
Contrast-enhanced ultrasound (CEUS) in diagnosis of Kikuchi disease: how to distinguish it from lymphoma?
Introduction
Kikuchi disease (KD), also called histiocytic necrotizing lymphadenitis, is a benign and self-limiting disease which is more common in young women and typically presents as lymphadenopathy (1). Ultrasonography is the most frequently utilized imaging modality for the evaluation of superficial lymphadenopathy benefited by the anatomical location (2). The diagnosis of KD in clinical practice is particularly challenging due to the atypical imaging characteristics that are easily confounded with lymphoma and the limited awareness among clinicians (3). However, the treatment strategies for KD and lymphoma diverge significantly. Hence, early and precise diagnosis is crucial for guiding appropriate therapeutic interventions. The definitive diagnosis predominantly depends on the pathological results of the core needle biopsy (CNB) or surgical excisional biopsy, an invasive operation (4). Several studies have suggested that enhanced computed tomography (CT) is beneficial for the diagnosis of KD. However, there is still a lack of robust diagnostic criteria and a sufficient number of supporting cases (5-7).
Recent advancements in contrast-enhanced ultrasound (CEUS) have enabled the evaluation of microvascularization in lymph nodes (LNs), demonstrating significant diagnostic potential (8). SonoVue (Bracco, Milan, Italy), an ultrasound (US) contrast agent, is a blood pool agent that effectively reflects the blood flow perfusion within LNs. Several studies have reported CEUS could help to differentiate benign and malignant LNs (2,8,9). However, the CEUS characteristics of KD have not been reported alone. In our study, we summarized the features of CEUS of KD, which could be helpful to differentiate it from lymphoma. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1672/rc).
Methods
This retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by ethics board of Shengjing Hospital of China Medical University (No. 2024PS1462K), and individual consent for this retrospective analysis was waived.
Patient selection
Patients with KD and lymphoma patients underwent both US and CEUS before CNB or surgery were collected from September 2019 until June 2024. Inclusive criteria were as follows: (I) superficial lymphadenopathy (neck, axilla or groin); (II) pathology results were obtained by CNB or surgery; (III) immunohistochemical results of the cases of lymphoma were obtained; (IV) the cases of KD had followed-up information within 1 month. Exclusive criteria were as follows: (I) uncertain histopathological results; (II) incomplete imaging data (Figure 1).

US and CEUS analysis
US and CEUS were performed using an L12-5 transducer (frequency 3–15 MHz) of Mindray Resona R9G (Mindray, Shenzhen, China). The mechanical index (MI) was 0.081 when CEUS was performed, with an intravenous bolus injection of suspension of SonoVue (Bracco). The US and CEUS images were analyzed by two physicians who had 10 and 4 years of experience in CEUS separately, being blind to the medical history of the images. Disagreements were resolved by consensus of by the judgement of a third expert who also specialized in CEUS.
The US characteristics were assessed as follows: location (neck, axilla or groin), laterality (unilateral or bilateral), number of involved LNs (single or several or multiple), perinodal fat hyper-echogenicity (absent or present), long diameter (LD), short diameter (SD), LD/SD, necrosis (absent or present), homogeneity of the echogenicity (homogeneity or heterogeneity), normal echogenic hilus (absent or present), margin (well-defined or ill-defined), matting (LNs adhere to each other or not). color Doppler flow images (CDFI) were described as follows: LN hilar vascularity, perinodal vascularity, mix vascularity or no vascularity.
The CEUS characteristics were assessed as follows: enhancement margin (well-defined or ill-defined), enhancement range (enlarged, same or reduced), enhancement pattern (centripetal enhancement, centrifugal enhancement or mixed enhancement), enhancement homogeneity (homogeneity or heterogeneity), perfusion defect (absent or present), numbers of perfusion defect (single, several or multiple), margin of perfusion defect (well-defined or ill-defined), percentage of perfusion defect (<10%, 10%–<50%, 50%–<90%, ≥90%) and reticulated enhancement (absent or present).
Statistical analysis
SPSS software (version 26.0, IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Continuous data were presented as mean ± standard deviation, while categorical data were presented as absolute numbers and percentages. Comparisons between groups were performed using Student’s t-test for continuous data and Pearson’s χ2 test or Fisher’s exact test for categorical data. Univariate analysis was used to identify features associated with KD, while multivariate logistic regression analysis with the forward method was employed to determine the optimal features for diagnosing KD. The diagnostic efficacy of model was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC) and 95% confidence intervals (CIs) of model was calculated.
Results
Basic characteristics
A total of 60 LNs were included. Among them, 22 were KD and 38 were lymphoma. All of the LNs with KD had returned to normal morphology within 1 month. The age of patients, LD and SD of LNs were different significantly between KD and lymphoma. The cut-off value of age was 40 years. All of the KD patients were less than 40 years old; 32 cases (84.2%) of lymphoma were more than 40 years old. The cut-off value of LD was 30.5 mm. The LD of 20 cases (90.9%) of KD were less than 30.5 mm. The LD of 24 cases (63.2%) of lymphoma were more than 30.5 mm. The cut-off value of SD was 14.5 mm. The SD of 21 cases (95.5%) of KD were less than 14.5 mm. The LD of 25 cases (65.7%) of lymphoma were more than 14.5 mm. There were no significant differences in sex, location, laterality, LD/SD and number of involved LNs (Table 1).
Table 1
| Characteristics | KD (n=22) | Lymphoma (n=38) | c2 or t | P value |
|---|---|---|---|---|
| Sex | 1.876 | 0.171 | ||
| Male | 7 (31.8) | 19 (50.0) | ||
| Female | 15 (68.2) | 19 (50.0) | ||
| Age (years) | 22.18±8.16 | 54.92±16.67 | 10.182 | <0.001* |
| Location | 1.283 | 0.257 | ||
| Neck | 17 (77.3) | 24 (63.2) | ||
| Axilla | 5 (22.7) | 2 (5.3) | ||
| Groin | 0 (0) | 12 (31.6) | ||
| Laterality | 1.371 | 0.242 | ||
| Unilateral | 9 (40.9) | 10 (26.3) | ||
| Bilateral | 13 (59.1) | 28 (73.7) | ||
| LD (mm) | 22.6±5.68 | 39.6±17.53 | 5.497 | 0.001* |
| SD (mm) | 10.0±2.99 | 19.9±13.90 | 4.209 | 0.001* |
| LD/SD | 2.34±0.43 | 2.23±0.52 | 0.895 | 0.374 |
| Number of involved LNs | 1.178 | 0.278 | ||
| Single [1] | 0 (0) | 2 (5.3) | ||
| Several [2–4] | 0 (0) | 0 (0) | ||
| Multiple [≥5] | 22 (100.0) | 36 (94.7) | ||
| Necrosis | 0.579 | 0.447 | ||
| Absent | 22 (100.0) | 37 (97.4) | ||
| Present | 0 (0) | 1 (2.6) | ||
| Homogeneity of the echogenicity | 1.482 | 0.224 | ||
| Homogeneity | 8 (36.4) | 20 (52.6) | ||
| Heterogeneity | 14 (63.6) | 18 (47.4) | ||
| Normal echogenic hilus | 9.502 | 0.002* | ||
| Absent | 12 (54.5) | 34 (89.5) | ||
| Present | 10 (45.5) | 4 (10.5) | ||
| Margin | 3.818 | 0.051 | ||
| Well-defined | 22 (100.0) | 34 (89.5) | ||
| Ill-defined | 0 (0) | 4 (10.5) | ||
| Matting | 1.155 | 0.282 | ||
| Absent | 12 (54.5) | 26 (68.4) | ||
| Present | 10 (45.5) | 12 (31.6) | ||
| Perinodal fat hyperechogenicity | 14.067 | 0.001* | ||
| Absent | 4 (18.2) | 26 (68.4) | ||
| Present | 18 (81.8) | 12 (31.6) | ||
| CDFI | 10.710 | 0.001* | ||
| LN hilar vascularity | 9 (40.9) | 2 (5.3) | ||
| Perinodal vascularity | 0 (0) | 1 (2.6) | ||
| Mix vascularity | 13 (59.1) | 35 (92.1) | ||
| No vascularity | 0 (0) | 0 (0) |
Data are presented as mean ± standard deviation or n (%). *, P value <0.05 indicates statistical significance. CDFI, color doppler flow images; KD, Kikuchi disease; LD, long diameter; LN, lymph nodule; SD, short diameter; US, ultrasound.
US and CEUS analysis
Significant differences were observed between the two groups in terms of normal echogenic hilus, perinodal fat hyper-echogenicity, and CDFI distribution. Lymphoma was more frequently associated with mixed vascularity and the absence of a normal echogenic hilum. In contrast, KD was characterized by increased LN hilar vascularity and perinodal fat hyper-echogenicity. There were no significant differences between the two groups in terms of necrosis, homogeneity of echogenicity, margin, and matting (Table 1).
In CEUS analysis, significant differences were noted in enhancement margin, enhancement pattern, perfusion defect, number of perfusion defects, margin of perfusion defects, percentage of perfusion defect, and reticulated enhancement. Lymphoma more frequently exhibited well-defined enhancement margins. Both KD and lymphoma predominantly showed a mixed enhancement pattern. However, centripetal enhancement was observed exclusively in lymphoma cases, whereas centrifugal enhancement occurred only in KD patients. Perfusion defects were more commonly observed in KD patients, typically presenting as single, smaller, and well-defined lesions. In contrast, perfusion defects in lymphoma were generally ill-defined and occupied a larger proportion of the lesion (Figures 2-4). Additionally, reticulated enhancement was more frequently associated with lymphoma (Figure 5). There was no significant difference in enhancement range and enhancement homogeneity between the two groups (Table 2).
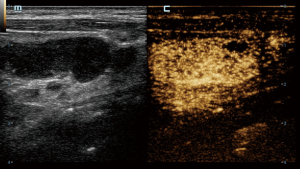
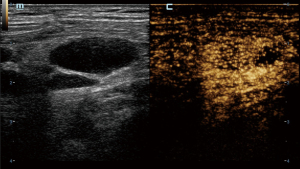
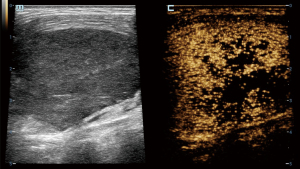
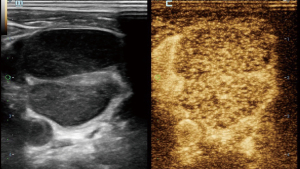
Table 2
| Characteristics | KD (n=22) | Lymphoma (n=38) | c2 value | P value |
|---|---|---|---|---|
| Enhancement margin | 12.073 | <0.001* | ||
| Well-defined | 9 (40.9) | 32 (84.2) | ||
| Ill-defined | 13 (59.1) | 6 (15.8) | ||
| Enhancement range | 2.297 | 0.13 | ||
| Enlarged | 10 (45.5) | 10 (26.3) | ||
| Same | 12 (54.5) | 28 (73.7) | ||
| Reduced | 0 (0) | 0 (0) | ||
| Enhancement pattern | 12.453 | 0.002* | ||
| Centripetal | 0 (0) | 7 (18.4) | ||
| Centrifugal | 3 (13.6) | 0 (0) | ||
| Mixed | 19 (86.4) | 31 (81.6) | ||
| Enhancement homogeneity | 1.826 | 0.177 | ||
| Homogeneity | 3 (13.6) | 11 (28.9) | ||
| Heterogeneity | 19 (86.4) | 27 (71.1) | ||
| Perfusion defect | 21.733 | <0.001* | ||
| Absent | 6 (27.3) | 33 (86.8) | ||
| Present | 16 (72.7) | 5 (13.2) | ||
| Numbers of perfusion defect | 9.242 | 0.002* | ||
| Single [1] | 15 (68.2) | 2 (5.3) | ||
| Several [2–4] | 1 (4.5) | 0 (0) | ||
| Multiple [≥5] | 0 (0) | 3 (7.9) | ||
| Margin of perfusion defect | 10.687 | 0.001* | ||
| Well-defined | 12 (54.5) | 0 (0) | ||
| Ill-defined | 4 (18.2) | 5 (13.2) | ||
| Percentage of perfusion defect | 4.112 | 0.043* | ||
| <10% | 10 (45.5) | 1 (2.6) | ||
| 10%–<50% | 6 (27.3) | 3 (7.9) | ||
| 50%–<90% | 0 (0) | 1 (2.6) | ||
| ≥90% | 0 (0) | 0 (0) | ||
| Reticulated enhancement | 22.969 | <0.001* | ||
| Absent | 21 (95.5) | 12 (31.6) | ||
| Present | 1 (4.5) | 26 (68.4) |
Data are presented as n (%). *, P value <0.05 indicates statistical significance. CEUS, contrast-enhanced ultrasound; KD, Kikuchi disease.
Univariate and multivariate analysis
Univariate analysis showed that age, LD, SD, normal echogenic hilus, perinodal fat hyperechogenicity, LN hilar vascularity, ill-defined enhancement margin, perfusion defect and reticulated enhancement absent were the independent predictors of KD. Multivariate analysis showed that age and SD were independent predictors of KD (Table 3). The sensitivity, specificity and accuracy of age were 86.8%, 95.5% and 90% respectively. The sensitivity, specificity and accuracy of SD were 65.8%, 95.5% and 76.7%. The sensitivity, specificity and accuracy of the predicted probability were 92.1%, 95.5% and 95%. The AUC of age, SD and predicted probability were 0.939, 0.809 and 0.974 respectively (Figure 6, Table 4).
Table 3
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Coefficient (β) | OR (95 % CI) | P value | Coefficient (β) | OR (95 % CI) | P value | ||
| Age (years) | −0.150 | 0.861 (0.798–0.929) | <0.001* | −0.208 | 0.812 (0.705–0.935) | 0.004* | |
| Long diameter (mm) | −1.649 | 0.192 (0.073–0.510) | <0.001* | ||||
| Short diameter (mm) | −2.476 | 0.084 (0.019–0.379) | 0.001* | −4.127 | 0.016 (0.001–0.451) | 0.015* | |
| Normal echogenic hilus | 1.958 | 7.083 (1.867–26.870) | 0.004* | ||||
| Perinodal fat hyperechogenicity | 2.277 | 9.750 (2.707–35.112) | <0.001* | ||||
| LN hilar vascularity | 2.523 | 12.462 (2.374–65.424) | 0.003* | ||||
| Ill-defined enhancement margin | 2.042 | 7.704 (2.280–26.032) | 0.001* | ||||
| perfusion defect | 2.868 | 17.600 (4.662–66.450) | <0.001* | ||||
| Reticulated enhancement absent | 3.818 | 45.500 (5.464–378.875) | <0.001* | ||||
*, P value <0.05 indicates statistical significance. CI, confidence interval; LN, lymph nodule; OR, odds ratio.
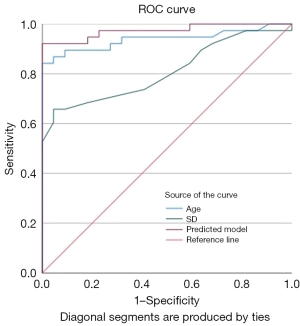
Table 4
| Characteristics | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| Age | 86.8 | 95.5 | 90 | 0.939 (0.876–1.000) | <0.001* |
| SD | 65.8 | 95.5 | 76.7 | 0.809 (0.702–0.916) | <0.001* |
| Predicted probability | 92.1 | 95.5 | 95 | 0.974 (0.938–1.000) | <0.001* |
*, P value <0.05 indicates statistical significance. AUC, area under the curve; CI, confidence interval; SD, short diameter.
Discussion
KD is reported to be more prevalent in young women (1). Our study included a total of 22 patients, with 15 females (68.2%) and 7 males (31.8%). This gender distribution difference was not statistically significant when compared to lymphoma. All KD patients in our study were under 40 years old, indicating that age is a highly specific diagnostic indicator for KD. According to our statistical analysis, the LNs in KD were generally smaller than those in lymphoma, with cut-off values for LD and SD being 30.5 and 14.5 mm, respectively. There was no statistical difference in the LD/SD ratio between the two conditions, suggesting that the LNs were almost oval in shape in both diseases.
The presence of a normal echogenic hilus is an important feature for distinguishing between benign and malignant LNs (10). In our study, the normal echogenic hilus differed statistically between the two groups, yet it was absent in 12 cases (54.5%) of KD. Several studies have reported that US can differentiate KD and lymphoma based on the characteristic of perinodal fat hyper-echogenicity, which is consistent with our findings (3,5). In our study, 12 lymphoma cases (31.6%) exhibited perinodal fat hyper-echogenicity. The increased echogenicity of perinodal soft tissue in lymphoma cases is due to infiltration by malignant cells, and the posterior echo of the LNs can be increased due to the marked hypo-echogenicity of lymphoma.
The characteristics of microcirculation, which differ between benign and malignant LNs, can be detected more precisely using CEUS. In our study, the enhancement margins of most KD (59.1%) were ill-defined, whereas most lymphoma cases (84.2%) exhibited well-defined margins. KD often involves the peripheral soft tissue of LNs, resulting in an ill-defined enhancement margin. In contrast, lymphomas are typically restricted by the LN capsules, leading to well-defined enhancement margins (11). Both KD and lymphoma can infiltrate adjacent soft tissues, causing indistinct borders and expanded enhancement ranges. While the enhancement patterns in these two diseases were predominantly mixed, centrifugal enhancement was a specific indicator of KD. Additionally, several KD cases with hilar LNs displayed hilar perfusion patterns, characterized by enhancement progressing from the center to the periphery. Our study found that perfusion defects were present in the majority (72.7%) of KD cases and in a minority (13.2%) of lymphoma cases, with a statistically significant difference. Although perfusion defects can occur in both diseases, their characteristics differ; in KD, the defects are usually single, small, and well-defined, whereas in lymphoma, the defects are typically ill-defined. Moreover, we identified a specific enhancement pattern in lymphoma, described as reticulated enhancement, which had not been reported in other studies. This pattern probably results from the reticulated echogenicity observed in grey-scale US images (12).
In our study, all of the US and CEUS characteristics were not included in the multivariate analysis. It is because the high correlation of age and SD with KD leads to the exclusion in the logistic regression equation. But US and CEUS presentations is helpful in differentiating KD and lymphoma. In univariate analysis, LD, SD, normal echogenic hilus, perinodal fat hyper-echogenicity, LN hilar vascularity, ill-defined enhancement margin, perfusion defect and reticulated enhancement absent were the independent predictors of KD.
In summary, the CEUS characteristics of KD can be delineated as follows: ill-defined enhancement margin, centrifugal or mixed perfusion pattern, and small, well-defined perfusion defects (<50%). Additionally, when considered combining with clinical features—such as younger patient age (<40 years) and increased echogenicity of the perinodal fat—these imaging characteristics can be helpful in the diagnosis of KD. CEUS is more likely to be accepted by patients compared to invasive CNB especially for parents of young persons.
In contrast, lymphoma could present as reticulated enhancement, a unique characteristic that could differentiate it from other conditions. Lymphoma typically presents with well-defined enhancement margins, a predominantly mixed perfusion pattern, and infrequent perfusion defects. When present, the defects tend to show ill-defined margins.
Other benign lymphadenopathy included tuberculous lymphadenitis and reactive hyperplasia. Tuberculous lymphadenitis is characterized by a primarily centripetal or mixed perfusion pattern, often accompanied by perfusion defects resulting from necrosis. These defects are usually extensive, and in certain cases, only a peripheral rim of perfusion remains (13). LNs with reactive hyperplasia, on the other hand, typically exhibits a hilar perfusion pattern with uniform enhancement (14).
This study is subject to several limitations. First, the sample size is relatively small, which may impact the generalizability of the findings. Second, there is a selection bias inherent in the study design, as CEUS was not performed in all cases of KD. In certain instances, diagnoses were made using grey-scale US in conjunction with patient age and clinical presentation. Consequently, the specific characteristics of CEUS in the context of KD need to be validated through studies with larger cohorts.
Conclusions
This study showed that combining with age, size of lymph nodules, US and CEUS features could effectively diagnose KD. Specifically, KD is characterized by ill-defined enhancement margins and typically presents with a single, small, well-defined perfusion defect on CEUS. In contrast, reticulated enhancement is a distinctive CEUS feature of lymphoma. The application of CEUS further enhances the diagnostic process for KD, playing a crucial role in differentiating it from lymphoma. By providing more comprehensive imaging information, CEUS facilitates more accurate diagnoses and helps reduce invasive procedures.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1672/rc
Funding: This work was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1672/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by ethics board of Shengjing Hospital of China Medical University (No. 2024PS1462K), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Perry AM, Choi SM. Kikuchi-Fujimoto Disease: A Review. Arch Pathol Lab Med 2018;142:1341-6. [Crossref] [PubMed]
- Huang S, Zhao Y, Jiang X, Lin N, Zhang M, Wang H, Zheng A, Ma X. Clinical Utility of Contrast-enhanced Ultrasound for the Diagnosis of Lymphadenopathy. Ultrasound Med Biol 2021;47:869-79. [Crossref] [PubMed]
- Lo WC, Chang WC, Lin YC, Hsu YP, Liao LJ. Ultrasonographic differentiation between Kikuchi's disease and lymphoma in patients with cervical lymphadenopathy. Eur J Radiol 2012;81:1817-20. [Crossref] [PubMed]
- Allin D, David S, Jacob A, Mir N, Giles A, Gibbins N. Use of core biopsy in diagnosing cervical lymphadenopathy: a viable alternative to surgical excisional biopsy of lymph nodes? Ann R Coll Surg Engl 2017;99:242-4. [Crossref] [PubMed]
- You SH, Kim B, Yang KS, Kim BK. Cervical necrotic lymphadenopathy: a diagnostic tree analysis model based on CT and clinical findings. Eur Radiol 2019;29:5635-45. [Crossref] [PubMed]
- Shim EJ, Lee KM, Kim EJ, Kim HG, Jang JH. CT pattern analysis of necrotizing and nonnecrotizing lymph nodes in Kikuchi disease. PLoS One 2017;12:e0181169. [Crossref] [PubMed]
- Lee S, Yoo JH, Lee SW. Kikuchi disease: differentiation from tuberculous lymphadenitis based on patterns of nodal necrosis on CT. AJNR Am J Neuroradiol 2012;33:135-40. [Crossref] [PubMed]
- Fu Y, Cui LG, Ma JY, Fang M, Lin YX, Li N. Development of a Novel Contrast-Enhanced Ultrasound-Based Nomogram for Superficial Lymphadenopathy Differentiation: Postvascular Phase Value. Ultrasound Med Biol 2024;50:852-9. [Crossref] [PubMed]
- Chen L, Chen L, Liu J, Wang B, Zhang H. Value of Qualitative and Quantitative Contrast-Enhanced Ultrasound Analysis in Preoperative Diagnosis of Cervical Lymph Node Metastasis From Papillary Thyroid Carcinoma. J Ultrasound Med 2020;39:73-81. [Crossref] [PubMed]
- Park JE, Ryu YJ, Kim JY, Kim YH, Park JY, Lee H, Choi HS. Cervical lymphadenopathy in children: a diagnostic tree analysis model based on ultrasonographic and clinical findings. Eur Radiol 2020;30:4475-85. [Crossref] [PubMed]
- Chae SY, Jung HN, Ryoo I, Suh S. Differentiating cervical metastatic lymphadenopathy and lymphoma by shear wave elastography. Sci Rep 2019;9:12396. [Crossref] [PubMed]
- Asai S, Miyachi H, Kawakami C, Kubota M, Kato Y, Shimamura K, Ando Y. Infiltration of cervical lymph nodes by B- and T-cell non-Hodgkin's lymphoma and Hodgkin's lymphoma: preliminary ultrasonic findings. Am J Hematol 2001;67:234-9. [Crossref] [PubMed]
- Zhao D, He N, Shao YQ, Yu XL, Chu J, Yang G. The diagnostic value of contrast-enhanced ultrasound for cervical tuberculous lymphadenitis. Clin Hemorheol Microcirc 2022;81:69-79. [Crossref] [PubMed]
- Yang JR, Song Y, Jia YL, Ruan LT. Application of multimodal ultrasonography for differentiating benign and malignant cervical lymphadenopathy. Jpn J Radiol 2021;39:938-45. [Crossref] [PubMed]


