
Metabolic abnormalities in the insula of patients with interictal migraine without aura: a prospective cross-sectional study
Introduction
Migraine is a prevalent and disabling neurological disorder that is characterized by recurrent headache attacks accompanied by nausea, phonophobia, and photophobia (1). As one of the five leading causes of years lived with disability worldwide, migraine also significantly increases the burden of disease on individuals and society (2). Researchers have proposed various hypotheses about the pathophysiological mechanisms of migraine, such as neuroinflammation (3), cortical spreading depression (4), subcortical dysfunction (5), and abnormal cortical excitability (6); however, these mechanisms are complex and remain unclear. In addition to recurrent headache attacks, migraine is also accompanied by a wide variety of affective, sensory, and cognitive symptoms, which involve the participation of multiple brain regions (7). As an important cortical hub for affection, sensation, autonomic function, and awareness, the insula is responsible for many aspects of migraine, and is considered an “active center” for migraine attacks (8).
Proton magnetic resonance spectroscopy (1H-MRS) is a non-invasive examination method often used to explore brain neurometabolic mechanisms in vivo (9). In recent years, many 1H-MRS studies have investigated and reported on brain neurometabolic abnormalities in migraineurs (6,10-17). These results mainly include increased (6,10) or decreased (11,16) glutamate (Glu) and glutamine (Gln) complex (Glx) or Glu, increased (12) or decreased (13) gamma-aminobutyric acid (GABA), decreased N-acetyl aspartate (NAA) (11,14,17,18) or NAA/creatine and phosphocreatine (Cr) (15,19), increased (11) or decreased (14) myo-inositol (Ins), and increased (14) or decreased (20) choline-containing compound (Cho) or Cho/Cr. However, most of these studies focused on cerebral regions such as the occipital lobe, thalamus, and anterior cingulate cortex (6,10-17). Indeed, only one study mentioned the left insula, but it found no significant metabolite differences between migraineurs and healthy controls (HCs), and the authors did not distinguish between types of migraine (21).
In clinical settings, migraine is classified as migraine with aura (MWA) and migraine without aura (MWoA) based on the presence or absence of an aura at the onset of the headache attack (1). MWoA is the most prevalent type of migraine and accounts for approximately 80% of all migraine headaches (22). In recent years, an increasing number of neuroimaging studies have shown that the insula is an important brain region in MWoA patients (23-25). For example, a structural study revealed that compared to HCs, the mean cortical thickness of the insula of MWoA patients was significantly decreased (23). Decreased regional homogeneity values have mainly been found in the insula of MWoA patients (24). Abnormal functional connectivity has also been found between the insular subregions of MWoA patients (25). In addition, a whole-brain assessment study of migraine patients showed that headache frequency affects brain cell structure and density (26). However, to date, no studies have examined the neurometabolic mechanisms in the insula of MWoA patients, and whether headache frequency affects these neurometabolites.
We speculated that there may be abnormal neurometabolic alterations in the insula during the interictal period of MWoA patients, and these neurometabolites may be affected by headache frequency. In this study, we aimed to explore possible neurometabolic abnormalities in the insula of MWoA patients during the interictal period via 1H-MRS, to assess the metabolite differences between high frequency (HF) and low frequency (LF) headache patients, and to determine the main clinical correlations of these metabolites. Our findings may provide further insights into neurometabolic mechanisms in the insula of MWoA patients. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2553/rc).
Methods
Participants
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the local Ethics Committee of the West China Hospital of Sichuan University (No. 2022301), and informed consent was obtained from all individual participants.
Between August 2021 and November 2022, 22 MWoA patients from the Neurology Department of West China Hospital were consecutively included in this prospective cross-sectional study by professional neurologists. In addition, 22 age-, sex-, and education-matched HCs were also included in the study. The diagnosis of MWoA was based on the criteria from the International Classification of Headache Disorders, 3rd edition (1). HCs were included in the study if they met the following inclusion criteria: had no personal history of migraine or any other type of headache, and had no chronic medical condition. Participants (both HCs and MWoA patients) were excluded from the study if they met any of the following exclusion criteria: (I) were aged <18 or >65 years; (II) had abnormal brain magnetic resonance imaging (MRI) findings; (III) had other types of headaches or neuropsychiatric disorders; (IV) had poor spectral qualities; and/or (V) had MRI contraindications. All the participants were right-handed. The MWoA patients were scanned during the interictal period of headache and stopped taking any medications for 72 hours before the scans.
The age, gender, and educational level of each participant were collected. Those with MWoA completed an additional clinical questionnaire that included questions about illness duration, headache frequency, painful side, family history, drug history, as well as three clinical scales: Hamilton Depression Rating Scale (HAMD), The headache-attributed lost time-90 days (HALT-90) scale, and visual analog scale (VAS). The HAMD scale is commonly used to assess depression in clinical settings (27). The HALT-90 scale is used to assess the disease burden: the negative effects of headaches on daily activities, including fatigue, cognitive function, and mental abnormality (28,29). The VAS is a 10-point scale used to assess the severity of pain (on which 0 indicates no pain and 10 indicates the worst possible pain) (30).
Image acquisition
The image scans of all participants were performed on a magnetic resonance scanner from Siemens Healthcare (Siemens Tim Trio 3.0-T, Erlangen, Germany) equipped with an 8-channel phased array head coil. Throughout the imaging interval, foam padding and mutated earplugs were used to minimize head movement and eliminate noise, respectively. Magnetization-prepared rapid gradient echo sequences were used to acquire high resolution sagittal T1-weighted imaging (T1WI) images, and the technical specifications were set as follows: repetition time (TR)/echo time (TE): 2,250/2.6 ms; flip angle: 9 degrees, slice thickness: 1 mm, field of view: 256×256 mm2, matrix size: 256×256, and total slices: 192. The axial and coronal T1WI images were subsequently reconstructed from the sagittal T1WI images to facilitate three-dimensional pinpointing of the spectral volume of interest (VOI). The MRS data acquisition was performed using the point resolved spectroscopy (PRESS) protocol, and the following settings were used: TR/TE: 2,000/30 ms; bandwidth: 1,200 Hertz; and average acquisitions: 128.
For each participant, the VOI was carefully placed in the left insula, under the supervision of a professional radiologist (Q.Y.) (the location of the VOI is shown in Figure 1). The fixed size of the VOI was 38×10×15 mm3; that is, (AP × RL × HF) where AP represents anterior-posterior, RL represents right-left, and HF represents head-foot. Pre-saturated bands were placed around the VOI to prevent outside signal interference. Spectral data both with and without water suppression were obtained. To accomplish water suppression, we used three chemical shift selection pulses before implementing the PRESS module. The signal from water within the tissue served as an intrinsic standard for rectifying eddy current distortions and the definitive quantification of metabolites. Fastmap shimming was used to achieve smaller line widths and higher signal-to-noise ratios (SNRs). Two researchers (L.W. and H.P.) independently inspected the images to ensure that all the participants were MRI negative. If any uncertainties arose, T2-weighted imaging (T2WI) and fluid attenuated inversion recovery (FLAIR) sequences were used to ensure that all the participants were MRI negative.
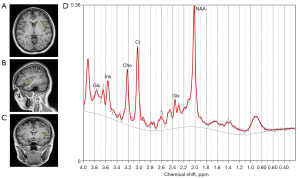
MRS data processing
The raw and unprocessed MRS data were exported and processed using the linear combination model (LCModel) software (version 6.3-1H; Provencher SW; http://s-provencher.com/lcmodel.shtml). Equipped with automated baseline and phase correction, the LCModel uses an internal reference, the unsuppressed tissue water signal, to produce absolute metabolic concentrations (31). The unit of absolute metabolites was mole per kilogram (mmol/kg) wet weight. The advanced normalization tool (ANT, https://stnava.github.io/ANTs/) was used to automatically segment all participants’ T1WI images into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Finally, to eliminate volume effects of the CSF in the VOI (the left insula), the total volume and the CSF volume in the VOI were determined and extracted based on their locations by overlaying the segmented whole-brain T1WI images on the VOI using the Gannet software (https://www.gabamrs.com/) in Matlab R 2018b. The following formula was then used to correct the raw metabolite levels in the VOI (32): , where Craw represents the uncorrected estimate of the metabolite concentration, and Vtotal and VCSF represent the total volume and CSF volume in the VOI, respectively.
Statistical analyses
The statistical analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). The figures were generated using GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA, USA). The Shapiro-Wilk test was used to test the normality of the continuous variables. Group differences in the demographic data between the HCs and MWoA patients were assessed using an independent sample t-test for both age and education level. While for sex, the Chi-squared test was used. Analysis of covariance (ANCOVA) was used to test for differences in metabolites between the HCs and MWoA patients, with age, sex, and years of education as covariates. A post-hoc analysis was performed after the ANCOVA for the five metabolites between the MWoA patients and HCs using the Bonferroni correction. The critical value of the Bonferroni correction P’ was set at a two-sided 0.05 level.
To explore the effect of headache frequency, the metabolic differences between those with LF and HF headaches were tested by ANCOVA with age, sex, years of education, disease duration, VAS score, HALT-90 score, and HAMD score as the covariates. “LF headache” was defined as a headache once within several months or less than once per month on average. “HF headache” was defined as headaches daily or weekly, or at least once or more per month on average. Pearson or Spearman correlation analyses were performed of the metabolites and the main clinical data of the MWoA patients, including disease duration, headache frequency, VAS score, HALT-90 score, and HAMD score. The significance level of the P value was set at a two-sided 0.05 level.
The sample size for the ANCOVA between the HCs and MWoA patients in this study was calculated using the G-Power program (version 3.1.9.2; a free statistical program available at http://www.gpower.hhu.de/). The specific parameter settings were as follows: effect size f=0.5, α error probability =0.05, power (1 − β error probability) =0.8, numerator degrees of freedom =1, number of groups =2, and number of covariates =3. The results showed that a minimum sample size of 34 would be enough to achieve 80% power at the 0.05 significance level. However, considering that some data of participants might need to be excluded because of poor spectral qualities, we increased the sample size as much as possible from the minimum sample size.
Results
Ultimately, 22 MWoA patients (2 males and 20 females; median age, 29 years; range, 19–48 years) and 22 HCs (2 males and 20 females; median age, 32 years; range, 18–44 years) were included in this study. Table 1 sets out the detailed clinical and demographic data of the participants; no significant differences were found between the groups in terms of age, sex, or education years. The mean disease duration was 5.75±3.30 years, and the mean headache frequency was 3.52±6.70 times per month. The mean VAS, HALT-90, and HAMD scores were 6.09, 15.09, and 6.50, respectively. Of these 22 MWoA patients, 15 had taken medications, mainly including analgesic drugs. Only one patient (named case 15) received medication for antimigraine prophylactic treatment. The detailed medication histories of the MWoA patients are shown in Table 2. To eliminate the possible effects of these medications on brain metabolic concentrations, all the MWoA patients were asked not to take any medication for 72 hours before the scans.
Table 1
| Parameters | HCs group (n=22) | MWoA group (n=22) | t/χ2 value | P |
|---|---|---|---|---|
| Age (years) | 32.09±10.97 | 29.50±7.14 | t=−0.929 | 0.358 |
| Sex (male/female) | 2/20 | 2/20 | χ2=0.000 | >0.99 |
| Education years | 17.05±3.63 | 15.68±3.15 | t=−1.330 | 0.191 |
| Family history (yes/no) | – | 9/13 | – | – |
| Medication history (treated/untreated) | – | 15/7 | – | – |
| Headache side (bilateral/unilateral) | – | 8/14 | – | – |
| Illness duration (years) | – | 5.75±3.30 | – | – |
| Headache frequency (times/month) | – | 3.52±6.70 | – | – |
| HF headaches | – | 15 | – | – |
| LF headaches | – | 7 | – | – |
| VAS score | – | 6.09±1.19 | – | – |
| HALT-90 score | – | 15.09±20.67 | – | – |
| HAMD score | – | 6.50±4.59 | – | – |
Data are presented as the mean ± SD or number. HCs, healthy controls; HALT-90, headache-attributed lost time-90 days; HAMD, Hamilton Depression Rating Scale; HF, high frequency; LF, low frequency; MWoA, migraine without aura; SD, standard deviation; VAS, visual analog scale.
Table 2
| Case | Age (years) | Sex (M/F) | Education years | Medication history | Medication names |
|---|---|---|---|---|---|
| 1 | 23 | F | 19 | Yes | Naproxen |
| 2 | 36 | F | 12 | Yes | Compound paracetamol tablets |
| 3 | 19 | M | 12 | No | – |
| 4 | 30 | F | 21 | Yes | Ibuprofen |
| 5 | 48 | F | 9 | No | – |
| 6 | 41 | F | 9 | No | – |
| 7 | 26 | F | 16 | Yes | Ibuprofen |
| 8 | 35 | F | 16 | No | – |
| 9 | 37 | F | 16 | Yes | Ibuprofen |
| 10 | 22 | F | 16 | Yes | Ibuprofen |
| 11 | 27 | F | 16 | Yes | Ibuprofen |
| 12 | 26 | F | 16 | Yes | Ibuprofen |
| 13 | 27 | F | 19 | Yes | Benorilate |
| 14 | 32 | F | 16 | Yes | Paracetamol, caffeine and aspirin powder; compound paracetamol tablets; ibuprofen |
| 15 | 37 | F | 16 | Yes | Flunarizine hydrochloride capsules |
| 16 | 30 | F | 12 | Yes | Rizatriptan benzoate tablets |
| 17 | 22 | F | 15 | Yes | Naproxen |
| 18 | 24 | M | 18 | No | – |
| 19 | 32 | F | 16 | No | – |
| 20 | 24 | F | 18 | Yes | Ibuprofen |
| 21 | 24 | F | 18 | No | – |
| 22 | 27 | F | 19 | Yes | Ibuprofen |
“Flunarizine hydrochloride capsules” is a drug for migraine prophylactic treatment, and the rest are analgetic drugs. F, female; M, male; MWoA, migraine without aura.
After processing the MRS data, the metabolites that met the spectral quality criteria were included in the next statistical analysis. Based on previous studies (33,34), the specific criteria for spectral quality are a SNR of not less than 10, a full width at half maximum of not more than 0.08 ppm, and a Cramer Rao lower bound of not more than 15%. We excluded the metabolites of two HF headache patients, who did not meet the inclusion criteria due to poor spectral quality. Thus, ultimately the metabolites of 20 MWoA patients (7 LF and 13 HF headache patients) and 22 HCs were included in the ANCOVA. A detailed flowchart of the inclusion process for the MWoA patients in this study is shown in Figure 2.

Using the G-Power program, a post-hoc power analysis based on the final sample size was performed for the ANCOVA of the metabolites between the HCs and MWoA patients. The specific parameter settings were as follows: effect size f=0.5, α error probability =0.05, total sample size (1 − β error probability) =42, numerator degrees of freedom =1, number of groups =2, and number of covariates =3. The result showed that the power value was 0.884. Using the G-Power program, a power analysis based on the statistical sample size was also performed for the ANCOVA of the metabolites between the HF and LF headache patients. The specific parameter settings were as follows: effect size f=0.5, α error probability =0.05, total sample size (1 − β error probability) =20, numerator degrees of freedom =1, number of groups =2, and number of covariates =7. The result showed that the power value was 0.532.
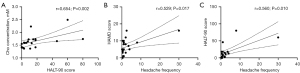
Differences in the metabolite concentrations in the insula between the different groups are summarized in Table 3 and Figures 3,4. The results revealed that the Ins, NAA, and Glx levels in the insula of the MWoA patients during the interictal period of headache were significantly lower than those of the HCs (P=0.017, 0.015, and 0.020, respectively). However, none of these results passed the Bonferroni correction (all P’>0.05). The Cr concentration (P=0.037) was obviously higher in the HF headache patients than the LF headache patients. The results of the correlation analyses between metabolites and the main clinical data of the MWoA patients are shown in Table 4 and Figure 5. The results revealed that headache frequency was positively correlated with the HALT-90 (r=0.560, P=0.010) and HAMD (r=0.529, P=0.017) scores. Moreover, the HALT-90 score was positively correlated with the Cho concentration (r=0.654, P=0.002). No other correlations reached statistical significance (P<0.05) between the metabolites and clinical data of the patients with MWoA.
Table 3
| Metabolites | HCs group (n=22) | MWoA group (n=20) | F† | P† | P’† | LF (n=7) | HF (n=13) | F‡ | P‡ |
|---|---|---|---|---|---|---|---|---|---|
| Ins | 6.21±1.14 | 5.16±1.14 | 6.252 | 0.017* | 0.085 | 5.39±1.18 | 5.21±1.19 | 3.341 | 0.095 |
| NAA | 6.59±1.12 | 5.70±1.23 | 6.533 | 0.015* | 0.075 | 5.30±1.76 | 5.97±0.82 | 0.743 | 0.407 |
| Cho | 1.76±0.26 | 1.63±0.28 | 0.692 | 0.411 | >0.99 | 1.57±0.30 | 1.69±0.27 | 1.583 | 0.234 |
| Cr | 6.45±0.77 | 6.06±0.72 | 1.588 | 0.215 | >0.99 | 6.01±0.91 | 6.16±0.67 | 5.757 | 0.037* |
| Glx | 14.36±2.17 | 12.88±1.63 | 5.864 | 0.020* | >0.99 | 13.14±2.12 | 12.74±1.39 | 3.653 | 0.085 |
Data are presented as the mean ± SD. The ANCOVA results revealed that during the interictal period of headache, the MWoA patients had significantly lower Ins, NAA, and Glx concentrations in the insula than the HCs, and the HF headache patients had significantly higher Cr concentration in the insula than the LF headache patients. The unit for each metabolite is mmol/kg wet weight. P indicates P value after the ANCOVA. P’ indicates P value after the Bonferroni correction. †, HC group vs. MWoA group. ‡, LF vs. HF. *, indicates P or P’<0.05. ANCOVA, analysis of covariance; Cho, choline-containing compound; Cr, creatine and phosphocreatine; Glx, glutamate and glutamine; HCs, healthy controls; HF, high frequency; Ins, myo-inositol; LF, low frequency; MWoA, migraine without aura; NAA, N-acetyl aspartate; SD, standard deviation.
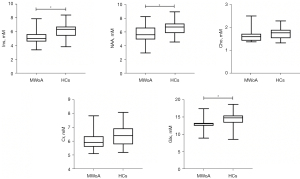
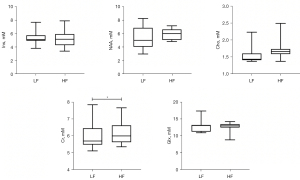
Table 4
| Metabolite | Illness duration | Headache frequency | VAS score | HALT-90 score | HAMD score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |||||
| Ins | 0.083 | 0.729 | 0.138 | 0.561 | 0.156 | 0.511 | 0.292 | 0.212 | 0.037 | 0.878 | ||||
| NAA | 0.167 | 0.482 | 0.297 | 0.204 | 0.026 | 0.915 | 0.155 | 0.515 | 0.417 | 0.068 | ||||
| Cho | 0.136 | 0.567 | 0.377 | 0.101 | 0.299 | 0.200 | 0.654 | 0.002* | 0.276 | 0.239 | ||||
| Cr | 0.198 | 0.402 | 0.272 | 0.246 | 0.251 | 0.286 | 0.433 | 0.056 | 0.436 | 0.054 | ||||
| Glx | −0.065 | 0.785 | 0.112 | 0.637 | −0.019 | 0.937 | 0.054 | 0.820 | −0.029 | 0.904 | ||||
| Illness duration | – | – | −0.089 | 0.710 | −0.157 | 0.509 | −0.009 | 0.970 | 0.099 | 0.677 | ||||
| Headache frequency | −0.089 | 0.710 | – | – | −0.137 | 0.564 | 0.560 | 0.010* | 0.529 | 0.017* | ||||
| VAS score | −0.157 | 0.509 | −0.137 | 0.564 | – | – | 0.190 | 0.422 | 0.001 | 0.997 | ||||
| HALT-90 score | −0.009 | 0.970 | 0.560 | 0.010* | 0.190 | 0.422 | – | – | 0.392 | 0.088 | ||||
| HAMD score | 0.099 | 0.677 | 0.529 | 0.017* | 0.001 | 0.997 | 0.392 | 0.088 | – | – | ||||
The correlation analysis results showed that the headache frequency of the MWoA patients was positively related to the HALT-90 and HAMD scores, and the HALT-90 score was positively correlated with Cho concentration. *, indicates P<0.05. Cho, choline-containing compound; Cr, creatine and phosphocreatine; Glx, glutamate and glutamine; HALT-90, headache-attributed lost time-90 days; HAMD, Hamilton Depression Rating Scale; Ins, myo-inositol; MWoA, migraine without aura; NAA, N-acetyl aspartate; VAS, visual analog scale.
Discussion
This study examined neurometabolic abnormalities in the insula between MWoA patients and HCs, and metabolic differences between LF and HF headache patients. The results showed that the concentrations of Ins, NAA, and Glx in the insula of the MWoA patients during the interictal period were significantly lower than those of the HCs. However, these metabolites must be interpreted with caution because they did not pass the Bonferroni correction. In addition, the HF headache patients had higher Cr levels than the LF headache patients. To the best of our knowledge, this study was the first to report on changes in the neurometabolic levels in the insula of MWoA patients, and neurometabolic differences in HF and LF headache patients during the interictal period. Our findings may extend understanding of the neurometabolic mechanisms in the insula of MWoA patients during the interictal period.
Niddam et al. found that compared with HCs, Ins was significantly reduced in the anterior cingulate cortex of patients with chronic migraines (14). Similarly, in our study, we found that Ins was significantly lower in the insula of the MWoA patients than the HCs. Ins is mainly present in glial cells and is considered a glial cell biomarker, which primarily provides information on the number and function of glial cells (14,17). Increased Ins usually represents glial proliferation and is always seen in lesions with glioma; conversely, reduced Ins reflects glial cell dysfunction or number reduction (14,17). Glial cell dysfunction or loss may affect the clearance of intrasynaptic neurotransmitters, neuronal synaptic plasticity, and synaptic generation, which can lead to migraine chronicity (14). Further, Ins plays a broad and important role in intracellular osmoregulation (35). An animal study found that when the brain is stimulated by abnormal signals, there is a brief imbalance in intracellular osmotic pressure, at which point Ins concentration decreases rapidly to compensate for this imbalance (36). However, the present study examined the inter-disease period of MWoA patients when there was no headache stimulus in their brains. Thus, the reduced Ins of the MWoA patients may not be due to a cellular osmotic imbalance but may be due to glial cell dysfunction or loss.
Previous studies reported that patients with migraine, including MWoA patients, had reduced NAA (11,14,17) or NAA/Cr (15) ratios. For example, studies have reported interictally reduced NAA concentrations in the thalamus (17) and occipital lobes (18) in MWoA patients relative to HCs. In our study, NAA was also significantly lower in the interictal insula of the MWoA patients than the HCs. NAA is a neuronal marker that mainly provides information on the quantity of neurons (32). A reduced NAA level suggests a decrease in the number of neurons (32). Further, NAA is also thought to reflect the function of neuronal mitochondria (37). It is mainly produced in neuronal mitochondria (37) and can enhance mitochondrial function by participating in adenosine triphosphate metabolism (38). Therefore, the decreased NAA level in this study also likely indicated neuronal mitochondrial dysfunction. In addition, studies have also shown that abnormal energy metabolism caused by neuronal mitochondrial dysfunction may be a trigger for migraine attacks (17,39).
Cr is a marker of mitochondrial energy metabolism that can decrease in hypermetabolic states and increase in hypometabolic states (40,41). In this study, there was no obvious change in the Cr of the MWoA patients compared with that of the HCs. This is consistent with previous research that revealed no significant change in Cr concentration in the visual cortex during the interictal period in MWoA patients compared with HCs (6). This may be because the present study was performed during the inter-disease phase, when the energy requirement is not large and the mildly impaired mitochondrial function can meet it, which is the same principle as that for patients with mild coronary artery disease, who have a normal blood supply to the myocardium in the resting state. Interestingly, we found that the HF headache patients had a significantly higher Cr level than the LF headache patients, indicating that hypometabolism (40,41) may be present in their brains during the interictal period. This may be due to more serious mitochondrial dysfunction in their brains caused by more frequent headaches, resulting in the blockage of Cr conversion and energy release process (17,39), which in turn can become the inducing factor for headaches (42). Of course, this hypothesis needs to be confirmed in future studies.
Glu is an important excitatory neurotransmitter in the brain. Its conversion with Gln between neurons and astrocytes is crucial for controlling excitation transmission (43). This study measured Glx (the complex of Glu and Gln, as they are difficult to distinguish in conventional 3.0-T MRS) (44). A 7.0-T MRS study by Zielman et al. measured cerebral Glu concentration, and reported that its level was obviously higher in the visual cortex during the interictal period of migraineurs without aura compared to HCs (6). In this study, we found that the Glx level in the insula in the interictal period was significantly lower in the MWoA patients than the HCs. A possible reason for the decreased Glx level is that the large energy consumptions required during headaches create an imbalance of the Glu-Gln cycle, and subsequently most neurotransmitter accumulation in the astrocytes in the form of Gln during the interictal period (43). In addition, a previous study revealed that migraine frequency could affect neuronal and glial structure and density (26). The reduced Glx level could also arise from a loss of neurons and astrocytes, and the subsequent reduction of neuron-astrocyte couplings (45).
In this study, similar to Zielman et al. (6), who found that Cho was unchanged in the visual cortex during the interictal period of migraineurs without aura, we did not observe any obvious changes in Cho concentration in the MWoA patients in comparison to the HCs. As an important component in cell membrane phospholipid metabolism, Cho mainly provides information on the integrity of cell structure and membrane turnover rate (i.e., the synthesis and degradation of phospholipids) (46). The stable Cho level implied a normal cell membrane turnover rate and undisrupted cell structure in the insula of the MWoA patients, which might be related to the implementation of scanning in the interictal period. However, we found that higher HALT-90 scores were correlated with higher Cho concentration. Higher HALT-90 scores represent a greater disease burden (28). Based on this correlation result, we speculate that as the disease burden increases, cytoarchitectural disruption and reactive proliferation may also occur in the insula of the MWoA patients, even during the interictal phase in the absence of headaches. However, this hypothesis needs to be confirmed in future studies.
In the correlation analyses, we also found that the headache frequency of the MWoA patients was positively related with the HAMD and HALT-90 scores. These results indicated that the more frequent the headaches, the more severe the depression and disease burden. Previous studies have reported that higher migraine frequency is associated with higher scores of depressions (47), and repeated migraine attacks could lead to a greater disease burden, such as anxiety, depression, cognitive decline, vascular endothelial injury, and even an increased risk of stroke (48,49), which further supports our findings.
This research had some limitations. First, the small sample size of the study might have limited its statistical power. Thus, larger sample size studies need to be conducted to confirm our findings. Second, due to ethical constraints, we only explored metabolic mechanisms during the interictal phase in MWoA patients. Third, due to the scanning time limitation, we only focused on the unilateral insula; thus, further research on the bilateral insula of MWoA patients is necessary.
Conclusions
Our study examined the neurometabolic mechanisms in the insula of MWoA patients, and the metabolic differences between HF and LF headache patients. Our results confirm that the insula is important in the pathophysiology of MWoA patients, even during the interictal period of headache. The dysfunction or loss of both neurons and glial cells, and excitatory neurotransmitter conversion imbalance may be the main pathophysiological mechanisms in the insula during the interictal period in MWoA patients. HF headaches lead to interictal hypometabolism, which may be related to more severe mitochondrial dysfunction.
Acknowledgments
We would like to thank the members of the Epilepsy Center at West China Hospital of Sichuan University for their assistance, and all the subjects who participated in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2553/rc
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2553/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the local Ethics Committee of the West China Hospital of Sichuan University (No. 2022301), and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1-211.
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. [Crossref] [PubMed]
- Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol 2019;15:483-90. [Crossref] [PubMed]
- Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol 2013;9:637-44. [Crossref] [PubMed]
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 2011;12:570-84. [Crossref] [PubMed]
- Zielman R, Wijnen JP, Webb A, Onderwater GLJ, Ronen I, Ferrari MD, Kan HE, Terwindt GM, Kruit MC. Cortical glutamate in migraine. Brain 2017;140:1859-71. [Crossref] [PubMed]
- The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629-808.
- Nieuwenhuys R. The insular cortex: a review. Prog Brain Res 2012;195:123-63. [Crossref] [PubMed]
- Yang Z, Wan X, Zhao X, Rong Y, Wu Y, Cao Z, Xie Q, Luo M, Liu Y. Brain neurometabolites differences in individuals with subjective cognitive decline plus: a quantitative single- and multi-voxel proton magnetic resonance spectroscopy study. Quant Imaging Med Surg 2021;11:4074-96. [Crossref] [PubMed]
- Bathel A, Schweizer L, Stude P, Glaubitz B, Wulms N, Delice S, Schmidt-Wilcke T. Increased thalamic glutamate/glutamine levels in migraineurs. J Headache Pain 2018;19:55. [Crossref] [PubMed]
- Dichgans M, Herzog J, Freilinger T, Wilke M, Auer DP. 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1. Neurology 2005;64:608-13. [Crossref] [PubMed]
- Peek AL, Leaver AM, Foster S, Oeltzschner G, Puts NA, Galloway G, Sterling M, Ng K, Refshauge K, Aguila MR, Rebbeck T. Increased GABA+ in People With Migraine, Headache, and Pain Conditions- A Potential Marker of Pain. J Pain 2021;22:1631-45. [Crossref] [PubMed]
- Bigal ME, Hetherington H, Pan J, Tsang A, Grosberg B, Avdievich N, Friedman B, Lipton RB. Occipital levels of GABA are related to severe headaches in migraine. Neurology 2008;70:2078-80. [Crossref] [PubMed]
- Niddam DM, Lai KL, Tsai SY, Lin YR, Chen WT, Fuh JL, Wang SJ. Brain metabolites in chronic migraine patients with medication overuse headache. Cephalalgia 2020;40:851-62. [Crossref] [PubMed]
- Zielman R, Teeuwisse WM, Bakels F, Van der Grond J, Webb A, van Buchem MA, Ferrari MD, Kruit MC, Terwindt GM. Biochemical changes in the brain of hemiplegic migraine patients measured with 7 tesla 1H-MRS. Cephalalgia 2014;34:959-67. [Crossref] [PubMed]
- Chan YM, Pitchaimuthu K, Wu QZ, Carter OL, Egan GF, Badcock DR, McKendrick AM. Relating excitatory and inhibitory neurochemicals to visual perception: A magnetic resonance study of occipital cortex between migraine events. PLoS One 2019;14:e0208666. [Crossref] [PubMed]
- Reyngoudt H, Achten E, Paemeleire K. Magnetic resonance spectroscopy in migraine: what have we learned so far? Cephalalgia 2012;32:845-59. [Crossref] [PubMed]
- Sarchielli P, Tarducci R, Presciutti O, Gobbi G, Pelliccioli GP, Stipa G, Alberti A, Capocchi G. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage 2005;24:1025-31. [Crossref] [PubMed]
- Dehghan A, Saatchian E, Sobhani M, Montazerabadi A. Neurochemical metabolite alterations of the occipital lobe in migraine without aura by proton magnetic resonance spectroscopy. Neuroradiol J 2020;33:410-5. [Crossref] [PubMed]
- Zhang L, Huang J, Zhang Z, Cao Z. Altered Metabolites in the Occipital Lobe in Migraine Without Aura During the Attack and the Interictal Period. Front Neurol 2021;12:656349. [Crossref] [PubMed]
- Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, Renshaw P, Burstein R, Borsook D. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain 2009;5:34. [Crossref] [PubMed]
- Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain 2022;23:34. [Crossref] [PubMed]
- Yu ZB, Peng J, Lv YB, Zhao M, Xie B, Liang ML, Li HT, Zhou ZH. Different mean thickness implicates involvement of the cortex in migraine. Medicine (Baltimore) 2016;95:e4824. [Crossref] [PubMed]
- Yu D, Yuan K, Zhao L, Liang F, Qin W. Regional homogeneity abnormalities affected by depressive symptoms in migraine patients without aura: a resting state study. PLoS One 2013;8:e77933. [Crossref] [PubMed]
- Yu ZB, Lv YB, Song LH, Liu DH, Huang XL, Hu XY, Zuo ZW, Wang Y, Yang Q, Peng J, Zhou ZH, Li HT. Functional Connectivity Differences in the Insular Sub-regions in Migraine without Aura: A Resting-State Functional Magnetic Resonance Imaging Study. Front Behav Neurosci 2017;11:124. [Crossref] [PubMed]
- Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA. Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008;48:1044-55. [Crossref] [PubMed]
- Demyttenaere K, Kiekens G, Bruffaerts R, Mortier P, Gorwood P, Martin L, Di Giannantonio M. Outcome in depression (II): beyond the Hamilton Depression Rating Scale. CNS Spectr 2021;26:378-82. [Crossref] [PubMed]
- Steiner TJ, Lipton RB. Lifting The Burden: The Global Campaign against Headache. The Headache-Attributed Lost Time (HALT) Indices: measures of burden for clinical management and population-based research. J Headache Pain 2018;19:12. [Crossref] [PubMed]
- Zhang S, Li J, Zhou D. A comparison of comorbid headache between patients with temporal lobe epilepsy and juvenile myoclonic epilepsy. Sci Rep 2023;13:16962. [Crossref] [PubMed]
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63:S240-52. [Crossref] [PubMed]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672-9. [Crossref] [PubMed]
- Tan Q, Sun H, Wang W, Wu X, Hao N, Su X, Yang X, Zhang S, Su J, Yue Q, Gong Q. Quantitative MR spectroscopy reveals metabolic changes in the dorsolateral prefrontal cortex of patients with temporal lobe epilepsy. Eur Radiol 2018;28:4496-503. [Crossref] [PubMed]
- Wan X, Liu L, Wang W, Tan Q, Su X, Zhang S, Yang X, Yue Q, Gong Q. 1H-MRS reveals metabolic alterations in generalized tonic-clonic seizures before and after treatment. Acta Neurol Scand 2022;145:200-7. [Crossref] [PubMed]
- Tan Q, Liu W, Wan X, Wang W, Su X, Sun H, Zhang S, Yue Q. Quantitative 1H-MRS reveals metabolic difference between subcategories of malformations of cortical development. Neuroradiology 2021;63:1539-48. [Crossref] [PubMed]
- Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 2002;82:736-54. [Crossref] [PubMed]
- Brand A, Leibfritz D, Richter-Landsberg C. Oxidative stress-induced metabolic alterations in rat brain astrocytes studied by multinuclear NMR spectroscopy. J Neurosci Res 1999;58:576-85.
- Hong S, Tomar JS, Shen J. Metabolic coupling between glutamate and N-acetylaspartate in the human brain. J Cereb Blood Flow Metab 2024;44:1608-17. [Crossref] [PubMed]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007;81:89-131. [Crossref] [PubMed]
- Sparaco M, Feleppa M, Lipton RB, Rapoport AM, Bigal ME. Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 2006;26:361-72. [Crossref] [PubMed]
- Pan JW, Kuzniecky RI. Utility of magnetic resonance spectroscopic imaging for human epilepsy. Quant Imaging Med Surg 2015;5:313-22. [Crossref] [PubMed]
- Xia Y, Fu Y, Xu H, Guan J, Yi H, Yin S. Changes in cerebral metabolites in obstructive sleep apnea: a systemic review and meta-analysis. Sci Rep 2016;6:28712. [Crossref] [PubMed]
- Caprio M, Moriconi E, Camajani E, Feraco A, Marzolla V, Vitiello L, Proietti S, Armani A, Gorini S, Mammi C, Egeo G, Aurilia C, Fiorentini G, Tomino C, Barbanti P. Very-low-calorie ketogenic diet vs hypocaloric balanced diet in the prevention of high-frequency episodic migraine: the EMIKETO randomized, controlled trial. J Transl Med 2023;21:692. [Crossref] [PubMed]
- Xie RG, Xu GY, Wu SX, Luo C. Presynaptic glutamate receptors in nociception. Pharmacol Ther 2023;251:108539. [Crossref] [PubMed]
- Novotny EJ Jr, Fulbright RK, Pearl PL, Gibson KM, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol 2003;54:S25-31. [Crossref] [PubMed]
- González de la Aleja J, Ramos A, Mato-Abad V, Martínez-Salio A, Hernández-Tamames JA, Molina JA, Hernández-Gallego J, Alvarez-Linera J. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 2013;53:365-75. [Crossref] [PubMed]
- Chen S, Lai L, Kang Z, Luo X, Zhang J, Li J. Imaging changes in neural circuits in patients with depression using (1)H-magnetic resonance spectroscopy and diffusion tensor imaging. Neural Regen Res 2012;7:1881-8. [Crossref] [PubMed]
- Duan S, Ren Z, Xia H, Wang Z, Zheng T, Li G, Liu L, Liu Z. Associations between anxiety, depression with migraine, and migraine-related burdens. Front Neurol 2023;14:1090878. [Crossref] [PubMed]
- Houts CR, Wirth RJ, McGinley JS, Gwaltney C, Kassel E, Snapinn S, Cady R. Content Validity of HIT-6 as a Measure of Headache Impact in People With Migraine: A Narrative Review. Headache 2020;60:28-39. [Crossref] [PubMed]
- Adelborg K, Szépligeti SK, Holland-Bill L, Ehrenstein V, Horváth-Puhó E, Henderson VW, Sørensen HT. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 2018;360:k96. [Crossref] [PubMed]


