
Comparative study on the efficacy and safety of ultrasound-guided transluminal (transvaginal/transrectal) and transabdominal core needle biopsy in patients with pelvic masses: a retrospective analysis
Introduction
Pelvic masses may originate from the uterus, ovary, intestine, etc., but most originate from female internal genitalia (1). Due to the deep location of the masses, patients usually have no symptoms or only have non-specific symptoms such as abdominal pain. Initial evaluation typically involves clinical assessment followed by imaging studies, and many cases are incidentally detected during routine health examination (1). Due to the occult onset, about 70–80% of patients with obvious clinical manifestations are already in an advanced stage or have distant metastasis at the time of diagnosis, and thus, the optimal treatment time has passed (2). Early diagnosis is crucial in deciding whether to undergo timely surgery and/or chemotherapy (3,4). The accurate preoperative assessment of pelvic masses plays a crucial role in guiding gynecologists’ treatment decisions and significantly improves patient prognosis.
Currently, imaging examinations combined with preoperative puncture biopsy are commonly used for the diagnosis of pelvic masses. Computed tomography (CT)-guided percutaneous biopsy is considered an effective and safe method for evaluating retroperitoneal and abdominal masses. However, when the masses are located deep in the pelvis, it has some limitations due to limited transabdominal accessibility or limited pelvic spatial imaging. Despite its imaging advantages, magnetic resonance imaging (MRI) is rarely used because it requires special non-magnetic equipment and experience (5). In recent years, ultrasound (US)-guided sampling methods (transabdominal, transvaginal, and transrectal) have attracted extensive attention because of their high flexibility and short operation times (6). US guidance can be used to observe the whole operation process in real time, select the appropriate puncture point, angle, path, and depth, accurately insert the needle into the lesion, and completely control the needle tip. Unlike CT guidance, under US guidance, neither the patient nor the operator is exposed to radiation, and it is an economic, effective, relatively safe, and simple diagnostic method. It provides an effective auxiliary basis for treatment plan formulation and the operation mode selection.
Currently, abdominal fine-needle aspiration is the most common method for the interventional US examination of pelvic masses in the clinic. However, research on this method has several limitations, including small sample sizes, and collected tissue samples of limited quantity and integrity (7). Further, the technical sensitivity of this method is low (8). Conversely, transabdominal core needle biopsy (CNB) has high value in tumor diagnosis and treatment, and can acquire pelvic mass samples that meet the requirements of pathological diagnosis (9). Some scholars have also used CNB for transvaginal (10) and transrectal pelvic masses sampling (11), and reported acceptable diagnostic accuracy in clinical practice.
To date, few direct comparisons have been conducted to determine which methods have higher diagnostic efficacy and a lower incidence of postoperative complications. Thus, this study sought to evaluate and compare the efficacy and safety of US-guided transluminal (transvaginal/transrectal) and transabdominal CNB in patients diagnosed with pelvic masses. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2585/rc).
Methods
General information
This study conducted a retrospective analysis of 305 patients with pelvic masses who were hospitalized at Sichuan Cancer Hospital & Institute from March 2018 to March 2021. The cohort comprised 290 females and 15 males, with ages ranging from 29 to 84 years (mean age: 55.9±11.3 years). Following an initial US evaluation, the patients were selected through a consensus reached by multidisciplinary team discussion. Surgical resection was performed in 284 patients, while the remaining 21 patients were ultimately diagnosed clinically based on the comprehensive evaluation of clinical data, imaging findings, and follow-up. In cases of benign results and in the absence of subsequent surgery, biopsies were considered accurate if the patients had been followed-up for at least 12 months without any suspicion or observation of a malignant condition based on clinical findings or imaging.
The indications for biopsies included: (I) a suspected primary gynecological disseminated tumor; (II) suspected recurrence or progression (in patients with a history of gynecological tumors); (III) a diagnosis of lesions inaccessible by gynecological exploration (e.g., tumors in the vaginal vault, myometrium, or cervix); (IV) suspected secondary metastasis (in patients with a history of non-gynecological tumors); (V) a diagnosis of a solitary lesion (in patients with no history of malignancy); and (VI) research purposes (in cases of known persistent inoperable tumors). Patients were excluded from the study if they met any of the following exclusion criteria: (I) had critical neurovascular structures in the adjacent tissues of the lesion that could not be avoided during the puncture approach; (II) had a known allergy to SonoVue; (III) had severe heart disease (New York Heart Association Class IV) or a critical illness; and/or (IV) had abnormal coagulation function or bleeding tendency.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Review Committee of Sichuan Cancer Hospital & Institute (No. SCCHEC-03-2018-029). All patients understood the US-guided biopsy process and signed the informed consent form prior to the examination.
US examination
The GE Logiq E9 and Philips EPIQ5 ultrasonic diagnostic instrument equipped with a vaginal probe (frequency 5–9 MHz) and abdominal probe (frequency 1–5 MHz) were used. Transabdominal or intraluminal US were used to examine the 305 cases of pelvic masses. If a mass was too large and its margins could not be shown by intraluminal US, combined transabdominal US and intraluminal US were employed. If multiple tumors were present, the tumors with the most complex ultrasonic morphology were included in the study. If the US characteristics of two tumors were similar, the largest tumor or the tumor easily detected by US was selected (12). First, conventional US was used to observe the morphology, size, internal echo of the pelvic mass, and its relationship with the surrounding tissues. Second, color doppler flow imaging (CDFI) was used to observe the internal blood flow and large vessels surrounding the lesion.
CEUS
The contrast agent was injected with sulfur hexafluoride microbubbles (SonoVue, Bracco, Italy), dissolved in 5 mL of 0.9% sodium chloride (NaCl), and fully oscillated to form sulfur hexafluoride (SF6) microbubble suspension. The section that best showed the lesion was selected for angiography, the probe was fixed, the real-time CEUS imaging mode was selected, the gain compensation and gain were adjusted appropriately, and the single-point focus was placed at the deepest part of the image. A bolus injection of 2.4 mL of SonoVue suspension was administered through a peripheral (antecubital) vein, followed immediately by a rapid flush with 5 mL of 0.9% NaCl solution. At the same time, the timer and image storage functions were used to observe the lesion perfusion process in real time, and images were recorded for 3 minutes.
Biopsy
The needle biopsy was performed by two experienced US physicians under the guidance of US. After routine CDFI and CEUS examinations, the contrast agent perfusion area was identified as the puncture target, avoiding adjacent blood vessels, nerves, cystic components, and necrotic areas.
US-guided transluminal CNB (transvaginal/transrectal)
The puncture procedure was performed using an 18-gauge automated core biopsy needle (Bard Max-Core™, Disposable Core Biopsy Instrument) with a needle length of 25 cm. The penetration depth was set to either 15 or 22 mm, depending on the size and location of the lesion.
Transvaginal approach
It was confirmed preoperatively that the patient was non-menstrual. The specific location and adjacent relationship of the pelvic masses were determined by transabdominal scanning with a low-frequency probe. The patient was placed in the lithotomy position for the puncture, with proper padding at the hips, the bladder was emptied, the vulva and vagina were disinfected according to the standards for routine surgical disinfection, towel was laid, and the guide probe was wrapped in a sterile endoscope cuff. To explore the position of the pelvic mass, a puncture frame was then mounted and gently placed in the patient’s vagina, generally in the area of the posterior fornix, according to the low-frequency probe’s guideline direction. Next, the structure and peripheral adjacency of the pelvic mass, and its orientation and distance relative to the vaginal wall puncture site were determined to examine the presence or absence of necrotic areas in the lesion. Avoiding necrotic areas, large blood vessels, and vital tissue organs, the angle was adjusted, and the needle was advanced along the needle path of the piercing frame. The screen displayed the position of the needle tip in real time, and once the target area was reached, the biopsy gun was excited for extraction (Figure 1). The quality of the tissue strip was observed macroscopically, and placed in fixative solution, and at the same time, the guided probe monitored whether there was bleeding in the lesion and surrounding tissues in real time.
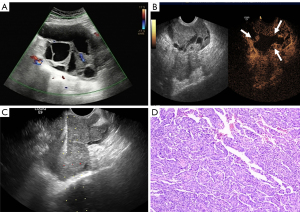
Transrectal approach
The patient underwent intestinal preparation one day before the examination. During the puncture procedure, the patient was placed in the left lateral decubitus position with their knees flexed towards the chest. The area around the anus was disinfected, and 150–200 mL of coupling agent was infused from the anus into the rectal cavity (13). Before the examination, the probe was surface coated with a coupling agent and then covered with a disposable probe cover. After securing the puncture holder and after outer coating with a sterile coupling agent, the probe was slowly inserted into the anus, and a multi-cut, multi-angle scan was used to determine the optimal puncture point and path. The probe was then fixed, and the needle was inserted along the guide hole of the puncture frame. The needle tip was observed reaching the lesion in real time by US (a safe distance between the needle tip and the lesion front ≥24 mm was required), and an excitation biopsy gun was used for sampling (Figure 2). At the same time, the guided probe monitored whether there was bleeding in the lesion or surrounding tissues in real time. According to the quality of the tissue strip, the sampling position was adjusted, materials were taken from multiple angles and points, and the above operation was repeated 3–5 times. The bleeding in the focus area, vagina and anus was observed. After the procedure, the operation area was filled with sterile iodophor sand strip and the patient received postoperative care instructions.
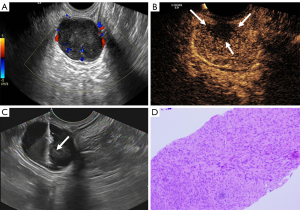
Transabdominal CNB group
A 16–18-gauge automated core biopsy needle (Bard Max-Core™, Disposable Core Biopsy Instrument) was used for the puncture, with a penetration depth of either 15 or 22 mm. The patient was directed to empty their bladder preoperatively, and then placed in a supine position. The location of the mass was located by US, and the body surface was marked to ensure that there were no important tissue organs and large vessels in the puncture path. The skin was routinely sterilized with iodophor, towel was laid, and the point of entry was determined. After local anesthesia with lidocaine hydrochloride (5 mL: 0.01 g), the lesion was percutaneously punctured under real-time ultrasonographic guidance of the parenchymal mass, and the area of contrast medium perfusion was selected. Next, the biopsy gun was excited, the biopsy needle was removed, and the quality of the tissue was visually observed. The percutaneous guided probe was used for real-time monitoring to determine whether there was any bleeding in the intralesional and peripheral tissues. According to the tissue strip mass, the site of extraction was adjusted, and multi angle multi-point extractions were made.
Post-biopsy evaluation
Generally, 2–5 tissue samples were obtained during each biopsy. Samples were considered adequate if they exceeded 0.5 cm in length (14). The tissue specimens were fixed in 10% formaldehyde solution and well-marked for pathology. Biopsy time was defined as the time from the first puncture to the end of the last puncture based on the statistical time of the imaging workstation. To assess the intensity of post-puncture pain, the visual analogue scale (VAS) (on which 0 indicates no pain and 10 indicates intolerable severe pain) was used. Intraoperative blood loss was estimated by visual inspection combined with the area method (15). Fixed size gauze was used. The puncture site was pressed or packed with gauze. A piece of fully saturated gauze (4 inches × 4 inches) was considered to contain 10 mL of blood, while the amount of blood in partially saturated pieces of gauze was calculated based on the assumption that 1 cm2 of gauze absorbed 0.1 mL of blood. These measurements were independently assessed and agreed upon by two investigators blinded to the procedural details.
Post-biopsy management and follow-up
After the transabdominal puncture, the puncture site was pressurized with an abdominal belt. A light liquid diet was maintained for 24 hours post-surgery, and the patients were observed to determine whether or not they had abdominal pain, puncture point bleeding, massive abdominal bleeding, intestinal tube injury, or vaginal bleeding, etc. Data on adverse events (including bleeding, blood transfusion, hospital readmission, emergency surgery, pelvic infection, or death) that occurred within 2 weeks after surgery were extracted from the electronic medical records.
Statistical analysis
All the statistical analyses were performed using SPSS 19.0 software. The quantitative variables are presented as the mean ± standard deviation (SD), while the categorical variables are expressed as the frequency or percentage. Diagnostic performance metrics, including the sensitivity (SEN), specificity (SPE), positive predictive value (PPV), negative predictive value (NPV), and accuracy (defined as the number of correctly diagnosed cases divided by the total number of cases in each group) were calculated and compared between the two groups. The volume of blood loss, pain levels, and incidence of postoperative complications were compared between the two groups. The statistical significance of intergroup differences was assessed using the independent-samples T-test for normally distributed data and the Wilcoxon rank-sum test for non-normally distributed data. The Pearson Chi-squared test and Fisher’s exact test were used for comparisons of categorical variables between two groups. A P value <0.05 was considered statistically significant.
Results
Histologic diagnosis
Transabdominal group
There were 5 benign lesions (5/151, 3.0%), 146 malignant lesions (146/151, 97.0%), 136 gynecologic lesions (136/151, 90.1%), and 15 non-gynecologic lesions (15/151, 9.9%). The malignant lesions included serous carcinoma (n=104), mucinous carcinoma (n=7), endometrioid carcinoma (n=2), clear cell carcinoma (n=5), carcinosarcoma (n=7), metastatic signet ring cell carcinoma (n=4), neuroendocrine carcinoma (n=3), enteric stromal tumor (n=6), cervical squamous cell carcinoma (n=4), pelvic lymphoma (n=3), metastatic prostate cancer (n=1). The benign lesions included endometrioid cystadenoma (n=1), struma ovarii (n=1), ovarian thecoma (n=1), pelvic pheochromocytoma (n=1), and pelvic schwannoma (n=1).
Transluminal group
There were 3 cases of benign lesions (3/154, 2.0%), 151 cases of malignant lesions (151/154, 98.0%), 135 cases of gynecological lesions (135/154, 87.7%), and 19 cases of non-gynecological lesions (19/154, 12.3%). The malignant lesions included serous carcinoma (n=72), mucinous carcinoma (n=6), endometrioid carcinoma (n=5), clear cell carcinoma (n=2), metastatic signet ring cell carcinoma (n=6), cervical squamous carcinoma (n=38, including 34 cases of cervical stump carcinoma), cervical lymphoma (n=1), fallopian tube carcinoma (n=1), endometrial carcinoma (n=2). Uterine leiomyosarcoma (n=1), neuroendocrine carcinoma (n=2), intestinal stromal tumor (n=6), urothelial carcinoma (n=1), malignant mesothelioma (n=1), and malignant peripheral schwannoma (n=1). There were also six cases of pelvic secondary tumors (including one case of metastasis of seminoma, one case of breast cancer, one case of lung cancer, and three cases of rectal cancer). The benign lesions included schwannomas (n=1), uterine leiomyomas (n=1), and pelvic abscesses (n=1).
US-related data characteristics
The long and short diameters of the transabdominal group were larger than those of the transluminal group (long diameter: 107.5±36.1 vs. 65.6±37.6 mm, short diameter: 74.7±26.6 vs. 45.0±23.0 mm, P<0.001). The diagnostic ability of transluminal CNB in deep pelvic lesions was better than that of transabdominal CNB (P<0.001). There were no significant differences in the biopsy time (98.2±28.7 vs. 94.5±27.7 s, P=0.25) and needle number (2.8±0.7 vs. 2.7±0.8, P=0.61) between the transabdominal CNB group and the transluminal CNB group. There was no significant difference in the post-puncture VAS scores (2.8±1.2 vs. 2.9±1.2, P=0.52), all of which indicated mild pain. There was no significant difference between the two groups in terms of blood loss (4.3±1.7 vs. 4.6±1.4 mL, P=0.21). Detailed data are reported in Table 1.
Table 1
| Characteristics | Transluminal group (n=154) | Transabdominal group (n=151) | P* |
|---|---|---|---|
| Age (years) | 55.9±10.9 | 56.0±11.6 | 0.95 |
| Male/female | 7/147 | 8/143 | 0.11 |
| Long diameter of tumor (mm) | 65.6±37.6 | 107.5±36.1 | <0.001 |
| Short diameter of tumor (mm) | 45.0±23.0 | 74.7±26.6 | <0.001 |
| Tumor location | |||
| Ovary and fallopian tube | 92 | 132 | <0.001 |
| Uterus | 43 | 4 | <0.001 |
| Non-gynecological location | 19 | 15 | 0.51 |
| Biopsy time (s) | 98.2±28.7 | 94.5±27.7 | 0.25 |
| Number of puncture needles | 2.8±0.7 | 2.7±0.8 | 0.61 |
| Bleeding parameters | |||
| Platelet (109/L) | 275.3±114.3 | 252.3±108.7 | 0.07 |
| PT (s) | 10.7±0.8 | 10.8±0.8 | 0.22 |
| INR | 0.9±0.1 | 0.9±0.1 | 0.17 |
| APTT (s) | 26.6±5.1 | 26.2±4.9 | 0.50 |
| Blood loss (mL) | 4.3±1.7 | 4.6±1.4 | 0.21 |
| VAS | 2.8±1.2 | 2.9±1.2 | 0.52 |
Data are presented as the number of patients or lesions or mean ± standard deviation. *, quantitative variables were compared using an independent-sample t-test. Pearson’s Chi-squared test and Fisher’s exact test were used for comparisons of categorical variables. APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; VAS, visual analogue scale.
Comparison of diagnostic capabilities
No significant difference in diagnostic accuracy was observed between the transluminal CNB group and the transabdominal CNB group (98.7% vs. 99.3%, respectively). The SEN, SPE, PPV, and NPV of the transluminal CNB group were 98.7% [95% confidence interval (CI): 95.2–99.8%], 100% (95% CI: 47.8–100%), 100% (95% CI: 97.5–100%), 71.4% (95% CI: 29.0–96.3%) respectively, while those of the transabdominal group were 99.3% (95% CI: 96.3–99.9%), 100% (95% CI: 29.2–100%), 100% (95% CI: 97.5–100%), 75.0% (95% CI: 19.4–99.4%), respectively. There were no significant differences in the SEN, SPE, PPV, and NPV between the two groups (Tables 2,3).
Table 2
| Groups | SEN (95% CI) (%) | SPE (95% CI) (%) | PPV (95% CI) (%) | NPV (95% CI) (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Transluminal group | 98.7 (95.2–99.8) | 100 (47.8–100) | 100 (97.5–100) | 71.4 (29.0–96.3) | 98.7 |
| Transabdominal group | 99.3 (96.3–99.9) | 100 (29.2–100) | 100 (97.5–100) | 75.0 (19.4–99.4) | 99.3 |
CI, confidence interval; CNB, core needle biopsy; NPV, negative predictive value; PPV, positive predictive value; SEN, sensitivity; SPE, specificity; US, ultrasound.
Table 3
| Final diagnosis | Transluminal group | Transabdominal group | |||||
|---|---|---|---|---|---|---|---|
| Malignant | Benign | Total | Malignant | Benign | Total | ||
| Malignant | 147 | 2 | 149 | 147 | 1 | 148 | |
| Benign | 0 | 5 | 5 | 0 | 3 | 3 | |
| Total | 147 | 7 | 154 | 147 | 4 | 151 | |
Data are presented as the number of patients or lesions.
Sufficient samples were obtained for histological analysis in all cases. No major complications were observed. In terms of minor complications, vaginal bleeding occurred in 5 patients (3.2%) and abdominal wall hematoma occurred in 2 patients (1.3%); however, these complications resolved spontaneously without further examination or treatment. No patient terminated the biopsy early because of pain or bleeding.
Discussion
Most pelvic masses originate from female internal genitalia. It is reported that about 20% of women will develop pelvic masses at some point in their life (16). However, most women are not aware of pelvic masses until they are diagnosed in a routine physical examination or gynecological examination. Pelvic masses are difficult to diagnose and treat (4). In this study, 88.9% of the pelvic masses originated from the uterus, ovary, and fallopian tube. The female ovaries and uterus have a good acoustic interface. When tumors and swelling occur in these structures, significant alterations in their internal architecture and morphology can be observed. US examinations can detect these changes in a timely manner and provide a preliminary assessment of the nature of the masses (17). However, the internal echo of malignant tumors is diverse. The masses are mostly substantive or mixed, and lack tissue specificity. Further, due to the misdiagnosis rate of US examinations, a combination of other diagnostic methods need to be employed. In various clinical situations (e.g., cases of malignant lesions of unknown origin found in imaging, and cases of suspected recurrence after treatment), non-surgical pathological diagnosis is still essential.
US-guided biopsy has been widely used in the clinical diagnosis of various tumors. This study performed a comparative analysis of the diagnostic efficacy and safety of transabdominal and transluminal US-guided biopsy techniques in the evaluation of pelvic masses. In this study, both modalities had diagnostic accuracy rates exceeding 95%, and no major procedure-related complications necessitating clinical intervention were observed. Further, the biopsy methods had high sensitivity in the detection of malignant lesions. The SEN of both the transluminal CNB and transabdominal CNB methods was very high (98.7% vs. 99.3%). Thus, in tumor patients, US-guided CNB is safe, accurate, and effective, and can replace invasive surgical biopsy.
Our results showed that the mass size (i.e., the long and short diameters) was significantly larger in the transabdominal CNB group than the transluminal CNB group. Transabdominal biopsy is the preferred approach for patients with a large mass volume adjacent to the abdominal wall and those that are not affected by the intestines or major blood vessels. Abdominal and pelvic mass coarse needle biopsy can obtain the amount of specimens required for pathological diagnosis, and has a high value in tumor diagnosis and treatment (9). For a mass with a deep anatomical position, the distance between the probe and pelvic organs is large, and will be affected by uncontrollable factors related to the patient’s body (e.g., flatulence, obesity, and abdominal wall tension). Currently, it is more difficult to show deep pelvic masses than superficial lesions by transabdominal US (18). In the past, this kind of pelvic mass could only be detected by laparoscopy, which was used to obtain pathological tissue. Laparoscopic exploration is an invasive examination, and the willingness of patients to undergo by laparoscopy is low. When transvaginal or transrectal US-guided biopsy is adopted, the probe is closer to the deep mass, the SEN to the lesion is higher, the ultrasonic window is improved, the biopsy distance is shortened, and the boundary, adjacent and internal echo of the mass can be more clearly displayed.
Some scholars (19,20) have applied the transvaginal pelvic mass biopsy technique in clinical practice, and confirmed it to be a safe and effective method. This technique has also been applied to the auxiliary biopsy of endogenous cervical tumors and recurrent cases of cervical cancer postoperatively (21), but research on its use is limited. In our study, 39 cases of cervical and stump recurrent cancer were diagnosed in the transluminal CNB group (including five cases of cervical cancer and 34 cases of stump recurrent cancer). Transluminal biopsy has obvious advantages compared with transabdominal biopsy.
Other scholars have attempted to biopsy pelvic masses by transrectal biopsy (11), which is a viable option for the diagnosis of pelvic masses. Transrectal biopsy was also performed in 11 patients in this study, as the lesions were located in the deep perirectal space, anterior sacral space, etc., or the patients were male. Our results showed that there was no significant difference in the SEN, SPE, PPV, NPV and accuracy between the transluminal CNB group and transabdominal CNB group. Nor was there any significant difference between the two groups in terms of the sampling success rate or the incidence of complications after puncture. Therefore, depending on the size of the mass, the location, the distance from the parietal peritoneum, and potential interference from bowel or major vessels, the selection of the puncture path can improve the success rate of puncture. Consistent with the research results of other scholars (6), we found that given the risk of intestinal injury, transvaginal or transrectal puncture biopsy is safe and feasible for pelvic masses with deep locations and small masses. US-guided biopsies can be performed in a suitable clinic, without anesthesia and with minimal patient discomfort (19,22). The combination of CDFI and CEUS helps to select the most suitable part of the tumor for biopsy during puncture.
CEUS is an advanced imaging modality developed on the basis of two-dimensional US and color doppler US. CEUS shows the vascular distribution patterns of the lesions. Non-enhancing areas on CEUS imaging correspond to necrotic tissue regions, which should be actively avoided during biopsy sampling. Conversely, hyper-enhanced areas should be prioritized for tissue sampling, as these zones typically represent viable tumor tissue with abundant vascularity (23). Over three years, 154 patients with pelvic masses underwent US-guided transvaginal or transrectal biopsies, and 151 patients with pelvic masses underwent US-guided transabdominal biopsies. As a result of our ongoing efforts to educate clinicians about this technology and its satisfactory results, and the practicability of US-guided transluminal or transabdominal biopsies to minimize invasive surgical biopsies, the number of cases is increasing every year.
Based on this study, we conclude that the following factors are key to the successful biopsy of pelvic masses: (I) depending on the size and location of the mass, the appropriate needle path should be selected to avoid adjacent organs and large vascular structures as far as possible; (II) a Bard automatic core biopsy needle with a strong ejection force should be used where possible, and a 16–18-gauge puncture needle should be used as the puncture needle, so that the specimen taken will be more continuous and complete, which will help to meet the pathological diagnosis requirements (24); (III) during puncture sampling, the area with color blood flow signal or the edge of the lesion should be selected as far as possible; (IV) to enable adequate and accurate diagnosis, at least two core biopsies should be taken; and (V) CEUS can be used to distinguish between active and necrotic areas in lesions, and thus is a good supplement to puncture biopsy. In addition, the selection of professionally trained and experienced operators can also improve the adequacy rate of biopsy materials, which was also shown by the research of Verschuere et al. (22).
Consistent with the results of Gao et al. (25), no serious complications were observed in this study. However, theoretically, transrectal and transvaginal approaches are not completely sterile, and there is still uncertainty about the need for prophylactic antibiotics, especially as the rectum is a relatively contaminated environment. Previous reports have suggested that transvaginal surgery may carry a risk of infectious complications (26). Vaginal flora or other pathogens may spread to the pelvic cavity through contaminated probes or biopsy needles (27). Therefore, in this study, oral antibiotics (e.g., third-generation cephalosporins) were used to prevent infection in the transluminal CNB group. Some scholars (28) have reported that the following methods can effectively reduce the incidence of complications: (I) implementing strict indication control prior to puncture. For transrectal biopsy, bowel preparation is required to ensure intestinal cleanliness; (II) implementing some preventive measures (e.g., diluted iodophor enemas in the rectal cavity before puncture, and oral antibiotics after surgery) to reduce the incidence of postoperative infection; and (III) in the process of puncture, attempting to fix the probe and keep the puncture needle parallel to the intestinal wall to reduce the scratch of the normal intestinal wall. Visual and accurate guidance of the needle tip throughout the operation also reduces the risk of complications (29). More research is needs to be conducted to determine the best preparation requirements for biopsy. In this study, three patients had false negative results; the puncture result was necrotic tissue, inflammatory exudate or mucus, but the pathological diagnosis was malignant after operation (including one case of lymphoma and two cases of ovarian adenocarcinoma). The reasons that sufficient samples were not obtained included mass cystic change or non-liquid tumor necrosis (10).
Our study also had some limitations. First, it was a single-center study. Additionally, some patients did not undergo surgery, and there might be data bias. Second, this was a retrospective study, and the number of benign cases was small; thus, further research with an increased sample size needs to be conducted. Third, biopsy has the risk of sampling error. When the biopsy result of highly suspected malignant lesions is non-diagnostic or benign, integrated clinical and imaging surveillance is necessary to minimize the risk of underestimation. Additionally, the possibility of false negative results could not be completely ruled out.
Conclusions
US-guided CNB of pelvic masses is a safe and effective procedure that offers high flexibility in the choice of the puncture route (transabdominal, transvaginal, and transrectal), has a short operation time, and can replace more invasive methods for the evaluation of pelvic lesions. The choice of transvaginal or rectal sampling biopsy for a pelvic mass with a deep location and small size may have high clinical utility.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2585/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2585/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Review Committee of Sichuan Cancer Hospital & Institute (No. SCCHEC-03-2018-029). All patients understood the US-guided biopsy process and signed the informed consent form prior to the examination.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vadlamudi S, Nagendra P. Role of imaging in the evaluation of pelvic masses in female patients. Int J of Adv Res 2024;10:1072-80.
- Jiang H. Clinical value of CT and B-ultrasound in the diagnosis of gynecological pelvic tumors. Chinese Community Doctors 2017;33:103-4.
- Sandri MT, Bottari F, Franchi D, Boveri S, Candiani M, Ronzoni S, Peiretti M, Radice D, Passerini R, Sideri M. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. Gynecol Oncol 2013;128:233-8. [Crossref] [PubMed]
- Hong MK, Ding DC. Early Diagnosis of Ovarian Cancer: A Comprehensive Review of the Advances, Challenges, and Future Directions. Diagnostics (Basel) 2025;15:406. [Crossref] [PubMed]
- Gupta S, Nguyen HL, Morello FA Jr, Ahrar K, Wallace MJ, Madoff DC, Murthy R, Hicks ME. Various approaches for CT-guided percutaneous biopsy of deep pelvic lesions: anatomic and technical considerations. Radiographics 2004;24:175-89. [Crossref] [PubMed]
- Arezzo F, Loizzi V, La Forgia D, Abdulwakil Kawosha A, Silvestris E, Cataldo V, Lombardi C, Cazzato G, Ingravallo G, Resta L, Cormio G. The Role of Ultrasound Guided Sampling Procedures in the Diagnosis of Pelvic Masses: A Narrative Review of the Literature. Diagnostics (Basel) 2021;11:2204. [Crossref] [PubMed]
- Malmström H. Fine-needle aspiration cytology versus core biopsies in the evaluation of recurrent gynecologic malignancies. Gynecol Oncol 1997;65:69-73. [Crossref] [PubMed]
- Pourabolghasem S, Dastranj A, Asghari N. Accuracy of fine needle aspiration of pelvic masses, A cyto-histological correlation. Int J Anat Appl Physiol 2016;2:39-42.
- Atallah S, Tilahun Y, Monson JR. Real-time stereotactic navigation for the laparoscopic excision of a pelvic neoplasm. Tech Coloproctol 2016;20:599-600. [Crossref] [PubMed]
- Mascilini F, Quagliozzi L, Moro F, Moruzzi MC, De Blasis I, Paris V, Scambia G, Fagotti A, Testa AC. Role of transvaginal ultrasound-guided biopsy in gynecology. Int J Gynecol Cancer 2020;30:128-32. [Crossref] [PubMed]
- Won SY, Kim HS, Park SY. Transrectal or transvaginal ultrasound guided biopsy for pelvic masses: external validation and usefulness in oncologic patients. Ultrasonography 2019;38:149-55. [Crossref] [PubMed]
- Sayasneh A, Wynants L, Preisler J, Kaijser J, Johnson S, Stalder C, Husicka R, Abdallah Y, Raslan F, Drought A, Smith AA, Ghaem-Maghami S, Epstein E, Van Calster B, Timmerman D, Bourne T. Multicentre external validation of IOTA prediction models and RMI by operators with varied training. Br J Cancer 2013;108:2448-54. [Crossref] [PubMed]
- Wu G, Gao Y, Hong H, Wang Y, Li J, Wu W, Liang D. Diagnostic value of 360° intraluminal ultrasound combined with couplant perfusion in rectal tumors. Chinese J Ultrasound Med 2020;36:1028-31.
- Nischal U. Nischal Kc, Khopkar U. Techniques of skin biopsy and practical considerations. J Cutan Aesthet Surg 2008;1:107-11. [Crossref] [PubMed]
- Anya SU, Onyekwulu FA, Onuora EC. Comparison of visual estimation of intra-operative blood loss with haemoglobin estimation in patients undergoing caesarean section. Niger Postgrad Med J 2019;26:25-30. [Crossref] [PubMed]
- Moore RG, Bast RC Jr. How do you distinguish a malignant pelvic mass from a benign pelvic mass? Imaging, biomarkers, or none of the above. J Clin Oncol 2007;25:4159-61. [Crossref] [PubMed]
- Grueneisen J, Umutlu L. Clinical evaluation of female pelvic tumors: Application fields of integrated PET/MRI. Radiologe 2016;56:605-611. [Crossref] [PubMed]
- Park BK. Ultrasound-guided genitourinary interventions: principles and techniques. Ultrasonography 2017;36:336-48. [Crossref] [PubMed]
- Pelayo-Delgado I, Sancho J, Pelayo M, Corraliza V, Perez-Mies B, Del Valle C, Abarca L, Pablos MJ, Martin-Gromaz C, Pérez-Vidal JR, Penades I, Garcia E, Llanos MC, Alcazar JL. Contribution of Outpatient Ultrasound Transvaginal Biopsy and Puncture in the Diagnosis and Treatment of Pelvic Lesions: A Bicenter Study. Diagnostics (Basel) 2023;13:380. [Crossref] [PubMed]
- Wood EJ, Pickhardt PJ, Elissa M, Mankowski Gettle L, Lubner MG. Ultrasound-guided transvaginal biopsies of pelvic lesions: diagnostic yield, safety profile, and technical considerations over a 20-year experience. Abdom Radiol (NY) 2023;48:1154-63. [Crossref] [PubMed]
- Peng W, Li F, Huang W, Cheng Z, Qiu R. Clinical application of transvaginal ultrasound-guided biopsy in the diagnosis of cervical cancer recurrence. Chinese J Ultrasound Med 2019;35:458-60.
- Verschuere H, Froyman W, Van den Bosch T, Van Hoefs M, Kaijser J, Van Schoubroeck D, Van Rompuy AS, Vergote I, Timmerman D. Safety and efficiency of performing transvaginal ultrasound-guided tru-cut biopsy for pelvic masses. Gynecol Oncol 2021;161:845-51. [Crossref] [PubMed]
- Li T, Lu M, Li Y, Yang W. Value of transrectal ultrasound-guided biopsy in endoscopy negative biopsy patients with rectal lesions. BMC Gastroenterol 2023;23:173. [Crossref] [PubMed]
- Du X, Jiang L, Sun Y, Guo J, Li Y, Su L, Hou Y. Clinical application of transvaginal pelvic mass biopsy with rectal guided probe. Journal of Hebei North University 2021;37:28-30. (Natural Science Edition).
- Gao C, Wang L, Zhang C, Li X. Transvaginal/transrectal ultrasound-guided aspiration biopsy for diagnosis of pelvic/pelvic floor tumors in females: A retrospective analysis. Exp Ther Med 2019;18:352-7. [Crossref] [PubMed]
- Mikamo H, Kawazoe K, Sato Y, Itoh M, Tamaya T. Ovarian abscess caused by Peptostreptococcus magnus following transvaginal ultrasound-guided aspiration of ovarian endometrioma and fixation with pure ethanol. Infect Dis Obstet Gynecol 1998;6:66-8. [PubMed]
- M’Zali F, Bounizra C, Leroy S, Mekki Y, Quentin-Noury C, Kann M. Persistence of microbial contamination on transvaginal ultrasound probes despite low-level disinfection procedure. PLoS One 2014;9:e93368. [Crossref] [PubMed]
- Li Y, Hu Z, Wang L, Lu M. Value of transrectal ultrasound guided biopsy for rectal tumors. J Cancer Control Treat 2022;35:262-5.
- Buonomo F, Bussolaro S, de Almeida Fiorillo C, Oliveira de Souza D, Giudici F, Romano F, Romano A, Ricci G. Ultrasound-Guided Tru-Cut Biopsy in Gynecological and Non-Gynecological Pelvic Masses: A Single-Center Experience. J Clin Med 2022;11:2534. [Crossref] [PubMed]


