RA, Bilardo CM, Van Den Bosch T, Bourne T, Brölmann HAM, Dueholm M, Hehenkamp WJK, Jastrow N, Jurkovic D, Kaelin Agten A, Mashiach R, Naji O, Pajkrt E, Timmerman D, Vikhareva O, Van Der Voet LF, Huirne JAF. Definition and sonographic reporting system for Cesarean scar pregnancy in early gestation: modified Delphi method. Ultrasound Obstet Gynecol 2022;59:437-49. [Crossref] [PubMed]
- Jauniaux E, Zosmer N, De Braud LV, Ashoor G, Ross J, Jurkovic D. Development of the utero-placental circulation in cesarean scar pregnancies: a case-control study. Am J Obstet Gynecol 2022;226:399.e1-399.e10. [Crossref] [PubMed]
- Sullivan LM, Massaro JM, D'Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 2004;23:1631-60. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594.
- Lin SY, Hsieh CJ, Tu YA, Li YP, Lee CN, Hsu WW, Shih JC. New ultrasound grading system for cesarean scar pregnancy and its implications for management strategies: An observational cohort study. PLoS One 2018;13:e0202020. [Crossref] [PubMed]
- Wu Y, Sun LF, Si YN, Luan XL, Gao YM. Clinical efficacy analysis of different therapeutic methods in patients with cesarean scar pregnancy. Taiwan J Obstet Gynecol 2021;60:498-502. [Crossref] [PubMed]
- De Braud LV, Knez J, Mavrelos D, Thanatsis N, Jauniaux E, Jurkovic D. Risk prediction of major haemorrhage with surgical treatment of live cesarean scar pregnancies. Eur J Obstet Gynecol Reprod Biol 2021;264:224-31. [Crossref] [PubMed]
- Xiang J, Cao Y, Zhou L, Yang H, Wu S, Li L. Evaluation of the necessity of laparoscopic repair of a uterine scar defect for cesarean scar pregnancy. J Int Med Res 2022;50:3000605211070753. [Crossref] [PubMed]
- Ou J, Peng P, Li C, Teng L, Liu X. Assessment of the necessity of uterine artery embolization during suction and curettage for caesarean scar pregnancy: a prospective cohort study. BMC Pregnancy Childbirth 2020;20:378. [Crossref] [PubMed]
- Shen F, Lv H, Wang L, Zhao R, Tong M, Lee AC, Guo F, Chen Q. A Comparison of Treatment Options for Type 1 and Type 2 Caesarean Scar Pregnancy: A Retrospective Case Series Study. Front Med (Lausanne) 2021;8:671035. [Crossref] [PubMed]
- Li C, Chen W, Xu H, Luo H. Obstetric outcomes in the expectant management of cesarean scar pregnancy with fetal heart activity: a single-center retrospective cohort study. Quant Imaging Med Surg 2024;14:6590-600. [Crossref] [PubMed]
- Kang SY, Park BJ, Kim YW, Ro DY. Surgical management of cesarean scar ectopic pregnancy: hysterotomy by transvaginal approach. Fertil Steril 2011;96:e25-8. [Crossref] [PubMed]
- Lu HY, Zhang WH, Shan J, Tian QS, Zhang XQ, Wu LC, Zhou YX, Li S, Peng YM, Li D, Hu LN. Study on 31 cases with cesarean scar pregnancy treated by transvaginal surgery. Zhonghua Fu Chan Ke Za Zhi 2011;46:917-22.
- Miller R, Gyamfi-Bannerman C. Publications Committee. Electronic address: pubs@smfm. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy. Am J Obstet Gynecol 2022;227:B9-B20. [Crossref] [PubMed]
- Xiao X, Feng Z, Li T, Qiao H, Zhu Y. Predictive nomogram of ultrasound indicators for the termination outcome of caesarean scar pregnancy. Sci Rep 2024;14:31378. [Crossref] [PubMed]
- Cheng XT, Liu YS, Song DY, Nie XC, Xiang YS, Niu JM. Risk factors associated with the failure of local methotrexate combined with minimally invasive surgery for late cesarean scar pregnancy. BMC Pregnancy Childbirth 2025;25:28. [Crossref] [PubMed]
- Liu G, Shang X, Qi Z, Xue F. Large intrauterine mass associated with cesarean scar pregnancy. Am J Obstet Gynecol 2016;214:126.e1-2. [Crossref] [PubMed]
- OuYang Z, Wu J, Wan Z. Pathogenesis and classification of Cesarean scar pregnancy: getting closer to the truth. Ultrasound Obstet Gynecol 2022;60:297-8. [Crossref] [PubMed]
- Ravi Selvaraj L, Rose N, Ramachandran M. Pitfalls in Ultrasound Diagnosis of Cesarean Scar Pregnancy. J Obstet Gynaecol India 2018;68:164-72. [Crossref] [PubMed]
- Jurkovic D, Knez J, Appiah A, Farahani L, Mavrelos D, Ross JA. Surgical treatment of Cesarean scar ectopic pregnancy: efficacy and safety of ultrasound-guided suction curettage. Ultrasound Obstet Gynecol 2016;47:511-7. [Crossref] [PubMed]
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol 2012;207:44.e1-13. [Crossref] [PubMed]


