
Stroke subtypes risk prediction and detection using retinal vascular structure and oxygen saturation analysis
Introduction
Stroke, a leading global health concern, is ranked as the second-leading cause of death and a major contributor to disability worldwide (1-3). The prevalence and severity of stroke, particularly ischemic strokes, which account for 88% of all cases, alongside hemorrhagic strokes, constituting approximately 12%, present significant challenges in medical research and public health (4). Pu et al.’s research has demonstrated that the global incidence of ischemic stroke reached 81.72 per 100,000 in 2020, with projections suggesting an increase to 89.32 by 2030 (5). Notably, in China, stroke has been the foremost cause of death from 1990 to 2017, highlighting its critical impact on public health systems (6).
Recent years have witnessed a growing interest in exploring the retinal structure’s changes following a stroke (7-9). The retina, given its unique position as a non-invasive window into the body’s vascular system, has emerged as a potential marker for neurological diseases, offering insights into both prognostic and clinical aspects. This potential is largely attributed to the similarities between the retinal and cerebral microvasculature, their shared embryological origins, and the intricate neuronal layers (10,11). Additionally, the direct link formed by axons from the optic nerve to the brain suggests a possible association between brain damage and retinal structural changes. Beyond structural alterations, the role of retinal vascular blood oxygen saturation (SO2) is increasingly being recognized as a possible indicator of cerebral functional changes. This interest is spurred by studies showing altered SO2 in retinal vessels among individuals with neurological conditions like Alzheimer’s disease (12).
However, current research on the use of retinal vasculature in the context of stroke faces several gaps. A notable deficiency is the lack of comprehensive studies examining the relationship between retinal vascular SO2 and both ischemic and hemorrhagic strokes. In hemorrhagic stroke research, although there is attention given to retinal vessel caliber, other potentially significant microvascular features such as retinal fractal dimension (FD), SO2, and indicators of vessel branching patterns remain largely unexplored (13-15). Similarly, in ischemic stroke research, there is a need for more detailed analyses of these vascular parameters and their association with stroke. Furthermore, the use of retinal vascular features as biomarkers for stroke classification is an area that has not been fully developed, despite its potential to significantly enhance the precision of stroke diagnosis and prognosis.
Our study addresses these gaps by focusing on both ischemic and hemorrhagic strokes and exploring the role of retinal vasculature in these conditions. By examining novel retinal vascular features and their correlations with stroke subtypes, this study aims to provide insights that could inform future research on stroke risk assessment and patient outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2712/rc).
Methods
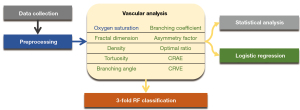
Figure 1 illustrates the workflow, starting with the initial data collection of fundus images from stroke patients and control subjects. Subsequently, proper deep learning methods are employed in the preprocessing procedure to accurately extract arteries, veins, optic disk (OD), and region of interest. Following this, vascular analysis is conducted to obtain parameters such as functional SO2 and vascular morphological features, including FD, density, etc. After obtaining these parameters, we conducted statistical analyses to compare the differences between stroke and control groups. Additionally, logistic regression was employed concurrently to establish the relationships between these parameters and the two stroke subtypes. Finally, stroke classification was performed through three-fold cross-validation using random forest (RF).
Data collection and study participants
Figure 2 summarizes the participant selection process for this study, conducted at the First Affiliated Hospital of the University of Science and Technology of China (USTC) from September 14, 2020 to September 14, 2021. The flowchart details the inclusion and exclusion criteria for Chinese individuals diagnosed with stroke (within six months) and healthy controls. All stroke diagnoses (hemorrhagic/ischemic subtypes) were confirmed by computed tomography (CT) and were based on the diagnostic criteria of cerebrovascular diseases in China (16). Cases with transient ischemic attack (TIA) cases explicitly excluded. Additionally, the control group also underwent CT scans to confirm the absence of stroke or other significant neurological conditions. Participants with Parkinson’s disease, Alzheimer’s disease, dementia, or diabetes history were excluded. Given the limited sample size, neither hemorrhagic nor ischemic stroke groups were subclassified within their respective categories, precluding detailed intra-group comparisons. This approach prioritized the identification of systemic retinal vascular differences between stroke subtypes (hemorrhagic vs. ischemic) and healthy controls, rather than delineating heterogeneity within each subtype.
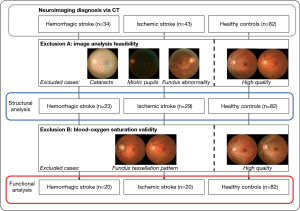
A total of 159 participants were initially enrolled, comprising 34 hemorrhagic stroke patients, 43 ischemic stroke patients, and 82 healthy controls. Retinal imaging was performed using a specialized fundus camera (OT-110M, Hefei Orbis Biotech Ltd., China) capable of simultaneous structural and blood SO2 analysis, the accuracy of which has been rigorously validated through a phantom experiment simulating the fundus (17).
As illustrated in Figure 2, the first exclusion step removed individuals with ocular conditions affecting image quality, including cataracts, miotic pupils, or fundus abnormalities (see representative retinal images in Figure 2, panels labeled “Exclusion A”). This step excluded 25 participants, leaving 134 individuals (23 hemorrhagic strokes, 29 ischemic strokes, 82 healthy controls) eligible for structural analysis of retinal vasculature. The second exclusion step focused on ensuring accurate blood SO2 measurements. Participants with fundus tessellation patterns (illustrated in Figure 2, “Exclusion B” panels) were excluded, resulting in 122 participants (20 hemorrhagic strokes, 20 ischemic strokes, 82 healthy controls) for functional analysis.
Written informed consent was obtained either from the participants or, in cases where participants lacked capacity, from their legal guardians. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by ethics committee of The First Affiliated Hospital of University of Science and Technology of China (No. 2020-KYLS-167). Trial registration details are available at the Chinese Clinical Trial Registry (ID: ChiCTR2000038731). Demographic characteristics of the final cohort are provided in Table 1.
Table 1
| Variables | Demographics | Study groups | ||
|---|---|---|---|---|
| Hemorrhagic stroke | Ischemic stroke | Controls | ||
| Structural analysis | Total | 23 | 29 | 82 |
| Gender (male/female) | 17/6*** | 20/9*** | 35/47 | |
| Age (years) | 49.6±11.0 | 57.2±12.8*** | 50.2±9.2 | |
| Hypertension | 17 (73.9)*** | 22 (75.9)*** | 32 (39.0) | |
| Functional analysis | Total | 20 | 20 | 82 |
| Gender (male/female) | 14/6*** | 14/6** | 35/47 | |
| Age (years) | 49.1±11.3 | 54.8±11.7 | 50.2±9.2 | |
| Hypertension | 15 (75.0)*** | 15 (75.0)*** | 32 (39.0) | |
Data are presented as n, mean ± standard deviation or n (%). **, P<0.05; ***, P<0.01.
Data preprocessing and SO2 level measurement
In our analysis of retinal vascular SO2 levels and structural features, we utilized advanced techniques. Firstly, we employed the U-net model for OD segmentation, followed by the CRU-net model for retinal artery and vein segmentation, known for their accuracy in our dataset (18). To facilitate a comprehensive comparison of SO2 changes between control and stroke groups, we utilized the macular-centered region of interest (MROI) proposed by Dou et al., building upon their detailed analysis methods for SO2 levels (19).
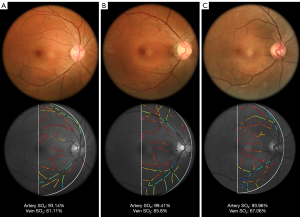
Figure 3 illustrates the original image and the resulting pseudocolor oxygen saturation level map.
Vascular structural features measurement
A subject’s retinal vascular structural features were analyzed using an array of quantitative measures. These measures included vascular density, which is the ratio of the area occupied by the arterial or venous vessels to the entire area of the MROI. Vascular caliber was determined using the revised Knudtson-Parr-Hubbard formula (20), which expresses the caliber of arterial and venous vessels as the central retinal arterial equivalent (CRAE) and central retinal venous equivalent (CRVE) respectively. Vascular tortuosity, which is defined as the integral of the curvature square divided by the total path length, was calculated separately for arterial and venous vessels (21). The FD of the retinal vascular network was determined using the box-counting method on skeletonized arterial and venous vessels (22). The branching angle (BA) at branching points was measured as the angle between two child blood vessels, as defined in the study by Baker et al. (7). The branching coefficient (BC) at branching points was calculated using the widths of the three vessels at the branching point, as defined in the study by Patton et al. (23). The asymmetry factor (AF) at branching points was used to describe the asymmetry between child vessels and was measured by the ratio of the square widths of the two child vessels. The optimal ratio (OR) at vessel bifurcations was also calculated to measure the relationship between parent and daughter vessels.
Statistical analysis
Statistical analyses were conducted to describe participant characteristics using mean values with standard deviations (SDs) for continuous variables and percentages for categorical variables. Categorical variables were compared between groups using the Chi-squared test, as summarized in Table 1. Differences in continuous variables were assessed by first evaluating normality via the Shapiro-Wilk test (full results in Table S1), where a P≥0.05 was considered indicative of normally distributed data. Normally distributed data were analyzed using the independent t-test, while non-normal data were compared via the Kolmogorov-Smirnov test. For all group comparisons (Chi-squared, t-test, and Kolmogorov-Smirnov), statistical significance was defined as P<0.05.
To investigate retinal vascular biomarkers in stroke subtypes, we developed two logistic regression models: Model 1 adjusted for age and sex, and Model 2 further adjusted for hypertension. Odds ratios with 95% confidence intervals (CIs) were calculated per SD increase in retinal parameters using R v4.1.2. Benjamini-Hochberg false discovery rate (FDR) correction was applied to address multiple comparisons, with significance defined as P<0.05 or FDR-adjusted q<0.05.
RF classifier
The RF classifier is an ensemble method that leverages a random selection of training samples and variables to generate a collection of decision trees. These decision trees, collectively forming the RF classifier, are utilized for making predictions. Each tree is constructed by randomly selecting a subset of training samples with replacement (24).
Before proceeding with RF classification, we first conducted RF parameter significance analysis to determine the most influential features in the dataset. The underlying principle of this analysis involves systematically altering the values of each feature and observing the resulting changes in the accuracy or out-of-bag error of the RF (24). This process yields importance scores for each feature, with higher scores indicating a greater impact on the classification outcome and vice versa. By employing this approach, we identified the key parameters significantly contributing to the classification task. Subsequently, features with elevated classification importance were meticulously chosen to constitute a refined input for the RF classifier. This strategic selection not only streamlined the input dimensionality but also bolstered the model's interpretability and classification performance. Ensuring that these selected features played a pivotal role in the accurate discrimination between the stroke and control groups.
To showcase the synergistic impact of merging blood vessel functional SO2 levels with vascular structural features on classifier performance, we conducted ablation experiments. Initially, we utilized isolated blood vessel functional SO2 and vascular structural features as inputs for the classifiers. Subsequently, we introduced a combined input comprising both functional SO2 and vascular structural features to the RF classifier.
To ensure the robustness of the experiment, threefold cross-validation was employed to assess the classifier’s performance at the individual level, and the number of trees in RF was fixed at 100.
Evaluation
Accuracy (Acc), Precision (Pre), Recall (Rec), and F1-score (F1) were employed to assess the effectiveness of classification. Their definitions are as follows:
where TP, TN, FP, and FN represent true positive, true negative, false positive, and false negative, respectively.
Results
Blood SO2 levels in stroke and control groups
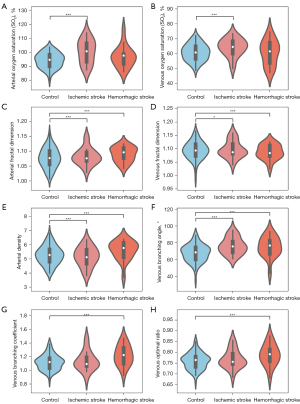
Figure 4A,4B present our findings on retinal blood SO2 level. In the ischemic stroke group, both arterial and venous SO2 levels were significantly elevated compared to the control group (arterial: 97.53%±6.93% vs. 93.01%±5.21%, P<0.01; venous: 65.48%±5.91% vs. 61.99%±5.33%, P<0.01). In contrast, the hemorrhagic stroke group showed no significant difference from controls (arterial: 96.00%±7.55% vs. 93.01%±5.21%, P=0.34; venous: 62.35%±6.82% vs. 61.99%±5.33%, P=0.80).
Structural features of retinal vasculature in stroke and control groups
Figure 4C-4F highlights the differences observed in retinal vascular structural features. The ischemic group exhibited significantly reduced FD in retinal arteries compared to controls (1.064±0.031 vs. 1.089±0.031, P<0.01), while venous FD showed a marginal reduction (1.079±0.034 vs. 1.102±0.032, P=0.06). The ischemic group exhibited significantly reduced arterial density compared to controls (4.62±0.82 vs. 5.49±0.63; P<0.01), alongside a notable decrease in venous density (6.38±1.01 vs. 6.87±0.87; P=0.03). Notably, the venous BA was significantly larger in the ischemic group than in the controls (75.18°±9.28° vs. 67.64°±10.05°, P<0.01), while there was no significant difference in arterial BA.
In the hemorrhagic stroke group, arterial and venous FD were significantly lower than those in the control group (1.063±0.029 vs. 1.089±0.031 for arteries, 1.079±0.034 vs. 1.102±0.032 for veins, both P<0.01). Arterial density was also significantly reduced in the hemorrhagic group (4.90±0.70 vs. 5.49±0.63 in controls, P<0.01). The venous BA was larger in the hemorrhagic group (76.82°±11.23° vs. 67.64°±10.05° in controls, P<0.01). Additionally, as shown in Figure 4G-4H, the venous BC was significantly higher in the hemorrhagic group (1.25±0.15 vs. 1.12±0.11 in controls, P<0.01), with a similar trend observed for the venous OR, while arterial BC and OR showed no significant differences.
Other vascular parameters such as vessel tortuosity, CRAE, CRVE, and AF did not show significant differences between the stroke groups and controls. For a detailed breakdown, refer to Table S2.
Ischemic stroke associations
For ischemic stroke patients compared to control subjects, Table 2 demonstrates that arterial SO2 remained notably associated with stroke incidence, with each SD increase corresponding to a nearly twofold increase in odds (odds ratio =1.916, 95% CI: 1.078–3.598; P=0.032), though its q value (0.144) exceeded the significance threshold after correction for 18 comparisons. Similarly, venous BA showed a strong positive association (odds ratio =2.066, 95% CI: 1.208–3.736; P=0.011), but its q value (0.066) also fell short of significance. In contrast, reduced arterial FD (odds ratio =0.364, 95% CI: 0.177–0.667; P=0.002; q value =0.018) and arterial density (odds ratio = 0.278, 95% CI: 0.122–0.557; P=0.001; q value =0.018) exhibited robust inverse correlations with stroke risk. However, arteriovenous calibers (CRAE/CRVE), tortuosity, and other geometric parameters (AF, OR, BC) showed no significant links. These findings underscore arterial SO2, venous BA, and arterial geometric markers (FD, density) as potential risk indicators, though the diminished significance of arterial SO2 and venous BA after multiplicity correction highlights the need for replication in larger cohorts to confirm their clinical relevance.
Table 2
| Retinal parameters | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | q value | Odds ratio (95% CI) | P | q value | ||
| SO2 per SD increase | |||||||
| Arterial | 1.979 (1.122–3.696) | 0.023* | 0.104 | 1.916 (1.078–3.598) | 0.032* | 0.144 | |
| Venous | 1.947 (1.040–3.980) | 0.049* | 0.147 | 1.894 (1.016–3.842) | 0.056 | 0.186 | |
| FD per SD increase | |||||||
| Arterial | 0.398 (0.201–0.711) | 0.004* | 0.036* | 0.364 (0.177–0.667) | 0.002* | 0.018* | |
| Venous | 0.693 (0.394–1.173) | 0.183 | 0.458 | 0.574 (0.310–1.009) | 0.062 | 0.186 | |
| Density per SD increase | |||||||
| Arterial | 0.282 (0.125–0.559) | 0.001* | 0.018* | 0.278 (0.122–0.557) | 0.001* | 0.018* | |
| Venous | 0.776 (0.432–1.361) | 0.382 | 0.458 | 0.651 (0.343–1.182) | 0.170 | 0.383 | |
| Tortuosity per SD increase | |||||||
| Arterial | 0.786 (0.442–1.309) | 0.375 | 0.458 | 0.845 (0.471–1.442) | 0.550 | 0.660 | |
| Venous | 1.181 (0.707–1.902) | 0.480 | 0.540 | 0.344 (0.122–0.850) | 0.681 | 0.721 | |
| BA per SD increase | |||||||
| Arterial | 0.999 (0.606–1.611) | 0.997 | 0.997 | 0.985 (0.592–1.616) | 0.952 | 0.952 | |
| Venous | 2.116 (1.260–3.762) | 0.007* | 0.042* | 2.066 (1.208–3.736) | 0.011* | 0.066 | |
| BC per SD increase | |||||||
| Arterial | 1.275 (0.809–2.026) | 0.296 | 0.458 | 1.282 (0.803–2.073) | 0.300 | 0.520 | |
| Venous | 1.248 (0.789–1.998) | 0.344 | 0.458 | 1.216 (0.757–1.986) | 0.422 | 0.584 | |
| AF per SD increase | |||||||
| Arterial | 1.053 (0.681–1.674) | 0.818 | 0.866 | 1.121 (0.723–1.778) | 0.609 | 0.685 | |
| Venous | 1.303 (0.820–2.093) | 0.265 | 0.458 | 1.198 (0.742–1.941) | 0.457 | 0.588 | |
| OR per SD increase | |||||||
| Arterial | 1.262 (0.802–2.027) | 0.320 | 0.458 | 1.271 (0.799–2.073) | 0.318 | 0.520 | |
| Venous | 1.303 (0.816–2.117) | 0.293 | 0.458 | 1.249 (0.768–2.074) | 0.375 | 0.563 | |
| CRAE per SD increase | 0.538 (0.291–0.933) | 0.034* | 0.122 | 0.617 (0.323–1.108) | 0.119 | 0.306 | |
| CRVE per SD increase | 0.733 (0.427–1.230) | 0.245 | 0.458 | 0.734 (0.428–1.231) | 0.247 | 0.494 | |
Model 1: adjusted for age and sex; Model 2: Model 1 plus additional adjustment for hypertension. Significant findings after logistic regression (P<0.05; q<0.05) are marked with asterisk. AF, asymmetry factor; BA, branching angle; BC, branching coefficient; CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; Density, vessel density; FD, fractal dimension; FDR, false discovery rate; OR, optimal ratio; SO2, oxygen saturation.
Hemorrhagic stroke associations
In analyses of hemorrhagic stroke risk (Table 3), neither arterial nor venous SO2 levels showed significant associations after blood pressure adjustment (Model 2: arterial SO2, odds ratio =1.499, P=0.135, q value =0.304; venous SO2, odds ratio =1.028, P=0.920, q value =0.920). However, both arterial and venous FD exhibited robust inverse associations with hemorrhagic stroke, surviving multiplicity correction (q values <0.01). Each SD decrease in arterial FD corresponded to a 64% reduction in stroke odds (odds ratio =0.357, 95% CI: 0.173–0.645; P=0.002), while venous FD showed a similar effect (odds ratio =0.360, 95% CI: 0.165–0.684; P=0.002). Arterial density demonstrated a nominally significant negative association (odds ratio =0.320, P=0.019), though its q value (0.057) approached but did not meet the significance threshold; venous density followed a comparable trend (odds ratio =0.464, P=0.031, q value =0.080). Strikingly, venous geometric markers—BA (odds ratio =2.604, 95% CI: 1.403–5.321; P=0.004, q value =0.014), BC (odds ratio =3.214, 95% CI: 1.768–6.600; P<0.001, q value <0.001), and OR (odds ratio =2.895, 95% CI: 1.609–5.800; P=0.001, q value =0.009)—showed strong positive associations with hemorrhagic stroke risk, surviving multiplicity correction. As with ischemic stroke, arteriovenous tortuosity and caliber (CRAE/CRVE) remained nonsignificant after adjustment (q values >0.05). These findings highlight vascular fractal geometry (FD) and venous structural markers (BA, BC, OR) as critical risk indicators for hemorrhagic stroke, independent of blood pressure.
Table 3
| Retinal parameters | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | q value | Odds ratio (95% CI) | P | q value | ||
| SO2 per SD increase | |||||||
| Arterial | 1.520 (0.901–2.648) | 0.121 | 0.727 | 1.499 (0.891–2.624) | 0.135 | 0.304 | |
| Venous | 0.980 (0.571–1.702) | 0.942 | 0.942 | 1.028 (0.600–1.789) | 0.920 | 0.920 | |
| FD per SD increase | |||||||
| Arterial | 0.353 (0.172–0.642) | 0.002* | 0.009* | 0.357 (0.173–0.645) | 0.002* | 0.009* | |
| Venous | 0.368 (0.177–0.668) | 0.003* | 0.009* | 0.360 (0.165–0.684) | 0.002* | 0.009* | |
| Density per SD increase | |||||||
| Arterial | 0.498 (0.271–0.864) | 0.003* | 0.009* | 0.320 (0.144–0.608) | 0.019* | 0.057 | |
| Venous | 0.642 (0.349–1.135) | 0.136 | 0.272 | 0.464 (0.219–0.898) | 0.031* | 0.080 | |
| Tortuosity per SD increase | |||||||
| Arterial | 0.741 (0.392–1.267) | 0.307 | 0.425 | 0.742 (0.384–1.298) | 0.331 | 0.397 | |
| Venous | 1.369 (0.838–2.295) | 0.187 | 0.306 | 1.314 (0.776–2.162) | 0.257 | 0.378 | |
| BA per SD increase | |||||||
| Arterial | 1.405 (0.857–2.335) | 0.176 | 0.306 | 1.435 (0.870–2.428) | 0.161 | 0.322 | |
| Venous | 2.694 (1.478–5.405) | 0.003* | 0.009* | 2.604 (1.403–5.321) | 0.004* | 0.014* | |
| BC per SD increase | |||||||
| Arterial | 1.258 (0.774–2.080) | 0.354 | 0.455 | 1.321 (0.810–2.217) | 0.271 | 0.378 | |
| Venous | 3.220 (1.791–6.507) | <0.001* | <0.001* | 3.214 (1.768–6.600) | <0.001* | <0.001* | |
| AF per SD increase | |||||||
| Arterial | 1.080 (0.659–1.761) | 0.754 | 0.798 | 1.051 (0.643–1.714) | 0.839 | 0.888 | |
| Venous | 0.825 (0.464–1.380) | 0.483 | 0.580 | 0.750 (0.416–1.265) | 0.306 | 0.393 | |
| OR per SD increase | |||||||
| Arterial | 1.302 (0.799–2.190) | 0.298 | 0.425 | 1.357 (0.821–2.310) | 0.235 | 0.378 | |
| Venous | 2.959 (1.651–5.890) | 0.001* | 0.009* | 2.895 (1.609–5.800) | 0.001* | 0.009* | |
| CRAE per SD increase | 0.661 (0.378–1.105) | 0.125 | 0.272 | 0.733 (0.410–1.268) | 0.273 | 0.378 | |
| CRVE per SD increase | 1.112 (0.677–1.844) | 0.674 | 0.758 | 1.083 (0.656–1.804) | 0.755 | 0.849 | |
Model 1: adjusted for age and sex; Model 2: Model 1 plus additional adjustment for hypertension. Significant findings after logistic regression (P<0.05; q<0.05) are marked with asterisk. AF, asymmetry factor; BA, branching angle; BC, branching coefficient; CRAE, central retinal artery equivalent; CRVE, central retinal vein equivalent; Density, vessel density; FD, fractal dimension; FDR, false discovery rate; OR, optimal ratio; SO2, oxygen saturation.
Optimization of RF classifier and performance enhancement through combined retinal vascular feature analysis
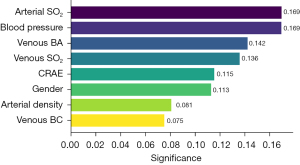
Using retinal vascular functional SO2 and structural features extracted from fundus images, and demographic information as comprehensive input, we applied the RF classifier to distinguish between stroke and control groups. We first conducted a parameter significance analysis using the RF classifier to determine the contribution of each parameter to the classification task. This analysis was essential for refining our parameter set to those with a significance level of 0.05 or higher. As detailed in Figure 5, significant parameters such as arterial SO2, blood pressure, venous BA, venous SO2, CRAE, gender, arterial density, and venous BC were identified. This refined subset of parameters, demonstrating higher classification importance, was then utilized in subsequent classification models.
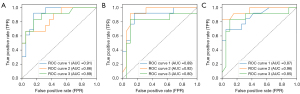
For the quantitative assessment of our classification strategy, we relied on the metrics outlined in Table 4. Qualitatively, the receiver operating characteristic (ROC) curves, as illustrated in Figure 6. When examining the functional features alone, the RF classifier achieved an accuracy of 78.70%±1.81%. Structural features on their own yielded a slightly higher accuracy of 79.81%±2.89%. However, when we combined both functional SO2 and structural features, the classifier’s accuracy notably increase to 81.96%±3.78%.
Table 4
| Metrics | Functional | Structural | Functional & structural |
|---|---|---|---|
| Accuracy, % | 78.70±1.81 | 79.81±2.89 | 81.96±3.78 |
| F1 score, % | 71.38±2.56 | 73.60±5.00 | 74.52±3.52 |
| Precision, % | 75.76±4.29 | 78.01±9.69 | 83.61±3.07 |
| Recall, % | 67.52±1.21 | 73.50±15.47 | 67.95±12.07 |
| AUC, % | 88.61±2.44 | 87.15±5.02 | 89.46±4.57 |
All metrics are expressed as mean ± standard deviation from threefold cross-validation. Combining both vascular functional and structural features, random forest outperforms these two features used alone. ‘Functional’ indicates input including arterial SO2 and venous SO2, while ‘Structural’ signifies input including central retinal arterial equivalent, arterial density, venous branching angle, and venous branching coefficient. AUC, area under the curve; SO2, oxygen saturation.
Discussion
Our study reveals significant differences in retinal blood vessel characteristics among stroke subtypes and the control group. Our novel use of retinal SO2 analysis in stroke patients highlights correlations between reduced arterial vessel density, altered arteriovenous networks, and increased venous BA with stroke risk. Integrating functional (SO2 level) and structural features significantly improved stroke classification accuracy, with a refined RF classifier reaching 81.96%±3.78% accuracy.
Comparison of ischemic and control group
In our comparison between the ischemic and control groups, logistic regression revealed a significant positive correlation between retinal artery SO2 and ischemic stroke, even after adjusting for blood pressure. However, retinal vein SO2 didn’t exhibit a significant relationship after this adjustment. The higher SO2 in retinal vessels within the ischemic group might be due to various factors like thinner retinal nerve fiber layers affecting oxygen uptake or concentration gradients between retinal artery and vein (25,26).
We assessed retinal structural features using global geometric parameters like FD, density, tortuosity, and caliber. Adjusted logistic regression showed significant negative associations between arteriovenous FD and ischemic stroke, aligning with previous findings (27). Reduction in vascular FD may indicate vessel narrowing linked to hypoxia, potentially associated with brain-related pathological processes such as atherosclerosis (28). Moreover, after blood pressure adjustment, arterial vessel density was significantly negatively associated with ischemic stroke. These anomalies in retinal arterial FD and density might correspond to cerebrovascular abnormalities, impacting vascular perfusion and the blood-brain barrier, which ultimately contributes to ischemic damage in the cerebral vasculature.
In analyzing retinal vessel branching patterns, only venous BA in the ischemic group was significantly higher than in controls, possibly due to intracerebral infarction affecting blood supply based on the assumption proposed by Murray (29). Moreover, despite our logistic regression analysis showing no significant correlation between the increase of venous BA and the occurrence of stroke, it is noteworthy that when comparing the mean odds ratio to 1, we observed a trend consistent with another study (27).
Comparison of hemorrhagic and control group
In comparing the hemorrhagic stroke group to controls, logistic regression analysis did not show significant odds ratios for arterial and venous SO2 levels, both before and after adjusting for blood pressure. Limited research on retinal oxygen levels post-hemorrhagic stroke suggests the need to understand the underlying compensatory mechanisms.
Regarding retinal structure analysis, hemorrhagic stroke consistently correlated with reduced arterial and venous FD in logistic regression, even after adjusting for blood pressure. Arterial vessel density consistently displayed a negative association with hemorrhagic stroke, while venous vessel density showed this connection after adjusting for blood pressure. The influence of blood pressure on retinal venous density in hemorrhagic stroke underscores its significance, although specific reasons require further exploration. Importantly, in analyzing retinal vessel CRAE and CRVE in hemorrhagic stroke, logistic regression revealed a significant association between decreased CRAE, increased CRVE, and hemorrhagic stroke. This aligns with existing literature focusing on retinal vessel caliber in hemorrhagic stroke, suggesting potential mechanisms like retinal hypoxia and endothelial dysfunction contributing to retinal venular dilation (13,15,30).
In assessing branching patterns, logistic regression revealed a significant positive correlation between venous BA, BC, OR, and hemorrhagic stroke. These venous irregularities imply an influence of hemorrhagic stroke on retinal venous branching patterns. Adhering to Murray’s principle of minimum work, the human circulatory system’s branching adheres to optimal design principles (31). Deviations from this in retinal vasculature branching might lead to microcirculatory impairment, increased shear stress, and pathogenesis. These anomalies may signify underlying vascular damage.
Hemorrhagic vs. ischemic stroke: contrasting vascular features with control groups
Our exploratory analyses comparing retinal vascular patterns between hemorrhagic and ischemic stroke subtypes revealed preliminary insights into shared and divergent vascular pathophysiology. While both subtypes exhibited overlapping associations with reduced arterial vessel density, diminished arteriovenous FD, and increased venous BA—suggesting common pathways of microvascular dysfunction—distinct trends emerged in parameters such as arteriovenous vessel density and arterial BA. For instance, venous BA showed stronger associations with hemorrhagic stroke (odds ratio =2.604, q value =0.014) compared to ischemic stroke (odds ratio =2.066, q value =0.066), while CRVE trends diverged between subtypes.
However, these comparisons should be interpreted with caution due to important limitations. The modest sample size of stroke subgroups limits the robustness of observed associations and reduces the ability to detect subtle differences between subtypes. Additionally, while hemorrhagic and ischemic strokes were analyzed as unified clinical categories in this study, their inherent pathophysiological heterogeneity may introduce unmeasured variability in retinal vascular signatures. We emphasize that these analyses are exploratory, designed to generate hypotheses rather than establish definitive mechanistic distinctions. Future studies with larger cohorts and granular phenotyping of stroke mechanisms are needed to validate and extend these preliminary findings.
Insights into stroke classification based on retinal vascular biomarkers
Our study harnessed the RF classifier to distinguish between stroke and control groups based on retinal functional SO2 levels and structural features extracted from fundus images. A parameter significance analysis identified key contributors to classification as illustrated in Figure 5. When considering these crucial functional, structural, and demographic features as input for the RF classifier, it achieved accuracies of 78.70%±1.81% with functional parameters alone, 79.81%±2.89% with structural features alone, and notably improved to 81.96%±3.78% when both functional SO2 and structural features were combined. The ROC curves showed superior classification ability with combined features, signifying the value of integrating diverse data types for more accurate predictions, crucial in medical diagnostics and stroke risk assessment, as illustrated in Figure 6. Rather than supporting immediate clinical implementation, our findings provide preliminary evidence that comprehensive retinal feature sets may merit further investigation as adjunctive biomarkers in stroke risk assessment.
Delving into vascular structural features, we identified their complementary role, adding sophistication to our stroke classification. Remarkably, significant differences in BA, a structural parameter, between stroke groups and the normal control group played a pivotal role in enhancing the overall classification performance. On the contrary, parameters such as CRAE did not exhibit significant differences between the stroke and control groups, yet their inclusion paradoxically contributed to improved classification performance.
This study not only contributes to the field by achieving precise stroke classification but also underscores the potential of blood vessel features as reliable biomarkers for cerebrovascular health. This first demonstration of combined structural-SO2 analysis in stroke reveals previously unexamined vascular relationships, though their clinical significance requires confirmation through longitudinal multi-center studies.
The distinct technical emphases of optical coherence tomography angiography (OCT-A) and the OT-110M system underscore their complementary yet non-interchangeable roles in retinal vascular assessment. OCT-A excels in high-resolution cross-sectional imaging of specific retinal microvascular layers, such as the superficial and deep capillary plexuses, within a limited scan area (typically 3 mm × 3 mm to 12 mm × 12 mm). While this capability enables detailed structural evaluation of localized vascular networks, it inherently restricts the detection of large-scale vascular patterns relevant to systemic conditions like stroke. In contrast, the OT-110M prioritizes functional and structural assessment by quantifying retinal vessel SO2 through spectral analysis of hemoglobin absorption—a biomarker inaccessible to OCT-A’s perfusion-based imaging. This functional divergence, combined with the OT-110M’s capacity for broader vascular coverage, renders OCT-A unsuitable as a gold standard for comparative analysis in this study. Rather, the two modalities address fundamentally different aspects of vascular pathophysiology: OCT-A captures microarchitectural details, whereas the OT-110M provides hemodynamic insights at a spatially comprehensive scale. Nevertheless, future research may benefit from integrating the strengths of both OCT-A and the OT-110M to develop a more holistic approach to retinal vascular assessment, potentially enhancing our understanding of cerebrovascular diseases.
Limitations
There are several limitations in this study. First, the generalizability of our findings may be constrained by the moderate sample size and the regional/ethnic homogeneity of the cohort. Second, the absence of longitudinal data limits our ability to track retinal biomarker dynamics in stroke progression or recovery. Most critically, the current sample size precluded subtype-specific analyses (e.g., cardioembolic vs. small vessel in ischemic stroke). Further subdivision of the dataset would have compromised statistical power. Future studies with expanded cohorts will prioritize granular subtyping to disentangle pathophysiology-specific retinal signatures.
Conclusions
This study identifies distinct retinal vascular features in stroke subtypes, demonstrating that integrating functional (SO2) and structural features significantly enhances classification accuracy. Notably, while hemorrhagic and ischemic strokes share some retinal vascular markers, subtype-specific divergence in branching patterns reveals distinct pathological mechanisms. These results indicate that retinal biomarkers could offer potential value in stratifying stroke risk. Further research could yield insights into other cerebrovascular disorders.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2712/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2712/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by ethics committee of The First Affiliated Hospital of University of Science and Technology of China (No. 2020-KYLS-167). The study was registered in the Chinese Clinical Trial Registry (URL: https://www.chictr.org.cn/index; Unique identifier: ChiCTR2000038731). Written informed consent was obtained either from the participants or, in cases where participants lacked capacity, from their legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Tu WJ, Wang LDSpecial Writing Group of China Stroke Surveillance Report. China stroke surveillance report 2021. Mil Med Res 2023;10:33. [PubMed]
- Mead GE, Sposato LA, Sampaio Silva G, Yperzeele L, Wu S, Kutlubaev M, Cheyne J, Wahab K, Urrutia VC, Sharma VK, Sylaja PN, Hill K, Steiner T, Liebeskind DS, Rabinstein AA. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int J Stroke 2023;18:499-531. [Crossref] [PubMed]
- Ye S, Pan H, Li W, Wang J, Zhang H. Development and validation of a clinical nomogram for differentiating hemorrhagic and ischemic stroke prehospital. BMC Neurol 2023;23:95. [Crossref] [PubMed]
- Pu L, Wang L, Zhang R, Zhao T, Jiang Y, Han L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke 2023;54:1330-9. [Crossref] [PubMed]
- Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [Crossref] [PubMed]
- Baker ML, Hand PJ, Wang JJ, Wong TY. Retinal signs and stroke: revisiting the link between the eye and brain. Stroke 2008;39:1371-9. [Crossref] [PubMed]
- Bakhoum CY, Madala S, Long CK, Adabifirouzjaei F, Freeman WR, Goldbaum MH, DeMaria AN, Bakhoum MF. Retinal vein occlusion is associated with stroke independent of underlying cardiovascular disease. Eye (Lond) 2023;37:764-7. [Crossref] [PubMed]
- Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res 2017;57:89-107. [Crossref] [PubMed]
- Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol 2001;46:59-80. [Crossref] [PubMed]
- London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 2013;9:44-53. [Crossref] [PubMed]
- Einarsdottir AB, Hardarson SH, Kristjansdottir JV, Bragason DT, Snaedal J, Stefánsson E. Retinal oximetry imaging in Alzheimer's disease. J Alzheimers Dis 2016;49:79-83. [Crossref] [PubMed]
- Menon S, Menon G. Role of retinal vessel caliber assessment in predicting hemorrhagic stroke—emerging concepts. J Stroke Med 2022;5:7-13. [Crossref]
- Baker ML, Hand PJ, Liew G, Wong TY, Rochtchina E, Mitchell P, Lindley RI, Hankey GJ, Wang JJMulti-Centre Retinal Stroke Study Group. Retinal microvascular signs may provide clues to the underlying vasculopathy in patients with deep intracerebral hemorrhage. Stroke 2010;41:618-23. [Crossref] [PubMed]
- Wieberdink RG, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, Breteler MM. Retinal vascular calibers and the risk of intracerebral hemorrhage and cerebral infarction: the Rotterdam Study. Stroke 2010;41:2757-61. [Crossref] [PubMed]
- Zeng J, Liu M, Cui L, et al. Diagnostic criteria of cerebrovascular diseases in China (version 2019). Chinese Journal of Neurology 2019;52:710-5.
- Chen H, Liu G, Zhang S, Shen S, Luo Y, Li J, Roberts CJ, Sun M, Xu RX. Fundus-simulating phantom for calibration of retinal vessel oximetry devices. Appl Opt 2019;58:3877-85. [Crossref] [PubMed]
- Zhang S, Zheng R, Luo Y, Wang X, Mao J, Roberts CJ, et al. Simultaneous arteriole and venule segmentation of dual-modal fundus images using a multi-task cascade network. IEEE Access 2019;7:57561-73.
- Dou P, Zhang Y, Zheng R, Ye Y, Mao J, Liu L, et al. Retinal imaging and analysis using machine learning with information fusion of the functional and structural features based on a dual-modal fundus camera. J Mech Med Biol 2021;21:2150030. [Crossref]
- Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003;27:143-9. [Crossref] [PubMed]
- Hart WE, Goldbaum M, Côté B, Kube P, Nelson MR. Measurement and classification of retinal vascular tortuosity. Int J Med Inform 1999;53:239-52. [Crossref] [PubMed]
- Liew G, Wang JJ, Cheung N, Zhang YP, Hsu W, Lee ML, Mitchell P, Tikellis G, Taylor B, Wong TY. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology 2008;115:1951-6. [Crossref] [PubMed]
- Patton N, Aslam TM, MacGillivray T, Deary IJ, Dhillon B, Eikelboom RH, Yogesan K, Constable IJ. Retinal image analysis: concepts, applications and potential. Prog Retin Eye Res 2006;25:99-127. [Crossref] [PubMed]
- Breiman L. Random forests. Machine Learning 2001;45:5-32. [Crossref]
- Little P. Penumbra Oxygen Metabolism and Acute Neuroinflammation in Ischemic Stroke: MRI and PET Imaging of a M2 Occlusion Model in Rat. Sweden: Karolinska Institutet; 2020.
- Hardarson S, Olafsdottir O, Karlsson R, Beach J, Eysteinsson T, Benediktsson J, et al. Retinal vessel oxygen saturation in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 2009;50:1328.
- Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, Wong TY, Ikram MK. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke 2013;44:2121-7. [Crossref] [PubMed]
- Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res 2011;1389:50-60. [Crossref] [PubMed]
- Murray CD. The physiological principle of minimum work applied to the angle of branching of arteries. J Gen Physiol 1926;9:835-41. [Crossref] [PubMed]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 2009;170:1323-32. [Crossref] [PubMed]
- Murray CD. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc Natl Acad Sci U S A 1926;12:207-14. [Crossref] [PubMed]


