
The role of imaging in predicting 3-month prognosis of primary intracerebral hemorrhage: a single-center, prospective observational study in tertiary care hospital
Introduction
Spontaneous or primary intracerebral hemorrhage (ICH), accounting for 85% of all cases of hemorrhagic strokes, is characterized by non-traumatic bleeding within the brain parenchyma, typically caused by small-vessel disease due to amyloid angiopathy or chronic, uncontrolled hypertension (1,2).
Although ICH accounts for approximately 10–20% of all stroke cases worldwide, it remains the most lethal stroke type, with a 30-day mortality rate of up to 40% and 1-year mortality rate of up to 50%. Despite recent advances in the interventional management of ischemic stroke patients, the beneficial effects of medical and interventional treatment on the survival and long-term prognosis of patients with ICH have lagged in recent clinical trials (3,4). Consequently, early accurate stratification of ICH outcomes in the emergency department is strongly desired and would facilitate the implementation of appropriate therapeutic options for affected patients (5). As a result, several prognostic tools encompassing different combinations of imaging markers were introduced to manage patients with primary ICH. Most notably, imaging markers of hemorrhage expansion held great promise in predicting prognosis in patients with ICH. These imaging signs include the spot sign, the spot-tail sign in computed tomography (CT) angiography, along with the island sign, blend sign, satellite sign (SS), black hole sign, and swirl sign in non-contrast computed tomography (NCCT). These imaging features are clinically important, as their presence elaborates the need for the initiation of anti-hematoma expansion treatments in the acute phase of ICH (6). The volume of the hemorrhage is another important prognostic indicator for 30-day mortality in primary ICH (7).
NCCT of the brain remains the imaging modality of choice in the acute setting (8). In addition to differentiating ICH from Ischemic stroke, NCCT provides detailed information about probable hemorrhage etiology and quantitative imaging features such as hemorrhage location and volume, midline structure shift, presence of intraventricular hemorrhage (IVH), and markers for early hematoma expansion including the black hole sign (BHS), the SS, and the blend sign (9,10). Baseline hematoma volume was found to be a well-known predictor of hematoma expansion and poor functional outcome in ICH (7,11,12). In addition, markers for early hematoma expansion were related to unfavorable clinical outcomes within 90 days in patients with ICH (13). Early, accurate identification of NCCT markers suggestive of hematoma expansion is crucial, as the implementation of anti-hematoma expansion therapies will be translated into more favorable clinical outcomes in ICH patients (14). In addition to radiological parameters, several clinical indicators were shown to be predictive of poor outcome in ICH, including advanced age, increased systolic blood pressure, and lower Glasgow Coma Scale (GCS) score at presentation (7,15,16). For instance, lower GCS at presentation, previous ischemic stroke, and history of smoking were correlated with ICH expansion and indicated poor prognosis (16). Therefore, it is imperative to note certain clinical and laboratory markers in the initial encounter of primary ICH patients to improve prognostication and medical decision-making in affected patients.
Consequently, considering the high morbidity and mortality of primary ICH and the importance of determining early prognosis using predictive models and the lack of similar studies in our country, this study aimed to establish a predictive model using relevant neuroimaging features and clinical parameters in determining the 90-day functional outcome in patients with primary ICH. This study provides an easy-to-employ prognostication model for clinicians in the emergency department and would improve medical decision-making in the management of patients with primary ICH. In our current study, we also employed a machine learning algorithm [support vector machine (SVM)] to evaluate the predictive role of certain imaging features for differentiating ICH patients with poor and good prognosis. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1299/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Ethics Committee of Isfahan University of Medical Sciences (approval date: 8/1/2021, protocol code IR.MUI.MED.REC.1400.446) and the National Research Ethics Board (approval number IR.MUI.MED.REC.1400.446). Written and verbal informed consent were obtained from participants after a complete explanation of the study. For patients with impaired consciousness, we obtained informed consent from their families. For such patients, according to our national medical ethical committee guidelines, we initially approached their first-degree male relative (if applicable) and patients’ spouses were approached for obtaining informed consent in other cases. For rare instances when neither a male relative nor a spouse were available, we obtained informed consent from the patients’ older children.
Patient selection and study design
In this prospective study, we recruited and analyzed imaging and clinical markers of 203 patients with primary ICH between September 2021 and October 2023 who were referred to and hospitalized at our university hospital (Al-Zahra General Hospital, Isfahan University of Medical Sciences, Iran), which is a large tertiary stroke center. Inclusion criteria were defined as: Patients aged ≥18 years with primary or spontaneous ICH confirmed on NCCT at the time of admission. The baseline NCCT was conducted in the early stage of ICH (within 6 hours from symptom onset). Patients were excluded from our study if they had any of the following: (I) traumatic brain injury, (II) secondary ICH due to hemorrhagic transformation following ischemic infarction, hemorrhage associated with brain tumor, arteriovenous malformation, cerebral cavernous malformation, dural arteriovenous fistula, intracranial venous thrombosis, or rupture of intracranial aneurysms, (III) presence of primary IVH, (IV) neurosurgical hematoma evacuation or external ventricular drainage prior to obtaining brain imaging, (V) CT images with severe artifact, and (VI) previous or current history of consumption of anticoagulant medications. Patients’ baseline demographic characteristics (age, gender), underlying comorbidities (history of hypertension, diabetes mellitus, central nervous system diseases such as ischemic stroke, cardiovascular diseases, and renal diseases), baseline GCS score, and smoking history were also collected using a defined paper research template at admission. Data regarding imaging features were also collected in our devised paper research templates and were transferred onto Excel sheets for analytical purposes.
Image data and evaluation
All images were acquired using the same scanning protocol in the 64-slice spiral CT scanner (Siemens). The protocol consisted of 120 KV, 280 mA, axial layer thickness 5 mm, and CTDIvol >40 mGy. For more consensus, all baseline NCCT images were simultaneously examined by an experienced radiologist (S.H.), who had 10 years of experience in neuroradiology and a radiology resident (T.J.) with 1 year of neuroimaging diagnosis experience and the following imaging features were evaluated using standardized definitions (17):
- Hemorrhage location: deep (basal ganglia, thalamus, internal capsule, corona radiata, or corpus callosum), brain hemisphere, cerebellum, brainstem, and others.
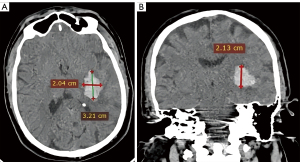
- Hemorrhage volume quantification: using the ABC/2 method (also known as Tada formula) for parenchymal ICH without IVH. In this method, A corresponds to the largest hematoma diameter on axial image, B corresponds to the largest perpendicular diameter to A on the same image slice, and C corresponds to the number of slices in which the hematoma was observed multiplied by the slice thickness (Figure 1) (18). For ICH with an intraventricular component, only the parenchymal component was assessed using this formula. All the lengths were measured in cm and volumes in mL.
- Presence of midline shift: The midline of the brain was determined by drawing a line connecting the anterior and posterior borders of the falx cerebri. The maximum vertical distances from the center of the midbrain, septum pellucida, pineal calcification, and the falx cerebri to the brain midline were measured and recorded as the midline shifts in mm. If different midline shift values were present in multiple slices, we chose the maximum value from all recorded midline shift values. As previous studies have reported different thresholds for midline shift (13,16,19), in this study we have also selected 3 mm as the cutoff value for further analyzing its association with the prognosis of primary ICH, where (yes/no; yes defined as a midline shift greater than 3 mm). We also evaluated whether different midline sift values were correlated with prognosis.
- The presence or absence of markers of hematoma expansion; including the blend sign, the SS, and the BHS. The criteria for each of the relevant imaging signs were as follows (20):
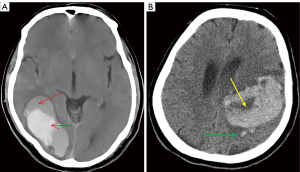
- Blend sign: uneven densities with an attenuation difference of at least 18 Hounsfield units (HU) between the areas with different densities (Figure 2A).
- SS: any small hematoma completely isolated from the main hematoma observed at least in one image slice. The shortest distance between this small hematoma and the main hematoma was 1–10 mm (Figure 2B).
- BHS: hypodense area that is encapsulated within a hyperattenuating hematoma, with a density difference of at least 28 HU between 2 areas of different densities (Figure 2B).
Images with poor quality or artifacts were excluded from our study.
Outcomes
After the initial NCCT of the brain was obtained and stored in the picture archiving and communication system (PACS) for future comparison, patients were followed at 90 days and their functional neurological outcome was evaluated using the modified Rankin Scale (mRS) (21,22) at 90 days post-discharge via telephone interview conducted by a trained medical staff (23). Our trained medical staff was blind to the patients’ clinical and imaging data and performed the interview using a structured, Persian-translated version of mRS. This approach was employed to ensure adequate follow-up and reduce attrition. Based on mRS, a binary outcome was defined as a good prognosis (mRS ≤2) or a poor prognosis (mRS ≥3) (24).
Statistical analysis
The sample size was calculated 203 using logistic regression model and considering type one error rate of 5% and statistical power of 80% previous similar studies (25). Continuous and categorical data are reported as mean ± standard deviation (SD) for normally distributed data [median (range) for non-normally continuous variables] and frequency (percentages), respectively. The normality of continuous data was evaluated using the Kolmogorov-Smirnov test and Q-Q plot. Non-normally positive skewed data were subjected to logarithmic transformation for normalization. Continuous normally and non-normally distributed data were compared between two groups using independent samples t-test and non-parametric Mann-Whitney U test, respectively, while categorical variables by using the chi-squared test.
In this study, we tested the predictive value of various imaging features and clinical and demographic characteristics using chi-squared test and logistic regression. Those predictors that were significantly associated with dependent variable in univariate analysis were entered into multivariable logistic regression. Results of logistic regression were reported as odds ratio (OR) and 95% confidence interval (CI) for OR. During logistic regression modeling, we adjusted the predictive role of imaging features for potential confounding variables, such as age and gender. We also used machine learning algorithm SVM to evaluate the predictive role of imaging features for determining patient prognosis. Features we used in SVM were those predictors which we identified them as significant predictors of poor prognosis in univariate and multivariable analyses. To ensure the generalizability and robustness of the models, the dataset is split into training and test sets, where the training set comprised 70% of samples is used to build the model, and remaining, i.e., 30% was considered as the test set, which was used for evaluating its performance. Moreover, the hyperparameters of the algorithms are tuned using 5-fold cross-validation within the training set, wherein the data is divided into five subsets, and the model is trained five times, each time using four subsets for training and one subset for validation. After finding the best values for hyperparameters, we use several metrics, including area under the receiver operator characteristic curve (AUC), sensitivity, specificity, and accuracy to determine the best performing model. All conventional statistical analyses were performed using SPSS version 26 (IBM Corp., SPSS Statistics for Windows, Version 26.0., Armonk, NY, USA) and SVM was run by using R free statistical software version 4.4 (R Core Team, 2023).
Results
Baseline characteristics
A total of 203 patients [female 78 (38.4%), male 125 (61.6%)] with primary ICH were identified during our study period, among whom 119 patients (58.6%) developed unfavorable prognosis in terms of functional neurological outcomes based on mRS. There was no statistically significant association between sex, history of hypertension, diabetes mellitus, cardiovascular diseases, renal diseases, previous ischemic stroke, elevated systolic blood pressure, and smoking (P>0.05). However, for age, diastolic blood pressure, and GCS score at admission there were significant differences between the two groups (P<0.05); indicating that advanced age (mean 68±14.35), higher diastolic blood pressure (97.37±24.67), and lower GCS score (mean 9.82±4.92) at admission were associated with poor prognosis in primary ICH patients. The demographic and clinical characteristics of patients are shown in Table 1.
Table 1
| Characteristics | Favorable prognosis (N=84) | Poor prognosis (N=119) | P value |
|---|---|---|---|
| Gender | 0.122 | ||
| Female | 27 (32.1%) | 51 (42.9%) | |
| Male | 57 (67.9%) | 68 (57.1%) | |
| Age (years), mean ± SD | 60.89±14.56 | 68±14.35 | 0.001 |
| SBP at admission (mmHg), mean ± SD | 161.74±31.16 | 170.46±34.9 | 0. 064 |
| DBP at admission (mmHg), mean ± SD | 91.69±15.47 | 97.37±24.67 | 0.046 |
| Baseline GCS score, mean ± SD | 12.92±3.46 | 9.82±4.92 | <0.001 |
| Disease history | |||
| DM | 0.983 | ||
| Yes | 26 (41.3%) | 37 (58.7%) | |
| No | 58 (41.4%) | 82 (58.6%) | |
| HTN | 0.654 | ||
| Yes | 59 (40.4%) | 87 (59.6%) | |
| No | 25 (43.9%) | 32 (56.1%) | |
| CNS disease | 0.531 | ||
| Yes | 20 (37.7%) | 33 (62.3%) | |
| No | 64 (42.7%) | 86 (57.3%) | |
| Cardiovascular disease | 0.576 | ||
| Yes | 19 (38%) | 31 (62%) | |
| No | 65 (42.5%) | 88 (57.5%) | |
| Renal disease | 0.326 | ||
| Yes | 1 (20%) | 4 (80%) | |
| No | 83 (41.9%) | 115 (58.1%) | |
| Smoking | 0.176 | ||
| Yes | 13 (54.2%) | 11 (45.8%) | |
| No | 71 (39.7%) | 108 (60.3%) | |
| Hematoma volume (mL) | |||
| Mean ± SD | 20.56±29.34 | 45.54±50.15 | 0.001 |
| Median | 9 | 30 | |
| ICH location | |||
| Brainstem | 0.117 | ||
| Yes | 5 (25%) | 15 (75%) | |
| No | 79 (43.2%) | 104 (56.8%) | |
| Cerebral hemispheres | 0.760 | ||
| Yes | 30 (40%) | 45 (60%) | |
| No | 54 (42.2%) | 74 (57.8%) | |
| Basal ganglia | 0.065 | ||
| Yes | 30 (34.1%) | 58 (65.9%) | |
| No | 54 (47%) | 61 (53%) | |
| Thalamus | 0.342 | ||
| Yes | 19 (35.8%) | 34 (64.2%) | |
| No | 65 (43.3%) | 85 (56.7%) | |
| Cerebellum | 0.613 | ||
| Yes | 8 (36.4%) | 14 (63.6%) | |
| No | 76 (42%) | 105 (58%) | |
| Midline shift >3 mm | <0.001 | ||
| Yes | 14 (20.9%) | 53 (79.1%) | |
| No | 70 (51.5%) | 66 (48.5%) | |
| Midline shift (mm) | <0.001 | ||
| Mean ± SD | 1.07±2.55 | 4.4±5.62 | |
| Median [range] | 0 [0, 11] | 0 [0, 22] | |
| CT imaging signs | |||
| Black hole sign | <0.001 | ||
| Yes | 7 (15.2%) | 39 (84.8%) | |
| No | 77 (49%) | 80 (51%) | |
| Satellite sign | 0.001 | ||
| Yes | 24 (28.2%) | 61 (71.8%) | |
| No | 60 (50.8%) | 58 (49.2%) | |
| Blend sign | 0.026 | ||
| Yes | 20 (30.3%) | 46 (69.7%) | |
| No | 64 (46.7%) | 73 (53.3%) | |
Independent t-test, ANOVA test, and Chi-squared test were used for statistical analysis. ANOVA, analysis of variance; CNS, central nervous system; CT, computed tomography; DBP, diastolic blood pressure; DM, diabetes mellitus; GCS, Glasgow Coma Scale; HTN, hypertension; ICH, intracerebral hemorrhage; NCCT, non-contrast computed tomography; SBP, systolic blood pressure; SD, standard deviation.
NCCT imaging markers and prognosis
Among NCCT imaging features obtained at baseline, the location of hemorrhage was not associated with different prognosis among patients (all P>0.05). The volume of the hemorrhage was considerably associated with 90-day prognosis (P=0.001), patients with larger hematoma volume at baseline (median 30 mL, 45.54±50.15 mL) experiencing unfavorable outcomes at 90 days and those with smaller bleeding volume (median 9 mL, 20.56±29.34 mL) developing favorable prognosis. Among 203 patients, 67 patients (33%) had midline shift >3 mm at initial NCCT imaging, of which 53 patients (79.1%) developed poor prognosis and 14 patients (20.9%) showed favorable prognosis and this association was shown to be statistically significant (P<0.001). When evaluating the amount of midline shift in mm, we found that higher midline shift values (4.4±5.62 mm) were inversely associated with prognosis at 3 months (P<0.001). Among CT imaging signs evaluated in this study, SS was the most frequently observed sign among patients (85, 41.9%), followed by the blend sign (66, 32.5%), and BHS (46, 22.7%). The presence of any of these imaging signs on initial NCCT was associated with worse outcome (all had P<0.05). Baseline imaging features of patients are demonstrated in Table 1.
Predictive accuracy of CT imaging features using logistic regression
The results of the logistic regression analysis (Table 2) for imaging characteristics shown to be statistically significant in prognosis determination, showed that higher volume of bleeding increases the risk for developing poor 90-day outcome (OR =0.981, 95% CI: 0.972–0.991, P<0.001). In addition, patients with midline shift greater than 3 mm were approximately 4 times more likely to experience unfavorable prognosis compared to patients without midline shift (OR =4.015, 95% CI: 2.038–7.911, P<0.001). Moreover, the presence of BHS was associated with a 5.3-fold increased risk of worse prognosis in primary ICH patients (OR =5.362, 95% CI: 2.262–12.714, P<0.001). SS and blend sign also correlated with up to 2-fold increased risk of unfavorable prognosis (OR =2.629, 95% CI: 1.451–4.764, P<0.001 and OR =2.016, 95% CI: 1.081–3.760, P=0.026; respectively). The final fitted logistic regression model containing the aforementioned resulted 75% correct classification rate.
Table 2
| Imaging marker | Odds ratio (95% CI) | P value | Importance rate with machine learning |
|---|---|---|---|
| Volume | 0.981 (0.972–0.991) | <0.001 | 100% |
| Midline shift >3 mm | |||
| Yes | 4.015 (2.038–7.911) | <0.001 | 54% |
| No | 1 (reference) | ||
| Black hole sign | |||
| Yes | 5.362 (2.262–12.714) | <0.001 | 63.1% |
| No | 1 (reference) | ||
| Satellite sign | |||
| Yes | 2.629 (1.451–4.764) | <0.001 | 20.4% |
| No | 1 (reference) | ||
| Blend sign | |||
| Yes | 2.016 (1.081–3.760) | 0.026 | 15.6% |
| No | 1 (reference) |
CI, confidence interval; NCCT, non-contrast computed tomography; SVM, support vector machine.
For determining the importance rate of each imaging sign in differentiating patients based on their 90-day mRS score, we used the SVM machine learning algorithm. Among the statistically significant imaging markers, hemorrhage volume was shown to have the highest importance rate in determining prognosis (importance rate: 100%). Other imaging signs in decreasing order of importance included, BHS (importance rate: 63.1%), midline shift greater than 3 mm (importance rate: 54%), SS (importance rate: 20.4%), and blend sign (importance rate: 15.6%) (Table 2).
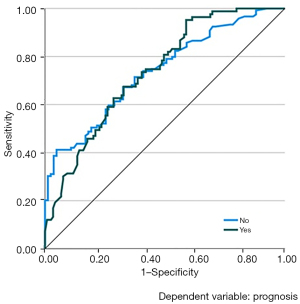
We also utilized the SVM method using the above imaging features to establish the 90-day prediction model with the best performance. In the training set, the sensitivity and specificity were 47.3% and 76.2%, respectively, with the diagnostic accuracy rate of 64.7%. In the validation set, the sensitivity and specificity were 75% and 68.6%, respectively, and the diagnostic accuracy rate was 71.4%. The AUC of our model was calculated to be 0.737 (P<0.001) (Figure 3). All these results indicate relatively high correct predictive ability of the fitted model.
Discussion
In this prospective study, we established a prognostication model for predicting the functional outcome of patients with primary ICH. The proposed model consisting of certain radiological features obtained from initial NCCT (hemorrhage volume, presence of midline shift, and the presence of markers of hematoma expansion such as blend sign, the BHS, and the SS) displayed considerable discriminative accuracy between unfavorable and favorable functional outcome of primary ICH based on mRS at 3 months. Machine learning-based analysis using the SVM algorithm also demonstrated classification performance and stability of these imaging parameters across all nested cross-validation runs, indicating sufficient generalizability of our results. The presence of certain clinical conditions was also associated with unfavorable prognosis, including higher diastolic blood pressure at presentation, advanced age, and lower GCS score at admission. Several predictive models encompassing radiological and clinical characteristics were proposed to determine the outcome of primary ICH patients at admission. There is growing interest in the early application of CT scan-based prognostication scoring systems for evaluation of patients suspected of having ICH and it has shown acceptable discriminative ability equal to computed tomography angiography (CTA) for predicting neurological outcomes (16,26).
It is a well-established paradigm that hemorrhage volume profoundly impacts functional clinical outcome for ICH patients (7). Several studies have proposed different cut-off values for hematoma volume for prognostication, with the majority reporting baseline volumes of 20–30 mL or greater suggestive of hematoma expansion and neurological deterioration, whereas baseline volumes of 10 mL or less were associated with more favorable clinical outcome and lower probability of hematoma expansion (11,27-29). In line with these findings, we found that patients with poor prognosis had a mean baseline ICH volume of 45.54±50.15 mL. Machine learning-based assessment of predictive performance also showed the greatest predictive power for ICH volume, with OR =0.981, 95% CI: 0.972–0.991, P<0.001 and the importance rate of 100%; further indicating hemorrhage volume as the strongest indicator of 90-day outcome for primary ICH. When the bleeding sites were compared between patients with unfavorable and favorable prognosis within 3 months of the index ICH, we found no statistically significant association between hemorrhage location and functional outcome; discouraging the results of previous studies (29-33). The INTERACT2 study showed that hemorrhages originating from the thalamus, posterior limb of the internal capsule, and cerebellum were associated with poor functional outcome, specifically thalamic and internal capsule bleeding had the greatest association with major disability or death (31). In another study of Park et al. (29), bleeding within the posterior limb of internal capsule and thalamus were independent predictors of poor outcome in primary ICH. The proposed mechanisms for poor outcome associated with deep hemorrhages include functional coagulation differences, different anatomical structure injury, and higher hematoma expansion in deep bleeding (30,32). Contrary to this, results of The FAST trial showed that lobar ICH was associated with larger baseline hemorrhage volume, greater expansion of hemorrhage, and worse 90-day functional outcome in unadjusted analysis; however when adjusting for factors influencing outcome, patients with deep ICH paradoxically had worse outcome in 3 months (33). Despite the reported discrepancy and complex relationship between location of bleeding and 90-day functional outcome in the literature, our study showed no significant relationship between bleeding location and prognosis; although patients with hemorrhage in the basal ganglia had worse outcome compared to other locations, this association was not statistically significant. The discrepancy observed in our study might be due to shorter follow-up period compared to previous studies.
Our study also demonstrated that the presence of midline shift correlated with poor outcome (OR =4.015, 95% CI: 2.038–7.911, P<0.001, and importance rate 54%), with the mean value of 4.4±5.62 mm observed among patients with poor prognosis. Previous studies evaluating the association between midline shift value and clinical outcome in primary ICH patients reported different cut-off values ranging from 3.7 to 6 mm, to be a clinically significant threshold for worse outcome (34-36). In accordance with previous studies, our study found a low threshold for poor prognosis in terms of midline shift (34,35). In this study, NCCT-based markers of hematoma expansion; the blend sign, the BHS, and the SS were all significantly associated with poor outcome in primary ICH (P<0.05), with the BHS being the most important predictor on machine learning analysis (importance rate: 63.1%) followed by the SS (importance rate: 20.4%) and the blend sign (importance rate: 15.6%). This finding is in correlation with previous studies indicating that the BHS on initial NCCT is independently associated with poor outcome at 90 days with high specificity (37-39). The appearance of BHS on NCCT indicates that the presence of bleeding spots of varying age within the heterogeneous hematoma and risk for future hematoma growth. Additionally, Li et al. (40) found that blend sign may be identified on initial NCCT with high specificity (95.5%) for predicting hematoma expansion and poor outcome. The development of blend sign indicates an area of mixed hemorrhage with different bleeding times with possible re-bleeding and hematoma expansion (16). In another study in patients with primary ICH, the BHS and the blend sign were independent predictors of poor outcome, with the blend sign demonstrating higher accuracy (AUC for blend sign: 0.660 vs. BHS: 0.620) (39). Furthermore, the SS was an independent predictor of hematoma expansion and poor outcome in previous studies (41,42). Collectively, there is a wide heterogeneity in the reported predictive performance metrics of these NCCT imaging markers (17). It is important to consider that all these imaging markers represent different points of a continuum sharing a similar pathophysiological change and that these imaging-based markers should be used in combination with other important prognosis indicators, such as clinical variables to improve their predictive accuracy.
In this study, we also evaluated the influence of several clinical indicators in the prognosis of primary ICH patients. In line with previous studies (15,43), advanced age at presentation correlated with poor 90-day functional outcome, with the cut-off value of 68 years for differentiating between favorable and unfavorable prognosis. Gender was not a predictor of prognosis between the 2 groups in our study. Several studies have found that females was associated with higher ICH scores and worse prognosis, due to the more intense inflammatory responses generally present in females and various socioeconomic factors that may limit females` access to adequate medical care (44,45). This observed discrepancy might be attributable to variations in female study population, as the majority of female patients have equal access to healthcare services in our hometown. Additionally, in line with the results of our study, Amer et al. (15) conducted a study on 70 patients with primary ICH and evaluated the clinical and radiological parameters in determining prognosis. They found that gender was not a determining predictive indicator of worse functional outcome.
The association between hypertension and development of ICH was extensively investigated in the literature; and elevated systolic blood pressure at admission correlated with poor prognosis (46,47). Our study showed that patients with elevated diastolic blood pressure at presentation (97.37±24.67 mmHg) had unfavorable 90-day outcome and high systolic blood pressure values and hypertension per se were not statistically different among the 2 groups. This may imply that the nominal value of blood pressure at admission is not the only indicator of outcome in affected patients, rather the dynamic changes in blood pressure during follow-up and hospitalization period may profoundly impact final prognosis (48). Additionally, the intensive blood pressure lowering during hospitalization might have lowered the risk of hematoma expansion and poor outcome among our study participants.
While there may be a modest association between diabetes mellitus and the development of ICH and its prognosis (49), no correlation with the final outcome was reported in other studies (15,50). Our study also showed that diabetes mellitus was not correlated with unfavorable outcome. It is imperative to note that uncontrolled diabetes mellitus might profoundly facilitate atherosclerosis and cause worse prognosis in patients with primary ICH. However, patients in our study, had adequately controlled blood glucose levels at hospitalization and their prognosis was not inversely affected.
Despite the underlying detrimental alterations of the cerebrovascular system of patients with ICH, a history of cerebrovascular diseases was not correlated with prognosis in our study. In a study of Wong et al. (51), it was shown that stroke recurrence rate was high in patients with primary ICH (7.01%) during the 5-year follow-up period. Other studies have also found a hemorrhagic stroke recurrence rate between 7% and 12% (52,53). We assume that short follow-up period of our study participants hampered the detection of poor outcome in this patient population compared to previous studies. In our study, a significant inverse association was found between GCS score at admission and outcome, which is in line with previous studies showing that low GCS at admission was found to be independently associated with a worse short-term and long-term prognosis (54,55).
Contrary to previous studies, we found no correlation between underlying cardiovascular and renal diseases and 90-day functional outcome of primary ICH patients. Ghoshal et al. (56) found that chronic kidney disease increases the risk of ICH. Amer et al. also found that baseline elevated blood urea nitrogen levels inversely correlated with outcome (15). The existing discrepancy in our study could be explained in part by the medical condition of patients with underlying renal and cardiovascular diseases at admission. Patients with underlying kidney and cardiovascular diseases had well-controlled disease at hospitalization and their comorbidity was adequately controlled by medical therapy. As a result, this patient population did not experience worse outcome compared to other individuals without renal and cardiovascular disorders.
The impact of smoking on the functional outcome of primary ICH remains controversial. While some studies found a worse prognosis in tobacco users who develop ICH (57,58), other studies found no difference in functional outcome (59,60). Our study also showed no difference in the 90-day functional outcome of patients based on their smoking status.
We developed a predictive model for the first time for differentiating ICH patients with poor and good prognosis by using the SVM method. SVM is a model-free method and an efficient approach in classification problems without any assumption regarding the distribution and interdependency of the predictors. Due to its flexibility, it performs better than traditional statistical methods such as logistic regression, particularly in situations that include multiple risk factors with interrelationships or multicollinearity, low sample size, and a limited knowledge of underlying biological relationships among risk factors. Despite remarkable aforementioned advantages and generalization capacity of the SVM, it has some weaknesses, such as feature selection challenges, algorithmic complexity that affects the training time of the classifier particularly in large data sets, development of optimal classifiers for categorical dependent variables with more than two categories and its unreliable performance in unbalanced data sets (61). In other words, high dimensional input vectors often reduce the computational efficiency and significantly slow down the classification process. Our study data set more benefits from the advantages of SVM instead of its weakness, however, if we used a larger sample size, we could develop a predictive model with more stable and reliable results, because we could able to train and test our model in subsets with larger sample size.
The major limitations of our current study were a short follow-up period of 3 months, functional outcome assessment being limited to the mRS, and a lack of laboratory markers assessment to establish a more comprehensive predictive model. We also did not evaluate other radiological markers of prognostication in ICH patients. Future studies are required to evaluate the predictive performance of all the relevant radiological and laboratory indicators in predicting prognosis of primary ICH in our patients particularly using machine learning-based image segmentation.
Conclusions
In summary, we evaluated the predictive performance of several NCCT imaging markers and clinical conditions in determining the 90-day prognosis of patients with primary ICH. This predictive model could be utilized by clinicians in the emergency department for prognostication purposes. Hemorrhage volume was associated with the most prognostication capability, followed by the BHS, midline shift, SS, and blend sign. Among clinical indicators, advanced age, elevated diastolic blood pressure, and low GCS score correlated with poor prognosis. On the other hand, hemorrhage location, females, history of diabetes mellitus, cardiovascular diseases, renal disease, and cerebrovascular accidents did not correlate with worse prognosis in our patients. Further study is required to evaluate the impact of other laboratory and imaging indicators on prognosis of patients with primary ICH. Furthermore, future studies with larger sample size are suggested to evaluate the predictive performance of all the potentially relevant radiological and laboratory indicators in predicting prognosis of primary ICH particularly using machine learning-based image segmentation approaches.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1299/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1299/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and approved by the Institutional Ethics Committee of Isfahan University of Medical Sciences (approval date: 8/1/2021, protocol code IR.MUI.MED.REC.1400.446) and the National Research Ethics Board (approval number IR.MUI.MED.REC.1400.446). Informed consent was obtained from all subjects or patients’ families involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGurgan IJ, Ziai WC, Werring DJ, Al-Shahi Salman R, Parry-Jones AR. Acute intracerebral haemorrhage: diagnosis and management. Pract Neurol 2020;21:128-36. [Crossref] [PubMed]
- Raposo N, Zanon Zotin MC, Seiffge DJ, Li Q, Goeldlin MB, Charidimou A, Shoamanesh A, Jäger HR, Cordonnier C, Klijn CJ, Smith EE, Greenberg SM, Werring DJ, Viswanathan A. A Causal Classification System for Intracerebral Hemorrhage Subtypes. Ann Neurol 2023;93:16-28. [Crossref] [PubMed]
- Law ZK, Appleton JP, Bath PM, Sprigg N. Management of acute intracerebral haemorrhage - an update. Clin Med (Lond) 2017;17:166-72. [Crossref] [PubMed]
- Al-Kawaz MN, Hanley DF, Ziai W. Advances in Therapeutic Approaches for Spontaneous Intracerebral Hemorrhage. Neurotherapeutics 2020;17:1757-67. [Crossref] [PubMed]
- Unmet Needs and Challenges in Clinical Research of Intracerebral Hemorrhage. Stroke 2018;49:1299-307. [Crossref] [PubMed]
- Huang YW, Huang HL, Li ZP, Yin XS. Research advances in imaging markers for predicting hematoma expansion in intracerebral hemorrhage: a narrative review. Front Neurol 2023;14:1176390. [Crossref] [PubMed]
- Hillal A, Ullberg T, Ramgren B, Wassélius J. Computed tomography in acute intracerebral hemorrhage: neuroimaging predictors of hematoma expansion and outcome. Insights Imaging 2022;13:180. [Crossref] [PubMed]
- Li Q, Yakhkind A, Alexandrov AW, Alexandrov AV, Anderson CS, Dowlatshahi D, Frontera JA, Hemphill JC, Ganti L, Kellner C, May C, Morotti A, Parry-Jones A, Sheth KN, Steiner T, Ziai W, Goldstein JN, Mayer SA. Code ICH: A Call to Action. Stroke 2024;55:494-505. [Crossref] [PubMed]
- Li Q, Yang WS, Wang XC, Cao D, Zhu D, Lv FJ, Liu Y, Yuan L, Zhang G, Xiong X, Li R, Hu YX, Qin XY, Xie P. Blend sign predicts poor outcome in patients with intracerebral hemorrhage. PLoS One 2017;12:e0183082. [Crossref] [PubMed]
- Shakya MR, Fu F, Zhang M, Shan Y, Yu F, Sun S, Lu J. Comparison of Black Hole Sign, Satellite Sign, and Iodine Sign to Predict Hematoma Expansion in Patients with Spontaneous Intracerebral Hemorrhage. Biomed Res Int 2021;2021:3919710. [Crossref] [PubMed]
- Lin F, He Q, Tong Y, Zhao M, Ye G, Gao Z, Huang W, Cai L, Wang F, Fang W, Lin Y, Wang D, Dai L, Kang D. Early Deterioration and Long-Term Prognosis of Patients With Intracerebral Hemorrhage Along With Hematoma Volume More Than 20 ml: Who Needs Surgery? Front Neurol 2021;12:789060. [Crossref] [PubMed]
- Yuan L, Shen YQ, Xie XF, Yang WS, Li R, Deng L, Li Q, Xie P. Combination of ultra-early hematoma growth and blend sign for predicting hematoma expansion and functional outcome. Clin Neurol Neurosurg 2020;189:105625. [Crossref] [PubMed]
- Du C, Liu B, Yang M, Zhang Q, Ma Q, Ruili R. Prediction of Poor Outcome in Intracerebral Hemorrhage Based on Computed Tomography Markers. Cerebrovasc Dis 2020;49:556-62. [Crossref] [PubMed]
- Haupenthal D, Schwab S, Kuramatsu JB. Hematoma expansion in intracerebral hemorrhage - the right target? Neurol Res Pract 2023;5:36. [Crossref] [PubMed]
- Amer HA, El-Jaafary SIM, Sadek HMAE, Fouad AM, Mohammed SS. Clinical and paraclinical predictors of early neurological deterioration and poor outcome in spontaneous intracerebral hemorrhage. Egypt J Neurol Psychiatr Neurosurg 2023;59:74. [Crossref] [PubMed]
- Wang P, Wu F, Wang Y, Du F, Yang X, Li J, Sheng J, Yu H, Jiang R. Computed tomography and clinical parameters predict intracerebral hemorrhage expansion. Medicine (Baltimore) 2022;101:e28912. [Crossref] [PubMed]
- Ducroux C, Nehme A, Rioux B, Panzini MA, Fahed R, Gioia LC, Létourneau-Guillon L. NCCT Markers of Intracerebral Hemorrhage Expansion Using Revised Criteria: An External Validation of Their Predictive Accuracy. AJNR Am J Neuroradiol 2023;44:658-64. [Crossref] [PubMed]
- Hillal A, Sultani G, Ramgren B, Norrving B, Wassélius J, Ullberg T. Accuracy of automated intracerebral hemorrhage volume measurement on non-contrast computed tomography: a Swedish Stroke Register cohort study. Neuroradiology 2023;65:479-88. [Crossref] [PubMed]
- Xu XM, Zhang H, Meng RL. Cranial midline shift is a predictor of the clinical prognosis of acute cerebral infarction patients undergoing emergency endovascular treatment. Sci Rep 2023;13:21037. [Crossref] [PubMed]
- Sporns PB, Schwake M, Kemmling A, Minnerup J, Schwindt W, Niederstadt T, Schmidt R, Hanning U. Comparison of Spot Sign, Blend Sign and Black Hole Sign for Outcome Prediction in Patients with Intracerebral Hemorrhage. J Stroke 2017;19:333-9. [Crossref] [PubMed]
- Pożarowszczyk N, Kurkowska-Jastrzębska I, Sarzyńska-Długosz I, Nowak M, Karliński M. Reliability of the modified Rankin Scale in clinical practice of stroke units and rehabilitation wards. Front Neurol 2023;14:1064642. [Crossref] [PubMed]
- Baker WL, Sharma M, Cohen A, Ouwens M, Christoph MJ, Koch B, Moore TE, Frady G, Coleman CI. Using 30-day modified rankin scale score to predict 90-day score in patients with intracranial hemorrhage: Derivation and validation of prediction model. PLoS One 2024;19:e0303757. [Crossref] [PubMed]
- Chen XW, Shafei MN, Abdullah JM, Musa KI. Reliability of Telephone Interview for Assessment of Long-Term Stroke Outcomes: Evidence from Interrater Analysis. Neuroepidemiology 2019;52:214-9. [Crossref] [PubMed]
- Nawabi J, Kniep H, Elsayed S, Friedrich C, Sporns P, Rusche T, Böhmer M, Morotti A, Schlunk F, Dührsen L, Broocks G, Schön G, Quandt F, Thomalla G, Fiehler J, Hanning U. Imaging-Based Outcome Prediction of Acute Intracerebral Hemorrhage. Transl Stroke Res 2021;12:958-67. [Crossref] [PubMed]
- Chung GH, Goo JH, Kwak HS, Hwang SB. The comprehensive comparison of imaging sign from CT angiography and noncontrast CT for predicting intracranial hemorrhage expansion: A comparative study. Medicine (Baltimore) 2022;101:e31914. [Crossref] [PubMed]
- Morotti A, Boulouis G, Nawabi J, Li Q, Charidimou A, Pasi M, et al. Using Noncontrast Computed Tomography to Improve Prediction of Intracerebral Hemorrhage Expansion. Stroke 2023;54:567-74. [Crossref] [PubMed]
- Leasure AC, Sheth KN, Comeau M, Aldridge C, Worrall BB, Vashkevich A, Rosand J, Langefeld C, Moomaw CJ, Woo D, Falcone GJ. Identification and Validation of Hematoma Volume Cutoffs in Spontaneous, Supratentorial Deep Intracerebral Hemorrhage. Stroke 2019;50:2044-9. [Crossref] [PubMed]
- Nakagawa K, King SL, Seto TB. Optimal Hematoma Volume Cut Points to Predict Functional Outcome After Basal Ganglia and Thalamic Hemorrhages. Front Neurol 2018;9:291. [Crossref] [PubMed]
- Park JS, Jang HG. Analysis of the association between location and patient prognosis in spontaneous intracerebral hemorrhage in the basal ganglia and thalamus: A retrospective single-center study. Medicine (Baltimore) 2022;101:e32000. [Crossref] [PubMed]
- Roh D, Boehme A, Young C, Roth W, Gutierrez J, Flaherty M, Rosand J, Testai F, Woo D, Elkind MSV. Hematoma expansion is more frequent in deep than lobar intracerebral hemorrhage. Neurology 2020;95:e3386-93. [Crossref] [PubMed]
- Delcourt C, Sato S, Zhang S, Sandset EC, Zheng D, Chen X, Hackett ML, Arima H, Hata J, Heeley E, Al-Shahi Salman R, Robinson T, Davies L, Lavados PM, Lindley RI, Stapf C, Chalmers J, Anderson CS. INTERACT2 Investigators. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 2017;88:1408-14. [Crossref] [PubMed]
- Roh D, Chang T, Zammit C, Wagener G, Reynolds AS, Yoh N, Elkind MSV, Doyle K, Boehme A, Eisenberger A, Francis RO, Park S, Agarwal S, Connolly ES, Claassen J, Hod E. Functional Coagulation Differences Between Lobar and Deep Intracerebral Hemorrhage Detected by Rotational Thromboelastometry: A Pilot Study. Neurocrit Care 2019;31:81-7. [Crossref] [PubMed]
- Kuohn LR, Witsch J, Steiner T, Sheth KN, Kamel H, Navi BB, Merkler AE, Murthy SB, Mayer SA. Early Deterioration, Hematoma Expansion, and Outcomes in Deep Versus Lobar Intracerebral Hemorrhage: The FAST Trial. Stroke 2022;53:2441-8. [Crossref] [PubMed]
- Yang WS, Li Q, Li R, Liu QJ, Wang XC, Zhao LB, Xie P. Defining the Optimal Midline Shift Threshold to Predict Poor Outcome in Patients with Supratentorial Spontaneous Intracerebral Hemorrhage. Neurocrit Care 2018;28:314-21. [Crossref] [PubMed]
- Park J, Goh DH, Sung JK, Hwang YH, Kang DH, Kim Y. Timely assessment of infarct volume and brain atrophy in acute hemispheric infarction for early surgical decompression: strict cutoff criteria with high specificity. Acta Neurochir (Wien) 2012;154:79-85. [Crossref] [PubMed]
- Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2005;76:1534-8. [Crossref] [PubMed]
- Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, Wei X, Xie P. Black Hole Sign: Novel Imaging Marker That Predicts Hematoma Growth in Patients With Intracerebral Hemorrhage. Stroke 2016;47:1777-81. [Crossref] [PubMed]
- Yu Z, Zheng J, Ma L, Guo R, Li M, Wang X, Lin S, Li H, You C. The predictive accuracy of the black hole sign and the spot sign for hematoma expansion in patients with spontaneous intracerebral hemorrhage. Neurol Sci 2017;38:1591-7. [Crossref] [PubMed]
- Li R, Yang M. A comparative study of the blend sign and the black hole sign on CT as a predictor of hematoma expansion in spontaneous intracerebral hemorrhage. Biosci Trends 2017;11:682-7. [Crossref] [PubMed]
- Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X, Chen JJ, Zhang LJ, Qin XY, Xie P. Blend Sign on Computed Tomography: Novel and Reliable Predictor for Early Hematoma Growth in Patients With Intracerebral Hemorrhage. Stroke 2015;46:2119-23. [Crossref] [PubMed]
- Shimoda Y, Ohtomo S, Arai H, Okada K, Tominaga T. Satellite Sign: A Poor Outcome Predictor in Intracerebral Hemorrhage. Cerebrovasc Dis 2017;44:105-12. [Crossref] [PubMed]
- Yu Z, Zheng J, Ali H, Guo R, Li M, Wang X, Ma L, Li H, You C. Significance of satellite sign and spot sign in predicting hematoma expansion in spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 2017;162:67-71. [Crossref] [PubMed]
- Mahdy ME, Ghonimi NA, Elserafy TS, Mahmoud W. The NIHSS score can predict the outcome of patients with primary intracerebral hemorrhage. Egypt J Neurol Psychiatr Neurosurg 2019;55:21.
- Yang L, Han J, Qin C, Shou W. Sex-Based Differences in the Outcomes of Intracerebral Haemorrhage: A Systematic Review and Meta-Analysis. Cerebrovasc Dis 2024;53:753-66. [Crossref] [PubMed]
- Hsieh JT, Ang BT, Ng YP, Allen JC, King NK. Comparison of Gender Differences in Intracerebral Hemorrhage in a Multi-Ethnic Asian Population. PLoS One 2016;11:e0152945. [Crossref] [PubMed]
- Lattanzi S, Silvestrini M. Blood pressure in acute intra-cerebral hemorrhage. Ann Transl Med 2016;4:320. [Crossref] [PubMed]
- Divani AA, Liu X, Di Napoli M, Lattanzi S, Ziai W, James ML, Jafarli A, Jafari M, Saver JL, Hemphill JC, Vespa PM, Mayer SA, Petersen A. Blood Pressure Variability Predicts Poor In-Hospital Outcome in Spontaneous Intracerebral Hemorrhage. Stroke 2019;50:2023-9. [Crossref] [PubMed]
- Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, Rosand J. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. JAMA 2015;314:904-12. [Crossref] [PubMed]
- Boulanger M, Poon MT, Wild SH, Al-Shahi Salman R. Association between diabetes mellitus and the occurrence and outcome of intracerebral hemorrhage. Neurology 2016;87:870-8. [Crossref] [PubMed]
- Hesami O, Kasmaei HD, Matini F, Assarzadegan F, Mansouri B, Jabbehdari S. Relationship between intracerebral hemorrhage and diabetes mellitus: a case-control study. J Clin Diagn Res 2015;9:OC08-10. [Crossref] [PubMed]
- Wong YS, Tsai CF, Ong CT. Risk factors for stroke recurrence in patients with hemorrhagic stroke. Sci Rep 2022;12:17151. [Crossref] [PubMed]
- Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke 2000;31:123-7. [Crossref] [PubMed]
- Nakase T, Yoshioka S, Sasaki M, Suzuki A. Clinical features of recurrent stroke after intracerebral hemorrhage. Neurol Int 2012;4:e10. [Crossref] [PubMed]
- Al-Mufti F, Thabet AM, Singh T, El-Ghanem M, Amuluru K, Gandhi CD. Clinical and Radiographic Predictors of Intracerebral Hemorrhage Outcome. Interv Neurol 2018;7:118-36. [Crossref] [PubMed]
- Suthar NN, Patel KL, Saparia C, Parikh AP. Study of clinical and radiological profile and outcome in patients of intracranial hemorrhage. Ann Afr Med 2016;15:69-77. [Crossref] [PubMed]
- Ghoshal S, Freedman BI. Mechanisms of Stroke in Patients with Chronic Kidney Disease. Am J Nephrol 2019;50:229-39. [Crossref] [PubMed]
- Chen CJ, Ding D, Ironside N, Buell TJ, Southerland AM, Koch S, Flaherty M, Woo D, Worrall BB. ERICH Investigators. Cigarette Smoking History and Functional Outcomes After Spontaneous Intracerebral Hemorrhage. Stroke 2019;50:588-94. [Crossref] [PubMed]
- Ironside N, Chen CJ, Pucci J, Connolly ES. Effect of Cigarette Smoking on Functional Outcomes in Patients with Spontaneous Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis 2019;28:2496-505. [Crossref] [PubMed]
- Schupper AJ, Khorasanizadeh M, Rossitto CP, Foster LD, Kellner CP, Suarez JI, Qureshi AI, Majidi S. ATACH‐2 trial investigators. Cigarette Smoking as a Risk Factor for Hematoma Expansion in Primary Intracerebral Hemorrhage: Analysis From a Randomized Clinical Trial. J Am Heart Assoc 2023;12:e030431. [Crossref] [PubMed]
- Sembill JA, Sprügel MI, Gerner ST, Beuscher VD, Giede-Jeppe A, Stocker M, Hoelter P, Lücking H, Kuramatsu JB, Huttner HB. Influence of Prior Nicotine and Alcohol Use on Functional Outcome in Patients after Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis 2018;27:892-9. [Crossref] [PubMed]
- Cervantes J, Garcia-Lamont F, Rodríguez-Mazahua L, Lopez A. A comprehensive survey on support vector machine classification: Applications, challenges and trends. Neurocomputing 2020;408:189-215.


