
Reassessing the role of cervical magnetic resonance imaging in diagnosing demyelinating disorders: a case misclassified as medullary infarction
Introduction
Inflammatory demyelination of the central nervous system (CNS) is precipitated by neurological lesions, predominantly featuring the degradation, disintegration, and even exfoliation of the myelin sheath enveloping nerve fibers, whereas the underlying axons and neurons remain comparatively undamaged. This condition has emerged as a pivotal contributor to non-traumatic neurological impairment among the young population (1). The imaging hallmarks of demyelinating diseases and infarct lesions within the medulla oblongata can exhibit striking similarities, and the inherent challenge of promptly identifying ischemic lesions in the medulla oblongata during magnetic resonance imaging (MRI) examinations markedly heightens the risk of misdiagnosis.
In clinical settings, patients presenting with acute symptoms and negative laboratory findings for medullary demyelination are frequently misclassified as having experienced a stroke. This report describes the case of a patient with a stroke-mimicking onset, chiefly exhibiting unilateral limb numbness and paresis. Subsequent to a meticulous review of the patient’s history and a re-evaluation of the cervical spine MRI, the diagnosis was amended and confirmed as demyelination. To the best of our knowledge, this is the first detailed case study documenting the initial misdiagnosis of lower medullary infarction, which was subsequently accurately identified as demyelination following a re-examination of the cervical spine MRI.
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
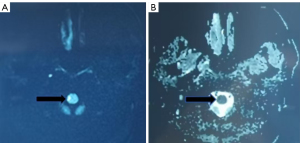
A 34-year-old male presented to the hospital with the acute onset of numbness and weakness in the right limbs. These symptoms in the right limbs had emerged subsequent to intense sweating on the day of symptom onset. During a computed tomography (CT) scan of the head at a local facility, the patient’s condition deteriorated progressively, culminating in an inability to stand and weight-bear on the right side. He was subsequently transferred to a tertiary care hospital, where the head CT scan excluded hemorrhage, and MRI of the head suggested diffuse changes in the right medulla oblongata (Figure 1). Head multimodal perfusion and computed tomography angiography (CTA) perfusion imaging indicated slightly delayed mean transit time (MTT) and time-to-peak (TTP) locally in the right medulla, with no significant abnormalities observed in cerebral blood volume (CBV) and cerebral blood flow (CBF). Cervical spine MRI revealed patchy signal changes on T1-weighted image (T1WI) and T2-weighted image (T2WI) sequences within the C7 vertebral body, as well as focal long T2 signal changes on the right side of the medulla, with indistinct margins. The patient’s medical history included fatty liver disease for 2 years, a history of occasional alcohol use, no history of smoking, and a moderate body habitus. Laboratory findings were as follows: white blood cell (WBC) count, 15.5×109/L; neutrophil percentage (NE%), 0.87%; lymphocyte percentage (LY%), 0.08%; neutrophil (NE) count, 0.77×109/L; triglycerides, 4.14 mmol/L.
Neurological examination findings included: alertness and clear speech, horizontal nystagmus bilaterally, muscle strength of 5/5 in the left limbs, 4/5 in the right upper limb, 3/5 in the proximal right lower limb, and 4/5 in the distal right lower limb. Tendon reflexes were intact in the left limbs and brisk in the right limbs. Hoffmann’s sign was positive on the right side. There was diminished pain, temperature, and crude touch sensation in the right side of the face, and reduced pain, absent temperature, and diminished crude touch sensation in the left limbs. The right limbs exhibited hyperesthesia to pain and crude touch. Vibration sense was preserved in the left limbs and absent in the right lower limb. No pathological signs were detected, and Romberg’s sign was positive (with both eyes open and closed).
The initial diagnosis was acute cerebral infarction. Intravenous thrombolytic therapy did not result in an appreciable improvement in symptoms. Admission laboratory studies, including coagulation profile, cardiac biomarkers, B-type natriuretic peptide (BNP), liver function tests, renal function tests, homocysteine levels, blood glucose levels, infection markers, thyroid function tests, rheumatoid factor panel, folic acid, vitamin B12, antinuclear antibody (ANA) panel, vasculitis screening, anticardiolipin antibodies, protein S activity, and protein C activity, all yielded normal or non-diagnostic results. Despite a 10-day treatment regimen consisting of antiplatelet therapy, circulation enhancement, and collateral circulation promotion, there was no clinical improvement observed.
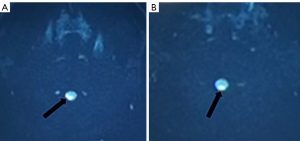
Review of the patient’s medical history revealed that the patient had experienced headaches 1 month preceding the onset of neurological symptoms, which occurred following vaccination. A subsequent re-examination of the brain MRI with diffusion-weighted imaging (DWI) (Figure 2) revealed diffuse areas of restriction in the medulla oblongata. The cervical spine MRI (Figure 3) demonstrated alterations within the cervical spinal cord extending from the medulla oblongata to the level of the C3 vertebral body. The enhanced cervical spine MRI (Figure 4) suggested the presence of demyelinating changes. Electromyography and nerve conduction studies indicated aberrant findings in the bilateral proprioceptive pathways, predominantly affecting the right side, as well as an abnormal blink reflex pathway (involving the brainstem medulla segment), again predominantly on the right side.
Analysis of the cerebrospinal fluid (CSF) revealed a total red blood cell count of 300×106/L, with a positive Pandy’s reaction. CSF biochemistry showed a total protein level of 667.7 mg/L. Both CSF and serum antibody tests against aquaporin-4 (AQP4), myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), and glial fibrillary acidic protein (GFAP)-IgG were negative. The CSF and serum albumin quotient (QAlb) was 8.7×103, with normal concentrations of IgG, IgA, and IgM. Quantitative analysis for oligoclonal bands (OCBs) in both the CSF and serum did not detect the presence of OCB. The CSF IgG index and the 24-hour intrathecal synthesis rate of IgG were within normal limits. Reibergram histogram suggested a straightforward disruption of the blood-brain barrier.
Diagnosis
Intramedullary demyelination extending from the medulla oblongata to the level of the C3 vertebral body. The patient was initiated on oral prednisone acetate tablets at a dosage of 60 mg once daily (with a decrement of 10 mg every 7 days) for anti-inflammatory therapy, vitamin B1 and B12 for neurotrophic support, and a comprehensive rehabilitation regimen including acupuncture, physical therapy, occupational therapy, and hand function training. After 1 week of treatment, there was a marked improvement in the patient’s limb strength, with the right upper limb achieving a muscle strength of 4+ and the right lower limb at a level of 4, which enabled independent transfers from a seated to a standing position. After 3 weeks of treatment, the patient regained the ability to eat independently. By the fourth week, the right upper limb muscle strength had recovered to a level of 5, and the right lower limb to a level of 4+, facilitating independent ambulation on level ground for discharge. Follow-up evaluations revealed no recurrence of symptoms, with continued improvement in strength. Following discharge, the patient continued to receive periodic acupuncture as part of their rehabilitation. At a 2.5-year follow-up, the patient had resumed working and living independently without any adverse events or unexpected complications. The changes in the Barthel index (BI) score for activities of daily living are detailed in Figure 5.
Discussion
In Asian countries, the rate of misdiagnosis for early-stage demyelinating diseases is significant, particularly in patients with acute symptom onset. The similarity between the initial symptoms and imaging findings of single-focus demyelination and stroke can lead to confusion. In the present case, the acute presentation, hyperlipidemia, and the imaging signal characteristics of the medulla oblongata were analogous to those of acute cerebral stroke, which resulted in an initial misdiagnosis of acute cerebral infarction. A review of the PubMed literature revealed that the first documented case of a demyelinating disease misdiagnosed as acute stroke was reported by Smith et al. following autopsy. Although their patient did not fulfill the clinical diagnostic criteria for multiple sclerosis (MS) among demyelinating diseases, post-mortem pathological analysis confirmed the presence of medullary demyelination (2). Qiu et al. described in 2009 a detailed case of a patient initially misdiagnosed with lateral medullary syndrome, who was subsequently diagnosed with MS, a demyelinating disease, upon readmission due to a recurrence (3). Research indicates that spinal cord lesions in non-demyelinating diseases are uncommon, particularly in the setting of ischemic stroke (4). A literature review by Sun et al. revealed that lateral medullary infarction typically occurs in the middle and upper segments of the medulla oblongata, whereas medial medullary infarction lesions are usually found in the upper segment of the medulla oblongata (5). Guo et al. found that medullary infarction predominantly affects middle-aged and elderly men, with lesions primarily located in the middle and upper parts of the medulla oblongata, and the majority of cases are unilateral (6). In contrast, medullary demyelination diseases are more common in young women, with lesions frequently situated in the lower part of the medulla oblongata, and there is a propensity for these lesions to extend into the spinal cord (6).
This patient was a young male without any apparent vascular risk factors, save for hyperlipidemia, and imaging revealed no plaques or stenosis within the cranial and cervical vessels. Given the bilateral nature of the symptoms and physical examination findings, subsequent brain MRI re-examinations paradoxically depicted only a unilateral lower medullary lesion. Consequently, for young patients in whom non-unilateral medullary “infarction” is suspected, it is imperative to entertain the possibility of lower medullary and cervical spinal cord involvement and to pursue timely re-examination of the cervical spine MRI.
Upon physical examination, the patient’s right medullary lesion foreshadowed left-sided limb dysfunction; however, the observed right limb weakness was incongruent with this expectation. Initially, it was hypothesized that there might be a delayed imaging manifestation of acute stroke; however, a re-examination of the brain MRI continued to show the lesion in the same locale, and the cervical spine MRI indicated an extensive spinal cord lesion. In light of the patient’s medical history, a demyelinating lesion extending from the medulla oblongata to C3 was suspected. The patient’s left corticospinal tract lesion accounted for right limb weakness, whereas the lesion affecting the right trigeminal spinal nucleus and spinothalamic lateral tract resulted in diminished sensation on the right face and left limb. Damage to the cerebellar spinal tract was manifested by a positive Romberg’s sign, and the lesion of the inferior olive of the medulla oblongata gave rise to nystagmus (7). These findings collectively accounted for the patient’s symptoms, leading to a diagnosis that was unambiguous in terms of both localization and nature.
This case underscores the importance for neurologists to be strongly suspicious of initial misdiagnosis when a lesion does not align with the clinical symptoms and physical examination results. Relevant diagnostic tests should be promptly re-evaluated. Early diagnosis and treatment of demyelinating diseases are crucial for preventing disease progression and irreversible secondary axonal degeneration (8). Rectifying the diagnostic course in a timely manner and administering more appropriate treatment can significantly enhance the patient’s quality of life.
This study, through the analysis of the diagnostic and therapeutic journey of a patient initially misdiagnosed, exposes the pitfalls that neurologists may encounter when faced with stroke-like presentations and symptoms. It underscores the critical importance of promptly rectifying the diagnostic trajectory. The findings offer novel perspectives for clinicians in the diagnosis and management of medullary pathologies. Nevertheless, this report is based on a single case, and future research should aim to expand the sample size to examine the imaging timing and morphology of medullary demyelinating lesions in comparison to those of medullary infarction. Such an investigation will delineate the distinguishing features between the 2 conditions and facilitate the development of diagnostic techniques that are grounded in imaging anatomical morphology and imaging expediency.
Acknowledgments
None.
Footnote
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2896/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu W, Lucchinetti CF. The pathological spectrum of CNS inflammatory demyelinating diseases. Semin Immunopathol 2009;31:439-53. [Crossref] [PubMed]
- Smith DB, DeMasters BK. Demyelinating disease presenting as Wallenberg’s syndrome. Report of a patient. Stroke 1981;12:877-8. [Crossref] [PubMed]
- Qiu W, Wu JS, Carroll WM, Mastaglia FL, Kermode AG. Wallenberg syndrome caused by multiple sclerosis mimicking stroke. J Clin Neurosci 2009;16:1700-2. [Crossref] [PubMed]
- Bot JC, Barkhof F. Lycklama à Nijeholt G, van Schaardenburg D, Voskuyl AE, Ader HJ, Pijnenburg JA, Polman CH, Uitdehaag BM, Vermeulen EG, Castelijns JA. Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 2002;223:46-56. [Crossref] [PubMed]
- Sun AP, Liu XY, Sun QL, Chen L, Liu XR, Fan DS. Clinical, imaging features and prognosis of medullary infarction. Chin Med J (Engl) 2016;55:361-5.
- Guo YZ. Clinical and imaging comparison of medullary infarction and medullary demyelinating disease. Liaoning: China Medical University; 2013.
- Eggers C, Fink GR, Möller-Hartmann W, Nowak DA. Correlation of anatomy and function in medulla oblongata infarction. Eur J Neurol 2009;16:201-4. [Crossref] [PubMed]
- Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol 2014;10:435-46. [Crossref] [PubMed]





