
Skeletal muscle fatty deposition in young and middle-aged adults with metabolic dysfunction-associated fatty liver disease: a magnetic resonance proton density fat fraction study
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a prevalent cause of chronic liver disease in China (1-3) and has a spectrum of histologically distinct conditions, ranging from metabolic dysfunction-associated fatty liver (MAFL) to metabolic dysfunction–associated steatohepatitis (MASH). The progression of MAFLD poses a substantial risk of fibrosis, liver cirrhosis, and potentially hepatocellular carcinoma (4). Despite extensive research, the pathogenesis of MAFLD remains incompletely understood, with insulin resistance (IR) emerging as a key factor linked to the onset of MAFLD (5,6).
Sarcopenia and MAFLD have a similar pathophysiological mechanism involving IR. Consequently, it has been speculated that MAFLD gives rise to sarcopenia. Studies have discovered that fatty infiltration within skeletal muscles diminishes muscle strength, increasing the susceptibility to osteoporotic fractures, reducing mobility, and raising mortality risk (7-10). Hence, timely assessment and intervention targeting fatty deposition in skeletal muscles are critical to treating patients with MAFLD.
The correlation between skeletal muscle fatty deposition in young and middle-aged adults with MAFLD remains inadequately explored, while the potential mediating role of blood glucose in the interplay between MAFLD and sarcopenia has not been extensively examined. There is thus an urgent need to comprehensively characterize the dynamics between MAFLD, skeletal muscle health, and metabolic factors in different age groups.
Various methods have been employed to quantitatively measure the extent of fatty deposition, including biopsy, ultrasound, dual-energy X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) (11-13). Although biopsy is considered the gold standard for assessing fatty deposition, its invasiveness limits its routine clinical use. Ultrasound examination can assess fatty deposition in the liver by comparing echoes with those of the kidney, but its subjectivity hinders its application in muscle evaluation (13). The use of dual-energy X-ray and CT is restricted due to ionizing radiation concerns (11,12). Meanwhile, MRI is a non-radiation technique than can provide in vivo quantitative assessment of fatty deposition in living subcutaneous and intramuscular tissue. Proton density fat fraction (PDFF) is an MR-based biomarker of hepatic steatosis, representing the proportion of MR-visible fat protons to the total of MR-visible fat and water protons (14). PDFF is commonly used to measure the hepatic fat fraction (HFF) in patients with MAFLD, enabling precise assessment of hepatic and muscle fat content in a single examination (15).
We hypothesized that individuals with MAFLD exhibit more pronounced fatty deposition in skeletal muscles and that blood glucose level mediates the relationship between the fatty deposition in the liver and that in the skeletal muscles. We conducted a retrospective study that analyzed the PDFF data in patients with and without MAFLD to determine the link between the degree of fatty deposition in the liver and that in the skeletal muscles in the lumbar regions. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1696/rc).
Methods
Study design
Patients with suspected MAFLD who underwent MRI-PDFF at Shuguang Hospital Affiliated with the Shanghai University of Traditional Chinese Medicine between January 2020 and August 2023 were included in this study. Initially, a total of 1,919 participants were enrolled (Figure 1). The following exclusion criteria were applied: (I) a history of excessive ethanol consumption (>7 and >14 beverages per week for females and males, respectively); (II) positive serologic findings for hepatitis B or C; (III) liver tumor or liver tumor after surgery, other types post-chemotherapy, or liver inflammatory lesions; (IV) age over 50 years old (as aging is closely associated with increased accumulation of lipids in no-adipose tissues and organs (16), participants over 50 years old were excluded to minimize age-related effects); (V) MRI scan with metal artifacts or motion artifacts; and (VI) no imaging of the third lumbar vertebrae.
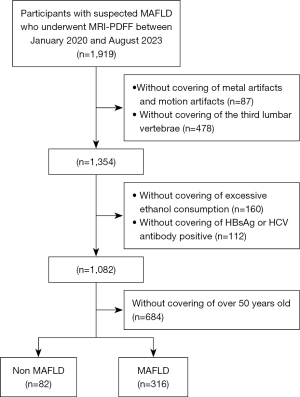
All participants were categorized into four groups based on HFF. The severity of hepatic fatty deposition was classified as follows: G0, HFF <5%; G1, 5%≤ HFF <10%; G2, 10%≤ HFF <25%; and G3, HFF ≥25%) (17). Due to the retrospective nature of this study, height and weight data were not available for any of the participants, and thus the body mass index could not be calculated.
This retrospective study was approved by the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (No. 2021-1062-137). The requirement for individual consent was waived due to the retrospective nature of the analysis. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Clinical and biochemical analyses
Blood biochemical examinations were conducted within 2 weeks before or after MR scans. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), albumin (ALB), and blood glucose were recorded. Blood samples were obtained from the median cubital vein.
MRI protocol
All MR scans were performed using a 3T MR system (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany) with an 18-channel body surface coil in combination with the table-mounted spine matrix coil. The sequences included three-dimensional (3D) volumetric interpolated breath-hold T1-weighted imaging (T1WI), fat-suppressed single-shot fast spin echo T2-weighted imaging (T2WI), and multi-echo Dixon. Multiecho Dixon was acquired during a single breath-hold of 13 s. The parameters of the MRI sequence are shown in Table 1.
Table 1
| Parameter | T1WI | T2WI | Dixon |
|---|---|---|---|
| Field of view (mm2) | 308×380 | 308×380 | 390×450 |
| No. of slices | 64 | 36 | 64 |
| Slice thickness (mm) | 3.0 | 5.0 | 3.5 |
| TE (msec) | 1.29 | 91 | 1.05, 2.46, 3.69, 4.92, 6.15, 7.38 |
| TR (msec) | 3.97 | 1,600 | 9.0 |
| Flip angle (degrees) | 9.0 | 160 | 4.0 |
| Bandwidth (Hz) | 1,040 | 698 | 1,080 |
| Acquisition matrix | 320×240 | 256×256 | 160×128 |
MRI, magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; TE, echo time; TR, repetition time.
Image analysis
All images were retrospectively reviewed and measured by two musculoskeletal radiologists (Q.W. and J.W.) with 10 years of experience in musculoskeletal imaging. All PDFFs measurements were acquired manually, with the mean value from two radiologists being used as the final result.
There were 38 patients with a more than 20% difference in measurement between the two radiologists, and in such cases, a third radiologist with 18 years of experience in musculoskeletal imaging remeasured the data. The measured value closest to the third radiologist’s value was used to calculate the mean value.
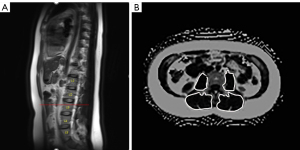
Liver fat was assessed using regions of interest (ROIs) that were as large as possible with a homogeneous signal, with large vessels and enlarged bile ducts being avoided. HFF was defined as the average PDFF of 9 ROIs, with the ROIs of HFF being manually selected to ensure a homogeneous signal and to avoid large vessels and enlarged bile ducts. The fatty deposition in the psoas major and paraspinal muscle, including the multifidus and erector spinae muscle, as well as the subcutaneous adipose tissue thickness (SATT), were measured at the upper level of the third lumbar vertebra following established protocols (18,19). The fat deposition in the psoas major muscle and paraspinal muscle was delineated and measured bilaterally on axial images, with the average of both sides being calculated as the psoas major fat fraction (PMFF) and paraspinal muscle fat fraction (PAMFF) to account for potential laterality bias due to factors such as dominant muscle usage, postural habits, or personal preference (Figure 2). SATT was quantified as the maximum thickness of the fat layer from the skin surface to the outer (anterior) edge of the rectus abdominis muscles on the same axial image (20).
Statistical analyses
Data analysis was conducted with SPSS version 23 (IBM Corp., Armonk, NY, USA). The measurement data were subjected to one-way analysis of variance (ANOVA), while the count data were analyzed through parametric analysis. A P value <0.05 was considered indicative of statistical significance. Pearson correlation analysis was used to determine the correlation of SATT, PMFF, and PAMFF with HFF. Additionally, Pearson correlation analysis was applied to assess the associations of PMFF, PAMFF, and SATT, with age and the biochemical variables of MAFLD. For the PDFF measurement values with a correlation with HFF, blood glucose level and HFF were analyzed by intermediary analysis. Specifically, HFF was treated as an independent variable, while PMFF and PAMFF were considered dependent variables, with glucoses acting as a mediator variable. The intermediary analysis was carried out using a simple mediation model developed by Andrew Hayes (http://processmacro.org/), with the significance of mediation effects determined by bootstrapped 95% confidence intervals (CIs) based on 5,000 iterations. The interobserver reliability of PDFF quantitative measurement was assessed using the intraclass correlation coefficient (ICC). An ICC value approaching 1 indicated excellent agreement between the two observers or observations.
Results
A total of 398 patients (250 males and 148 females) were enrolled in this study. Their mean age was 35.9±7.3 years, and the age range was 20–50 years. The baseline characteristics of the 398 participants are summarized in Table 2. No significant differences were observed between the four groups in terms of age, sex, or the levels of LDL-C and ALB. However, as the severity of fatty liver infiltration increased across the G0, G1, G2, and G3 groups, there was a notable elevation in the levels of total TC, TG, blood glucose, ALT, and AST, while the level of HDL-C decreased.
Table 2
| Variable | G0 (n=82) (HFF <5%) |
G1 (n=54) (5%≤ HFF <10%) |
G2 (n=149) (10%≤ HFF <25%) |
G3 (n=113) (HFF ≥25%) |
F | P |
|---|---|---|---|---|---|---|
| Age (years) | 35.7±8.2 | 36.6±8.0 | 35.7±7.2 | 35.9±6.4 | 0.204 | 0.894 |
| Sex (male:female) | 43:39 | 33:21 | 96:53 | 78:35 | – | 0.139 |
| TC (mmol/L) | 11.0±15.6¶ | 12.9±17.8¶ | 12.4±15.1¶ | 22.4±37.5 | 3.446 | 0.017 |
| LDL-C (mmol/L) | 2.6±1.1 | 2.9±1.0 | 3.5±4.9 | 3.4±1.7 | 1.331 | 0.264 |
| HDL-C (mmol/L) | 1.4±0.6¶ | 1.2±0.2†,¶ | 1.1±0.2† | 1.1±0.2†,‡ | 10.537 | 0.000 |
| TG (mmol/L) | 1.9±1.2‡,§,¶ | 2.0±1.0§,¶ | 2.5±1.6†,‡ | 2.8±2.1†,‡ | 5.348 | 0.001 |
| Blood glucose (mg/dL) | 5.3±0.6§,¶ | 5.5±1.2 | 5.9±1.9† | 6.1±2.0† | 3.849 | 0.010 |
| ALT (U/L) | 25.5±31.6§,¶ | 32.1±19.1 | 61.1±48.7†,‡,¶ | 97.7±52.9†,‡,§ | 33.535 | 0.000 |
| AST (U/L) | 16.1±8.5§ | 21.7±14.6§ | 32.2±25.3†,‡,¶ | 46.1±34.3†,‡,§ | 18.845 | 0.000 |
| ALB (g/L) | 44.3±3.2 | 43.9±7.6 | 45.3±2.9 | 45.6±3.6 | 2.217 | 0.086 |
Data are presented as the mean ± standard deviation. †, a statistical difference with G0; ‡, a statistical difference with G1; §, a statistical difference with G2; ¶, a statistical difference with G3. ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; HFF, hepatic fat fraction; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Fat fractions
The distribution of patients among the groups was as follows: there were 82 participants in the G0 group, 54 in the G1 group, 149 in the G2 group, and 113 in the G3 group. Statistical analysis revealed significant differences in PMFF and PAMFF between the four groups (P<0.05), while there was no significant difference in SATT (P>0.05) (Table 3). Specifically, PMFF and PAMFF in the G0 group (5.9%±1.9% and 7.7%±2.4%) were lower than those in the G1 group (7.1%±2.0% and 9.2%±2.4%), G2 (9.0%±2.9% and 10.6%±3.9%), and G3 group (9.7%±3.1% and 10.7%±2.5%) (P<0.05). Moreover, the PMFF and PAMFF levels in the G1 group were lower than those in the G2 and G3 group (P<0.05), while no significant statistical difference was observed between the G2 and G3 groups in terms of PMFF or PAMFF (P>0.05).
Table 3
| Variable | G0 (n=82) | G1 (n=54) | G2 (n=149) | G3 (n=113) | F | P |
|---|---|---|---|---|---|---|
| PMFF (%) | 5.9±1.9‡,§,¶ | 7.1±2.0†,§,¶ | 9.0±2.9†,‡,§ | 9.7±3.1†‡ | 40.342 | 0.000 |
| PAMFF (%) | 7.7±2.4‡,§,¶ | 9.2±2.4†,§,¶ | 10.6±3.9†,‡,§ | 10.7±2.5†,‡ | 20.021 | 0.000 |
| SATT (mm) | 27.3±9.3 | 29.9±12.1 | 29.8±10.9 | 27.6±8.7 | 1.806 | 0.145 |
Data are presented as the mean ±standard deviation. †, a statistical difference with G0; ‡, a statistical difference with G1; §, a statistical difference with G2; ¶, a statistical difference with G3. PAMFF, paraspinal muscle fat fraction; PMFF, psoas major fat fraction; SATT, subcutaneous adipose tissue thickness.
Correlation analysis
HFF showed a positive correlation between PMFF (r=0.475; P<0.001) and PAMFF (r=0.343; P<0.001) (Table 4 and Figure 3). After adjustments were made for the levels of ALT, AST, TC, LDL-C, HDL-C, TG, and ALB, these correlations remained significant (PMFF: r=0.332, P<0.001; PAMFF: r=0.392, P<0.001). However, there was no significant correlation between SATT and HFF (r=0.007; P=0.895).
Table 4
| Variable | PMFF (%) | PAMFF (%) | SATT (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |||
| Ages (years) | 0.155 | 0.002 | 0.107 | 0.033 | −0.301 | 0.000 | ||
| TC (mmol/L) | 0.108 | 0.063 | 0.094 | 0.105 | −0.075 | 0.196 | ||
| LDL-C (mmol/L) | 0.076 | 0.195 | 0.024 | 0.685 | −0.058 | 0.319 | ||
| HDL-C (mmol/L) | −0.022 | 0.703 | −0.066 | 0.260 | −0.001 | 0.986 | ||
| TG (mmol/L) | 0.029 | 0.619 | −0.054 | 0.358 | −0.171 | 0.003 | ||
| ALT (U/L) | 0.169 | 0.003 | 0.118 | 0.040 | 0.011 | 0.844 | ||
| AST (U/L) | 0.186 | 0.001 | 0.169 | 0.004 | 0.042 | 0.472 | ||
| ALB (g/L) | −0.087 | 0.136 | −0.168 | 0.004 | −0.145 | 0.013 | ||
| Fasting glucose (mg/dL) | 0.177 | 0.003 | 0.138 | 0.020 | 0.041 | 0.491 | ||
| HFF | 0.421 | <0.001 | 0.300 | <0.001 | −0.033 | 0.515 | ||
ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HFF, hepatic fat fraction; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAMFF, paraspinal muscle fat fraction; PMFF, psoas major fat fraction; SATT, subcutaneous adipose tissue thickness; TC, total cholesterol; TG, triglyceride.
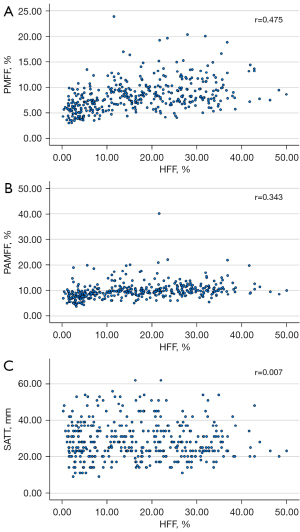
Furthermore, PMFF was positively correlated with age (r=0.155; P=0.002), ALT (r=0.169; P=0.003), AST (r=0.186; P=0.001) and blood glucose (r=0.177; P =0.003). PAMFF was positively correlated with age (r=0.107; P=0.033), ALT level (r=0.118; P=0.040), AST level (r=0.169; P=0.004), and blood glucose level (r=0.138; P =0.020) but negatively correlated with ALB level (r=–0.168; P=0.004). SATT was negatively correlated with age (r=–0.301; P=0.000), TG level (r=–0.171; P=0.003), and ALB level (r=–0.145; P=0.013).
In light of these results, HFF was considered to exhibit a positive correlation with blood glucose level (r=0.144; P=0.015). Both PMFF and PAMFF were positively correlated with blood glucose. Mediation analysis was conducted to investigate whether blood glucose level mediated the relationship between HFF and PMFF and that between HFF and PAMFF. The results indicated that blood glucose level partially mediated the relationship between HFF and PAMFF (indirect effect =0.0046; 95 % CI: 0.0004–0.0130) at 5,000 bootstrap samples. However, blood glucose level did not mediate the relationship between HFF and PAMFF (indirect effect =0.0050; 95 % CI: –0.001 to 0.0112) at 5,000 bootstrap samples.
Interobserver reproducibility
The interobserver agreement of PDFF was found to be good for the psoas major (ICC =0.802; 95% CI: 0.759–0.837), paraspinal muscle (ICC =0.779; 95% CI: 0.731–0.818), and SATT (ICC =0.861, 95% CI: 0.830–0.886).
Discussion
In this study, we investigated the extent of fat deposition in the psoas major muscle, paraspinal muscle, and subcutaneous adipose tissue in young and middle-aged patients with MAFLD using PDFF measurements. Our results revealed a progressive increase in fat deposition in the psoas major and paraspinal muscles with the severity of hepatic steatosis. Additionally, we found that blood glucose levels partially mediated the relationship between HFF and PAMFF.
Skeletal muscle plays a crucial role in the whole-body insulin-mediated glucose disposal, and a reduction in skeletal muscle mass can lead to decreased glucose uptake, contributing to IR (21). IR promotes the uptake of free fatty acids from adipose tissue into the liver, disrupts the suppression of gluconeogenesis, alters TGs transport, and inhibits beta-oxidation, ultimately resulting in hepatic TG accumulation (22). Moreover, skeletal muscle acts as an endocrine organ, releasing myokines, such as interleukin-6, which may have a protective effect against the development of MAFLD (23). Therefore, it is conceivable that diminished skeletal muscle mass could be a contributing factor to the pathogenesis MAFLD. The shared pathophysiological features between skeletal muscle and MAFLD suggest a potential mechanistic link between the two conditions.
In our study, it was observed that patients with MAFLD exhibited a higher level of blood glucose as compared to individuals without MAFLD, and the blood glucose levels increased progressively with the severity of MAFLD. Notably, a statistically significant difference in blood glucose level was observed between individuals classified as G0 and those categorized as G2 and G3, whereas no statistically significant difference was noted between the G0 and G1 categories. Additionally, both PMFF and PAMFF were found to be positively correlated with blood glucose levels. Further mediation analysis indicated that blood glucose partially mediated the association between PAMFF and fat deposition in patients with MAFLD, but not in the case of PMFF. This mediation analysis offers important insights into the potential mechanisms underlying the interplay between MAFLD and skeletal muscle fat deposition. Interestingly, in this study, blood glucose level was not identified as a mediator in the relationship between PMFF and MAFLD. This observation may be attributed to the fact that the fat content of the psoas major muscle is linked not only with MAFLD but also to other factors such as lumbar intervertebral disc degeneration and age. In our study, the higher the PMFF and PAMFF were, the higher the glucose level. Therefore, our study indirectly demonstrates that steatosis is associated with IR. Skeletal muscle is the primary tissue of insulin-mediated glucose utilization, and previous studies have reported that diabetes is significantly prevalent in adults with sarcopenia (24,25).
Several cross-sectional studies have investigated the relationship between skeletal muscle steatosis and MAFLD. For example, Kitajima et al. examined CT muscle attenuation in 208 Japanese patients with biopsy-proven MAFLD and found that intramuscular adipose tissue content increased significantly with the severity of MASH (26). Similarly, Hsieh et al. reported that severe fatty infiltration of skeletal muscle is associated with early MASH and more severe histological features (6,27). In our study; PMFF and PAMFF were positively correlated with HFF, which aligns with the results of these studies. However, we were unable investigate the relationship between hepatic inflammation and skeletal muscle fat content due to the lack of liver biopsies in our study. Our finding suggest a correlation between muscle fat content and hepatic steatosis, highlighting the need for further detailed analysis through liver biopsy to better understand this relationship. Body composition changes with age, leading to expected physiologic changes (28). Fat deposition typically increases with age, with a transition from peripheral to visceral accumulation (29). In males, testosterone plays a role in stimulating muscle regeneration. Testosterone levels decreases by approximately 1% per year, and insufficient testosterone can lead to decreased muscle mass and increased fat content (30,31). Meanwhile, around the age of 50 years, women typically enter menopause, which can impact body fat mass (32). Therefore, in our study, we excluded patients older than 50 years to focus on a specific age group.
Although MAFLD is often associated with obesity, it is important to note that not all patients with MAFLD are obese, and conversely, not all obese individuals have MAFLD. Approximately one-sixth of patients with MAFLD have a normal BMI (33). Therefore, we did not include BMI as a factor in our study and instead used SATT as a measure of obesity. However, we found that SATT was not significantly associated with the severity of MAFLD. This suggests that waist circumference, which can be indirectly reflected by subcutaneous fat thickness, may not fully capture obesity. In a study by Amevor et al. (20), which included patients with chronic liver disease under the age of 25 years, no significant differences in SATT were observed between chronic liver disease, sarcopenic, and non-sarcopenic groups. Choi et al. (34) reported a correlation of the severity of MAFLD with subcutaneous fat area and waist circumference. The discrepancy in findings between these studies may be attributed to the age of the patients included. Our study included patients under 50 years, with a mean age of 35.9 years, which is similar to the patient population in the study by Amevor et al. (20). Additionally, Younossi et al. noted that lean patients with MAFLD are generally younger than are overweight or obese patients, further supporting our research findings (33).
Our study involved several limitations that should be considered in the interpretation of the results. First, the retrospective nature of the analysis meant that we did not collect height or weight data, which could have provided additional insights into the relationship between fat deposition in skeletal muscles and MAFLD. Additionally, the cross-sectional design of the study limited our ability to establish causality between fat content in muscle fat deposition over time in relation to MAFLD progression. Furthermore, the lack of skeletal muscle measurement, such as gait speed or handgrip strength testing, is a limitation of this study. These measurements could have provided valuable information on the functional implications of muscle fat content in patients with MAFLD. Additionally, the impact of physical activity on muscle mass was not assessed, which could have provided important insights into the role of lifestyle factors in muscle fat deposition. Although we excluded age-related changes in fat content, we were unable to differentiate whether the observed increase in muscle fat content was due to MAFLD or a sedentary lifestyle. Future studies should consider the impact of physical activity on muscle fat content in individuals with MAFLD. Moreover, factors such as elevated homeostasis model assessment of IR, chronic inflammation, and vitamin D deficiency, which may contribute to muscle wasting in MAFLD, were not measured in this study. Including these factors in future research could provide a more comprehensive understanding of the relationship between muscle fat content and MAFLD. Finally, in skeletal muscle, lipids are either stored as adipocytes visibly located between muscle groups and muscle fibers and within the muscle fascicles—corresponding to intermuscular adipose tissue—as TGs stored in adipocytes not visibly located outside muscle fibers in the interstitium as extramyocellular lipids (EMCLs), or as lipid droplets in the cytoplasm within muscle fibers, known as intramyocellular lipids (IMCLs). Research suggests that EMCLs and IMCLs have different behaviors. However, PDFF cannot distinguish between them and can only be assessed through magnetic resonance spectroscopy (35).
Conclusions
Our study found there to be a significant association between fat content in skeletal muscles, as measured by PDFF, and HFF in patients with MAFLD. Further research is needed to investigate the progression of MAFLD in relation to changes in skeletal muscle fat content. By addressing the limitations of our study and conducting additional research, we can further elucidate the role of muscle fat deposition in the pathogenesis of MAFLD.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1696/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1696/coif). M.L. is currently employed at Siemens Healthineers Ltd. and reports that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (No. 2021-1062-137). Informed consent from participants was waived due to the retrospective nature of the analysis. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol 2005;43:508-14. [Crossref] [PubMed]
- Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, Hu PJ. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol 2007;13:6419-24. [Crossref] [PubMed]
- Cai W, Wu X, Zhang B, Miao L, Sun YP, Zou Y, Yao H. Serum uric acid levels and non-alcoholic fatty liver disease in Uyghur and Han ethnic groups in northwestern China. Arq Bras Endocrinol Metabol 2013;57:617-22. [Crossref] [PubMed]
- Fang J, Yu CH, Li XJ, Yao JM, Fang ZY, Yoon SH, Yu WY. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front Cell Infect Microbiol 2022;12:997018. [Crossref] [PubMed]
- Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, Fox CS. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33:863-70. [Crossref] [PubMed]
- Hsieh YC, Joo SK, Koo BK, Lin HC, Kim W. Muscle alterations are independently associated with significant fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2021;41:494-504. [Crossref] [PubMed]
- Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012;2012:629637. [Crossref] [PubMed]
- Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 2010;25:513-9. [Crossref] [PubMed]
- Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res 2012;2012:172957. [Crossref] [PubMed]
- Zhao Q, Zmuda JM, Kuipers AL, Jonnalagadda P, Bunker CH, Patrick AL, Youk AO, Miljkovic I. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing 2016;45:529-34. [Crossref] [PubMed]
- Lemos T, Gallagher D. Current body composition measurement techniques. Curr Opin Endocrinol Diabetes Obes 2017;24:310-4. [Crossref] [PubMed]
- Nachit M, Lanthier N, Rodriguez J, Neyrinck AM, Cani PD, Bindels LB, Hiel S, Pachikian BD, Trefois P, Thissen JP, Delzenne NM. A dynamic association between myosteatosis and liver stiffness: Results from a prospective interventional study in obese patients. JHEP Rep 2021;3:100323. [Crossref] [PubMed]
- Martín-Rodríguez JL, Arrebola JP, Jiménez-Moleón JJ, Olea N, González-Calvin JL. Sonographic quantification of a hepato-renal index for the assessment of hepatic steatosis in comparison with 3T proton magnetic resonance spectroscopy. Eur J Gastroenterol Hepatol 2014;26:88-94. [Crossref] [PubMed]
- Imajo K, Kessoku T, Honda Y, Hasegawa S, Tomeno W, Ogawa Y, Motosugi U, Saigusa Y, Yoneda M, Kirikoshi H, Yamanaka S, Utsunomiya D, Saito S, Nakajima A. MRI-Based Quantitative R2(*) Mapping at 3 Tesla Reflects Hepatic Iron Overload and Pathogenesis in Nonalcoholic Fatty Liver Disease Patients. J Magn Reson Imaging 2022;55:111-25. [Crossref] [PubMed]
- Ajmera V, Park CC, Caussy C, Singh S, Hernandez C, Bettencourt R, Hooker J, Sy E, Behling C, Xu R, Middleton MS, Valasek MA, Faulkner C, Rizo E, Richards L, Sirlin CB, Loomba R. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018;155:307-310.e2. [Crossref] [PubMed]
- Palikaras K, Mari M, Petanidou B, Pasparaki A, Filippidis G, Tavernarakis N. Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans. J Lipid Res 2017;58:72-80. [Crossref] [PubMed]
- Zhang Y, Lu D, Wang R, Fu W, Zhang S. Relationship between Muscle Mass/Strength and Hepatic Fat Content in Post-Menopausal Women. Medicina (Kaunas) 2019;55:629. [Crossref] [PubMed]
- Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer CC, Bosy-Westphal A, Müller MJ. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 2015;102:58-65. [Crossref] [PubMed]
- Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015;17:O20-6. [Crossref] [PubMed]
- Amevor AA, Yodoshi T, Trout AT, Dillman JR, Singh R, Jarvis R, Fei L, Liu C, Taylor A, Miethke A, Mouzaki M. Sarcopenia is highly prevalent in children with autoimmune liver diseases and is linked to visceral fat and parent-perceived general health. Liver Int 2022;42:394-401. [Crossref] [PubMed]
- Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819-29. [Crossref] [PubMed]
- Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, Jin SM, Hur KY, Jee JH, Lee MK, Kim JH. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018;68:1755-68. [Crossref] [PubMed]
- Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, Yin S, Lafdil F, Gao B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology 2011;54:846-56. [Crossref] [PubMed]
- Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123-31. [Crossref] [PubMed]
- Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;45:510-8. [Crossref] [PubMed]
- Kitajima Y, Hyogo H, Sumida Y, Eguchi Y, Ono N, Kuwashiro T, Tanaka K, Takahashi H, Mizuta T, Ozaki I, Eguchi T, Kimura Y, Fujimoto K, Anzai KJapan Nonalcoholic Fatty Liver Disease Study Group (JSG-NAFLD). Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol 2013;28:1507-14. [Crossref] [PubMed]
- Hsieh YC, Joo SK, Koo BK, Lin HC, Lee DH, Chang MS, Park JH, So YH, Kim WInnovative Target Exploration of NAFLD (ITEN) Consortium. Myosteatosis, but not Sarcopenia, Predisposes NAFLD Subjects to Early Steatohepatitis and Fibrosis Progression. Clin Gastroenterol Hepatol 2023;21:388-397.e10. [Crossref] [PubMed]
- Heo M, Faith MS, Pietrobelli A, Heymsfield SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999-2004. Am J Clin Nutr 2012;95:594-602. [Crossref] [PubMed]
- Hunter GR, Gower BA, Kane BL. Age Related Shift in Visceral Fat. Int J Body Compos Res 2010;8:103-8. [PubMed]
- Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol 2008;154:522-8. [Crossref] [PubMed]
- Yeap BB. Are declining testosterone levels a major risk factor for ill-health in aging men? Int J Impot Res 2009;21:24-36. [Crossref] [PubMed]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr 1999;70:405-11. [Crossref] [PubMed]
- Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319-27. [Crossref] [PubMed]
- Choi MH, Choi JI, Park MY, Rha SE, Oh SN, Jung SE, Byun JY, Kannengiesser S, Son Y. Validation of intimate correlation between visceral fat and hepatic steatosis: Quantitative measurement techniques using CT for area of fat and MR for hepatic steatosis. Clin Nutr 2018;37:214-22. [Crossref] [PubMed]
- Garcia-Diez AI, Porta-Vilaro M, Isern-Kebschull J, Naude N, Guggenberger R, Brugnara L, Milinkovic A, Bartolome-Solanas A, Soler-Perromat JC, Del Amo M, Novials A, Tomas X. Myosteatosis: diagnostic significance and assessment by imaging approaches. Quant Imaging Med Surg 2024;14:7937-57. [Crossref] [PubMed]


