
Transthoracic echocardiographic Doppler parameters evaluation of mechanical prosthetic tricuspid valve dysfunction
Introduction
Tricuspid valve (TV) replacement remains the definitive treatment for some patients with significant TV disease. The choice between mechanical and bioprosthetic valves is controversial, as each type carries its own risk-to-benefit ratio. Although bioprosthetic valves are more commonly used, research has shown comparable risks of early postoperative mortality, 5-year valve dysfunction, and reoperation rates between biological and mechanical TV prostheses (1). Mechanical prosthetic TV (MPTV) dysfunction is an uncommon but potentially lethal complication following TV replacement (2). Early detection and timely reoperation are vital to improving clinical outcomes.
Current guidelines recommend echocardiography as the first-line imaging modality for assessing MPTV function, particularly a comprehensive transthoracic 2-dimensional (2D), color, and Doppler echocardiography (3-5). Since the TV is positioned anteriorly, transthoracic echocardiography (TTE) is typically an excellent imaging tool for evaluating the TV (6). Although TTE-derived Doppler parameters have been shown to be effective in detecting dysfunction in prosthetic aortic and mitral valves (7,8), their utility in assessing tricuspid prostheses remains less explored. Diagnosing prosthetic TV dysfunction is challenging due to the lack of well-defined diagnostic criteria. The full range of Doppler parameters derived from TTE for normally functioning mechanical and bioprosthetic TVs have been published, including pressure half-time (PHTTV), peak early tricuspid diastolic velocity (E velocity), mean gradient (MGTV), the velocity-time integral (VTITV), the ratio (VTI ratio) of VTITV to the velocity-time integral of the left ventricular outflow tract (VTILOVT), and effective orifice area (EOATV) (6,9-11). According to current guidelines, the cutoff values of these Doppler parameters for identifying possible prosthetic TV dysfunction are extrapolated from data obtained in normally functioning prosthetic TVs (5). By comparing dysfunctional and normally functioning bioprosthetic TVs, our institution published a retrospective study on the use of TTE-derived Doppler parameters to accurately detect bioprosthetic TV dysfunction (12). However, quantitative echocardiographic Doppler data directly from dysfunctional mechanical TV prostheses remain limited. This study aimed to determine the accuracy and optimal cutoff values of TTE-derived Doppler parameters for detecting MPTV dysfunction. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2552/rc).
Methods
Patient selection
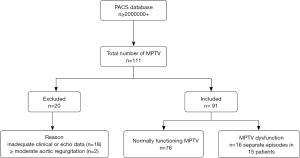
We retrospectively enrolled 111 patients who underwent mechanical TV replacement between January 2012 and December 2023. Data were retrieved from the picture archiving and communication systems (PACS) of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. All patients were regularly followed up at our hospital and demonstrated good medication adherence. Clinical, echocardiographic, and surgical data were collected from the hospital information system and the PACS systems. Patients were categorized into two groups based on the function of MPTV: the normal group [defined as no restricted leaflet motion, no paravalvular leak, and no more than mild transvalvular regurgitation, which were confirmed by transesophageal echocardiography (TEE) or at least 2 stable TTE studies for patients lacking TEE], and the MPTV dysfunction group [defined as aberrant prosthesis structure on 2D TEE (restricted leaflets with thrombus and/or pannus) with a MGTV >6 mmHg, with or without transvalvular regurgitation, and no paravalvular leak]. The MGTV threshold of 6 mmHg was selected based on the 2024 American Society of Echocardiography (ASE) guidelines (5). All cases of MPTV dysfunction were confirmed by TEE and/or surgical inspection. During the period from January 2012 to December 2023, no cases of paravalvular leak in a mechanical TV were observed at our center.
Of the 111 patients, 18 were excluded due to inadequate clinical or echocardiographic data, and 2 were excluded because of moderate or greater aortic regurgitation, which could influence the VTILVOT. Consequently, 91 patients were included in the final analysis, of whom 11 had mild aortic regurgitation and one had mild-to-moderate aortic regurgitation. The study cohort comprised 15 patients with 16 separate episodes of MPTV dysfunction (experimental group) and 76 patients with normal MPTV function (control group). The specific process is displayed in Figure 1. In the experimental group, detailed information on the type and size of the previously implanted mechanical TV prostheses was available for only 5 patients, all of whom had received St. Jude valves. Thus, the limited number of patients restricted the ability to perform subgroup analysis based on valve type and size. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 20230086) and informed consent was provided by all individual participants.
Echocardiography
TTE and TEE were performed using commercial echocardiographic equipment (Philips EPIQ 7C, IE33, Philips Medical Systems, Andover, MA, USA; and Vivid E9, GE Medical Systems, Horten, Norway). Comprehensive 2D, color, and Doppler echocardiography assessments were conducted in accordance with current guidelines (5). Careful evaluation of the MPTV was conducted from all available imaging windows. Standard transthoracic views included the parasternal right ventricle (RV) inflow view, the short-axis view at the aortic valve level, the apical four-chamber view, and the subcostal view. Standard transesophageal views comprised the mid-esophageal four-chamber view, the mid-esophageal inflow-outflow view, the mid-esophageal modified bicaval view, and the transgastric RV inflow-outflow view.
Conventional 2D echocardiographic parameters were measured according to the ASE recommendations (5,13). The diameter of the left ventricular outflow tract (DLVOT) was measured in the parasternal long-axis view. The diameter of the pulmonary artery (DPA) was measured in the short-axis view at the aortic valve level. The right atrial end-systolic diameter (RAESD) and right ventricular end-diastolic diameter (RVEDD) were measured in the apical four-chamber view. We used the modified biplane Simpson method to calculate the left ventricular ejection fraction (LVEF). In addition, 2D echocardiography was utilized to assess prosthesis mobility and morphology, as well as to determine the etiology of MPTV dysfunction (e.g., thrombus or pannus formation) (14).
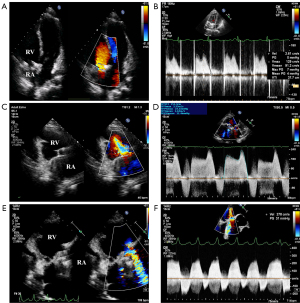
The blood flow patterns across the MPTV were evaluated using color and continuous-wave (CW) Doppler imaging. Representative transthoracic images are displayed in Figure 2. Hemodynamic parameters measured by CW Doppler at the MPTV position included PHTTV, E velocity, MGTV, and VTITV. The VTILVOT was calculated using pulsed-wave (PW) Doppler in the apical five-chamber view. The VTI ratio was derived as the ratio of VTITV to VTILVOT (VTI ratio =VTITV/VTILVOT). Stroke volume (SV) was calculated as the product of VTILVOT and the area of the left ventricular outflow tract (SV = VTILVOT × AREALVOT). The EOA of the MPTV was acquired using the continuity equation (EOATV = SV/VTITV). For individuals with atrial fibrillation, at least 5 cardiac cycles should be measured and averaged. All transthoracic measurements were independently remeasured by 2 investigators using an offline workstation (TomTec software, TOMTEC Imaging Systems, Unterschleißheim, Germany) to ensure reproducibility.
Statistical analysis
The software SPSS 27.0 (IBM Corp., Armonk, NY, USA) and MedCalc 20.022 (MedCalc Software, Ostend, Belgium) were used for data analysis and plotting. Continuous variables were compared using the student t-test or Mann-Whitney U test, depending on their distribution, and were expressed as mean ± standard deviation (SD) or median (interquartile range), respectively. Categorical variables were compared using the chi-square test and reported as frequencies and percentages. Logistic regression analysis was employed to find independent predictors of MPTV dysfunction, with results expressed as odds ratio (OR) and 95% confidence interval (CI). The receiver operating characteristic (ROC) curve was constructed to evaluate the predictive performance of risk factors. We deduced the optimal cutoff values using Youden index and calculated the sensitivity, specificity, and area under the curve (AUC). A P value <0.05 was considered statistically significant.
We randomly selected 20 participants to assess the reproducibility of MGTV and PHTTV measurements using intraclass correlation coefficient (ICC) and Bland-Altman analysis (15). For intra-observer reproducibility, the same investigator repeated the measurements 2 weeks later, blinded to the initial measurements. For inter-observer reproducibility, 2 experienced investigators completed the same procedure of measurement independently in a blinded manner. Excellent reproducibility was defined as an ICC >0.9, as previously described (16).
Results
Baseline characteristics
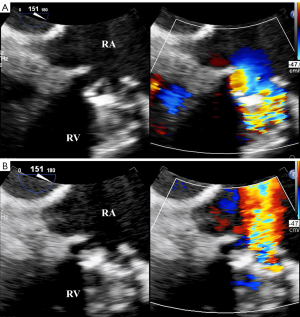
Among the 91 patients who underwent MPTV replacement, 15 patients experienced a total of 16 distinct episodes of mechanical TV dysfunction. Notably, one patient had 2 separate episodes of prosthetic dysfunction, whereas the remaining 14 patients each experienced a single episode. All patients in the MPTV dysfunction group exhibited abnormal leaflet motion and color flow patterns, which were verified by TEE (Figure 3). In the control group, 62 patients (81.6%) underwent intraoperative TEE, which confirmed normal prosthetic valve function, with TTE images subsequently collected within 3 postoperative days. The remaining 14 patients (18.4%) who did not undergo TEE had at least 2 stable TTE studies within 6 months, and showed no clinical evidence of a change in valvular function. All patients in the control group had normally functioning prostheses (representative transesophageal images of normally functioning MPTV are displayed in Figure 4).

A comparison of baseline clinical characteristics was performed between the MPTV dysfunction and control groups, including age, body surface area (BSA), sex, concomitant other valvular heart disease, and New York Heart Association (NYHA) functional class (Table 1). The MPTV dysfunction group had a mean age of 36.6 years and BSA of 1.5 m2, with a male predominance of 31.3%. No significant disparities were observed between the two groups in terms of age, BSA, sex, or concomitant other valvular heart disease (P>0.05). However, the control group demonstrated a higher prevalence of atrial fibrillation compared to the MPTV dysfunction group (56.6% vs. 25%, P=0.022). Additionally, the MPTV dysfunction group exhibited worse functional status, with a greater proportion of patients classified as NYHA III–IV compared to the control group (62.5% vs. 9.2%, P<0.001).
Table 1
| Variables | Dysfunction (n=16 episodes in 15 patients) | Normal (n=76 patients) | P value |
|---|---|---|---|
| Clinical | |||
| Age (years) | 36.6±13.5 | 43.6±10.7 | 0.051 |
| Male | 5 (31.3) | 35 (46.0) | 0.278 |
| BSA (m2) | 1.5±0.3 | 1.6±0.2 | 0.201 |
| Atrial fibrillation | 4 (25.0) | 43 (56.6) | 0.022* |
| Other valvular heart disease | 0.287 | ||
| Moderate or greater mitral regurgitation | 1 (6.3) | 3 (3.9) | |
| Moderate or greater pulmonary regurgitation | 1 (6.3) | 1 (1.3) | |
| NYHA class | <0.001* | ||
| I–II | 6 (37.5) | 69 (90.8) | |
| III–IV | 10 (62.5) | 7 (9.2) | |
| 2D echocardiography | |||
| DLVOT (cm) | 19.7±1.5 | 20.9±2.4 | 0.058 |
| LVEF (%) | 62±7.3 | 60.1±9.0 | 0.423 |
| DPA (cm) | 2.3±0.4 | 2.4±0.4 | 0.077 |
| RAESD (cm) | 6.0±2.0 | 5.0±1.1 | 0.068 |
| RVEDD (cm) | 4.6±1.1 | 4.3±1.0 | 0.182 |
| Doppler echocardiography | |||
| VLVOT (m/s) | 0.8±0.2 | 0.9±0.3 | 0.366 |
| PHTTV (ms) | 298.6±95.3 | 111.3±27.4 | <0.001* |
| E velocity (m/s) | 2.4±0.5 | 1.4±0.3 | <0.001* |
| MGTV (mmHg) | 14.5±6.0 | 3.2±1.5 | <0.001* |
| VTILVOT (cm) | 18.8±3.1 | 20.1±3.4 | 0.155 |
| VTITV (cm) | 112.4±43.7 | 37.7±9.7 | <0.001* |
| VTI ratio | 6.2±2.8 | 1.9±0.6 | <0.001* |
| SV (mL) | 58.3±12.1 | 65.7±12.3 | 0.031* |
| EOATV (cm2) | 0.6±0.3 | 1.8±0.6 | <0.001* |
Data were expressed as the mean ± SD or number (percentage). *, P<0.05. 2D, 2-dimensional; BSA, body surface area; DLVOT, diameter of the left ventricular outflow tract; DPA, diameter of the pulmonary artery; EOATV, effective orifice area of the mechanical prosthetic tricuspid valve; E velocity, early tricuspid diastolic velocity; LVEF, left ventricular ejection fraction; MGTV, mean gradient of the mechanical prosthetic tricuspid valve; NYHA, New York Heart Association; PHTTV, pressure half-time of the mechanical prosthetic tricuspid valve; RAESD, right atrial end-systolic diameter; RVEDD, right ventricular end-diastolic diameter; SD, standard deviation; SV, stroke volume; VLVOT, velocity of the left ventricular outflow tract; VTILVOT, velocity-time integral of the left ventricular outflow tract; VTITV, velocity-time integral of the mechanical prosthetic tricuspid valve; VTI ratio, the velocity-time integral of the mechanical prosthetic tricuspid valve to the velocity-time integral of the left ventricular outflow tract.
Surgical data and etiology of MPTV dysfunction
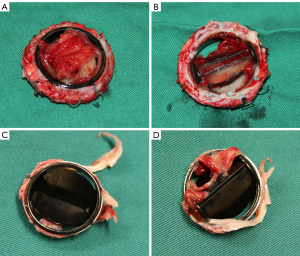
Among patients with MPTV dysfunction, 11 separate reoperations were performed in 10 patients at an average of 9.1 years following their previous surgery, including 10 separate TV replacements and 1 heart transplantation. Surgical inspection revealed that the causes of dysfunction were thrombus in 4 episodes (36.4%), a combination of pannus and thrombus in 6 episodes (54.5%), and pannus formation alone in 1 episode (9.1%). In our study, thrombus was identified as the primary culprit of MPTV dysfunction (90.9%), often coexisting with fibrotic pannus ingrowth. One case with thrombus and another with both thrombus and pannus are shown in Figure 5. Diagnosing MPTV thrombus using TTE or TEE was shown to be difficult. TTE detected thrombus in 4 of 16 episodes (25%), whereas TEE detected thrombus in 5 of 16 episodes (31%). Both modalities demonstrated low detection rates; TEE did not outperform TTE in visualizing MPTV thrombus in our study.
2D echocardiographic characteristics
Conventional 2D echocardiographic parameters were analyzed. Although the RAESD and RVEDD were larger in individuals with MPTV dysfunction compared to those with normal prosthetic function, no significant difference was found between the two groups. Similarly, no notable distinctions were observed in the DLVOT, LVEF, and DPA (P>0.05) (Table 1).
Doppler echocardiographic parameters analysis
All Doppler echocardiographic parameters derived from TTE were analyzed using univariate tests (Table 1). Compared to the control group, patients with MPTV dysfunction exhibited significantly elevated PHTTV (298.6±95.3 vs. 111.3±27.4 ms), E velocity (2.4±0.5 vs. 1.4±0.3 m/s), MGTV (14.5±6.0 vs. 3.2±1.5 mmHg), VTITV (112.4±43.7 vs. 37.7±9.7 cm), and VTI ratio (6.2±2.8 vs. 1.9±0.6), alongside a smaller EOATV (0.6±0.3 vs. 1.8±0.6 cm2). All of these above-mentioned parameters showed significant differences (P<0.001), suggesting that their potential utility as indicators for predicting MPTV dysfunction.
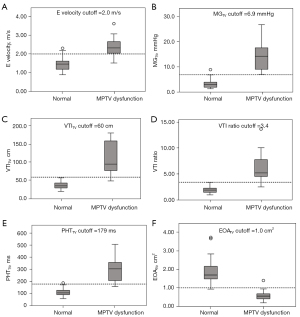
ROC curves were constructed to evaluate the predictive performance of these 6 Doppler parameters, including PHTTV, E velocity, MGTV, VTITV, VTI ratio, and EOATV (Figure 6). Optimal cutoff values for predicting MPTV dysfunction were deduced as follows: PHTTV, 179 ms; E velocity, 2.0 m/s; MGTV, 6.9 mmHg; VTITV, 60 cm; VTI ratio, 3.4; and EOATV, 1.0 cm2 (Table 2). All of these 6 parameters had excellent sensitivity and specificity in detecting MPTV dysfunction, with AUC values exceeding 0.95. MGTV, with a threshold of 6.9 mmHg, exhibited the best predictive capacity (AUC =0.997), demonstrating a sensitivity of 100% and a specificity of 99%, followed by PHTTV (AUC =0.993), VTITV (AUC =0.991), and VTI ratio (AUC =0.991). E velocity (AUC =0.953) and EOATV (AUC =0.986) also showed excellent predictive capacity in detecting MPTV dysfunction. Box plots comparing these above Doppler parameters between the two groups are displayed in Figure 7.
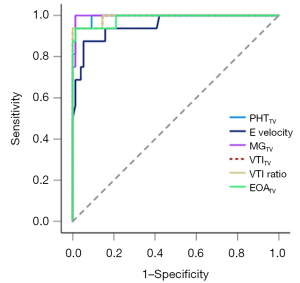
Table 2
| Variables | Cut-off | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|
| PHTTV (ms) | 179 | 94 | 99 | 0.993 (0.98–1.00) |
| E velocity (m/s) | 2.0 | 88 | 95 | 0.953 (0.89–1.00) |
| MGTV (mmHg) | 6.9 | 100 | 99 | 0.997 (0.99–1.00) |
| VTITV (cm) | 60 | 94 | 100 | 0.991 (0.97–1.00) |
| VTI ratio | 3.4 | 94 | 100 | 0.991 (0.97–1.00) |
| EOATV (cm2) | 1.0 | 94 | 99 | 0.986 (0.96–1.00) |
AUC, area under the curve; CI, confidence interval; EOATV, effective orifice area of the mechanical prosthetic tricuspid valve; E velocity, early tricuspid diastolic velocity; MGTV, mean gradient of the mechanical prosthetic tricuspid valve; MPTV, mechanical prosthetic tricuspid valve; PHTTV, pressure half-time of the mechanical prosthetic tricuspid valve; VTITV, velocity-time integral of the mechanical prosthetic tricuspid valve; VTI ratio, the velocity-time integral of the mechanical prosthetic tricuspid valve to the velocity-time integral of the left ventricular outflow tract.
These Doppler parameters were dichotomized using their optimal cutoff values and subsequently analyzed through logistic regression analysis. After the univariable logistic regression analysis, Doppler parameters indicative of MPTV dysfunction included PHTTV >179 ms, E velocity >2.0 m/s, MGTV >6.9 mmHg, VTITV >60 cm, VTI ratio >3.4, and EOATV <1.0 cm2 (P<0.001). The P values, OR, and 95% CI are shown in Table 3. Due to the limited sample size in the experimental group (n=15), multivariate logistic regression analysis was omitted to avoid potential confounding interactions among these 6 Doppler parameters.
Table 3
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| PHTTV >179.0 (ms) | 1,125.00 | 66.61–19,000.84 | <0.001* |
| E velocity >2.0 (m/s) | 54.00 | 11.87–245.59 | <0.001* |
| MGTV >6.9 (mmHg) | 555.00 | 47.24–6,520.75 | <0.001* |
| VTITV >60 (cm) | 1,125.00 | 66.61–19,000.84 | <0.001* |
| VTI ratio >3.4 | 1,125.00 | 66.61–19,000.84 | <0.001* |
| EOATV <1.0 (cm2) | 555.00 | 47.24–6,520.75 | <0.001* |
*, P<0.05. CI, confidence interval; EOATV, effective orifice area of the mechanical prosthetic tricuspid valve; E velocity, early tricuspid diastolic velocity; MGTV, mean gradient of the mechanical prosthetic tricuspid valve; MPTV, mechanical prosthetic tricuspid valve; PHTTV, pressure half-time of the mechanical prosthetic tricuspid valve; VTITV, velocity-time integral of the mechanical prosthetic tricuspid valve; VTI ratio, the velocity-time integral of the mechanical prosthetic tricuspid valve to the velocity-time integral of the left ventricular outflow tract.
Constructing a predictive model of MPTV dysfunction
Based on the findings, PHTTV, E velocity, MGTV, VTITV, VTI ratio, and EOATV were identified as valuable parameters for evaluating MPTV function. A multiparameter algorithm incorporating these parameters was developed to identify MPTV dysfunction (Figure 8). VTITV was excluded from the algorithm, as the VTI ratio theoretically provides superior diagnostic utility compared with VTITV.

Reproducibility
Our study demonstrated excellent intra- and inter-observer reliability for MGTV and PHTTV measurements, as evidenced by ICC >0.9 (Table S1). Bland-Altman plots were used to express the intra- and inter-observer consistency for the measurement of MGTV and PHTTV (Figure S1). Nearly all data points fell within the 95% CI, indicating good consistency.
Discussion
This retrospective study further underscores the utility of TTE-derived Doppler parameters in accurately identifying MPTV dysfunction. By comparing normal and abnormal prosthetic valve function, we established cutoff values suggestive of MPTV dysfunction: PHTTV, 179 ms; E velocity, 2.0 m/s; MGTV, 6.9 mmHg; VTITV, 60 cm; VTI ratio, 3.4; and EOATV, 1.0 cm2. This study provides quantitative echocardiographic Doppler data directly from dysfunctional MPTV prostheses, complementing the ASE guidelines (5), which recommend cutoff values for diagnosing MPTV stenosis or regurgitation based on data extrapolated from normally functioning prostheses.
The ASE guidelines highlight the importance of a significantly prolonged PHT in detecting prosthetic valve stenosis, with a PHT ≥130 ms indicating clinically significant MPTV stenosis (5). We found that a PHT >179 ms was suggestive of MPTV dysfunction; this cutoff value was longer than proposed in previous studies (6,17). In a recent study of 12 patients with MPTV dysfunction, Fadel et al. reported that a PHT >157 ms was frequently associated with MPTV dysfunction (17). Both our study and Fadel et al.’s study, which focused on dysfunctional prostheses, propose a longer PHT threshold value than suggested by the ASE guidelines. This indicates that cutoffs of Doppler parameters indicative of MPTV dysfunction deduced from normally functioning MPTV may be more sensitive but less specific than cutoffs obtained from dysfunctional valves. Compared to the cutoff value reported by Fadel et al., our study indicated a longer PHT for detecting MPTV dysfunction. The discrepancy in PHT threshold value may stem from differences in valve design. In Fadel et al.’s study, patients with MPTV dysfunction were implanted with either Medtronic ATS medical (Santa Ana, CA, USA) or CarboMedics valves (Burnaby, British Columbia, Canada), whereas our study did not impose restrictions on valve type or size. Doppler-derived hemodynamics vary with valve size and type, with newer-generation bileaflet mechanical valves offering better hemodynamics compared with some older mechanical valves (18). Several studies have reported a PHT threshold for different types of prosthetic valve dysfunction, with the value ranging from 119 to 179 ms (6,10,19). Unfortunately, our study was unable to subcategorize results by valve type and size due to limited data. A prolonged PHT has a high diagnostic value of MPTV pathologic obstruction, but it is not useful for distinguishing between normal and regurgitant valves, as a short PHT may occur in both circumstances. Since PHT is affected by right chamber compliance, loading conditions, and heart rate (5), it needs to be interpreted cautiously when used PHT as a stand-alone indicator of MPTV dysfunction.
We proposed that an E velocity >2.0 m/s or MGTV >6.9 mmHg was highly suggestive of MPTV dysfunction, with MGTV demonstrating the highest diagnostic accuracy (AUC =0.997). These cutoff values align with the latest ASE recommendations (5). Elevated velocities and gradients are associated with the severity of valve obstruction (20). However, elevated E velocity or MG alone does not prove intrinsic valve blockage but may be related to significant valve regurgitation, high flow states (e.g., hyperthyroidism, anemia, sepsis), or patient-prosthesis mismatch (PPM) (5,21), resulting in a low level of evaluation specificity. PPM occurs when a prosthetic valve is too small relative to the patient’s body size, leading to markedly elevated E velocity and MG despite normal prothesis function, thereby causing functional stenosis (5,22). In such cases, PHT typically remains within the normal range, aiding in distinguishing PPM from pathological stenosis (5).
VTITV and VTILVOT are both affected by SV. However, the VTI ratio is a less flow-related parameter, making it particularly valuable for detecting prosthetic valve dysfunction (7,23). In high flow states, the E velocity, MGTV, and VTITV demonstrate a significant increase, whereas the VTI ratio remains within the normal range. The VTI ratio will elevate in the context of valve stenosis or regurgitation (23), making it a useful parameter for confirming prosthetic valve dysfunction. Our study identified a VTI ratio >3.4 as indicative of MPTV dysfunction, which represents a cutoff value higher than that reported in a previous study (3.4 vs. 2.3) (17). The inclusion of prosthetic valves with different types have contributed to the disparity between these 2 studies. Blauwet et al. reported that the calculated cutoff value for VTI ratio predictive of MPTV dysfunction varies depending on valve type, with the threshold ranging from 2.0 to 3.1 (6). This value was found to be even higher in some older prosthesis valves (7).
The EOA has recently been recognized as a valuable parameter for assessing valvular dysfunction, with the continuity-derived EOA calculation method being routinely recommended. This method is most accurate in cases where there is mild or less tricuspid regurgitation and aortic regurgitation (5). Compared to other Doppler parameters, EOATV exhibited relatively lower specificity in this study, likely because continuity-derived EOATV requires measuring the area of the left ventricular outflow tract, which is more susceptible to measurement error or bias. We propose that an EOATV <1.0 cm2 is indicative of MPTV dysfunction; this cutoff value was comparable to Fadel et al.’s study (1.1 cm2) but smaller than the ASE guidelines (2.0 cm2) (5,17). The continuity-derived EOA should be calculated at early post-implantation and during follow-up to evaluate potential prosthesis stenosis. We propose that if the continuity-derived EOA <1.0 cm2 or has decreased from baseline, pathological MPTV obstruction should be suspected.
Since Doppler parameters derived from TTE are influenced by multiple factors, the diagnosis of MPTV dysfunction should not rely on a single parameter. The more abnormal Doppler parameters present, the higher the likelihood of MPTV dysfunction. This study emphasizes the clinical utility of a multiparameter algorithm incorporating PHTTV, E velocity, MGTV, VTI ratio, and EOATV to discriminate dysfunctional from normally functioning MPTV. Unlike Fadel et al.’s study, this is the first study to focus on patients with MPTV dysfunction confirmed by TEE and/or surgical inspection, thereby complementing existing research.
Limitations
First, this was a single-center, retrospective study involving a relatively small cohort of patients with MPTV dysfunction. Consequently, the generalizability of the proposed algorithm requires further validation through multi-center, large-scale, prospective studies to confirm these findings. Second, valve obstruction was present in all cases with MPTV dysfunction in this study, and data on MPTV regurgitation (defined as moderate or greater tricuspid regurgitation with unrestricted leaflet movement) were lacking. Third, selection bias is an inherent limitation of this research. Only patients with MPTV dysfunction identified by TEE were enrolled, potentially missing individuals with valve dysfunction who did not undergo TEE. Fourth, the study demonstrated higher thresholds for Doppler parameters in diagnosing MPTV dysfunction, suggesting that patients presented with clinically significant symptoms. However, given the disease progression, which typically ranges from asymptomatic to severe symptomatic states, a higher diagnostic threshold could result in delayed diagnosis. Finally, TTE-derived Doppler parameters can be influenced by valve type and size, whereas the limited information available for patients with MPTV dysfunction restricted the ability to perform subgroup statistical analysis based on valve type and size.
Conclusions
TTE-derived Doppler parameters, including PHTTV >179 ms, E velocity >2.0 m/s, MGTV >6.9 mmHg, VTITV >60 cm, VTI ratio >3.4, and EOATV <1.0 cm2, represent valuable diagnostic parameters for identifying MPTV dysfunction. We propose a multiparameter algorithm that combines PHTTV, E velocity, MGTV, VTI ratio and EOATV to differentiate malfunctioning MPTV from normally functioning prostheses. This algorithm may serve as a practical aid for clinicians in the routine evaluation of MPTV function. Further prospective studies are warranted to validate these findings and refine the diagnostic criteria.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2552/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2552/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 20230086) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Negm S, Arafat AA, Elatafy EE, Fawzy HF. Mechanical Versus Bioprosthetic Valve Replacement in the Tricuspid Valve Position: A Systematic Review and Meta-Analysis. Heart Lung Circ 2021;30:362-71. [Crossref] [PubMed]
- Rossello X, Muñoz-Guijosa C, Mena E, Camprecios M, Mendez AB, Borras X, Padro JM. Tricuspid valve replacement with mechanical prostheses: Short and long-term outcomes. J Card Surg 2017;32:542-9. [Crossref] [PubMed]
- Blauwet LA, Miller FA Jr. Echocardiographic assessment of prosthetic heart valves. Prog Cardiovasc Dis 2014;57:100-10. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Zoghbi WA, Jone PN, Chamsi-Pasha MA, Chen T, Collins KA, Desai MY, Grayburn P, Groves DW, Hahn RT, Little SH, Kruse E, Sanborn D, Shah SB, Sugeng L, Swaminathan M, Thaden J, Thavendiranathan P, Tsang W, Weir-McCall JR, Gill E. Guidelines for the Evaluation of Prosthetic Valve Function With Cardiovascular Imaging: A Report From the American Society of Echocardiography Developed in Collaboration With the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2024;37:2-63. [Crossref] [PubMed]
- Blauwet LA, Burkhart HM, Dearani JA, Malouf JF, Connolly HM, Hodge DO, Herges RM, Miller FA Jr. Comprehensive echocardiographic assessment of mechanical tricuspid valve prostheses based on early post-implantation echocardiographic studies. J Am Soc Echocardiogr 2011;24:414-24. [Crossref] [PubMed]
- Spencer RJ, Gin KG, Tsang MYC, Tsang TSM, Nair P, Lee PK, Jue J. Doppler Parameters Derived from Transthoracic Echocardiography Accurately Detect Bioprosthetic Mitral Valve Dysfunction. J Am Soc Echocardiogr 2017;30:966-973.e1. [Crossref] [PubMed]
- Thomassen HK, Cioffi G, Gerdts E, Einarsen E, Midtbø HB, Mancusi C, Cramariuc D. Echocardiographic aortic valve calcification and outcomes in women and men with aortic stenosis. Heart 2017;103:1619-24. [Crossref] [PubMed]
- Shapira Y, Nili M, Hirsch R, Vaturi M, Vidne B, Sagie A. Mid-term clinical and echocardiographic follow up of patients with CarboMedics valves in the tricuspid position. J Heart Valve Dis 2000;9:396-402.
- Sezai A, Shiono M, Akiyama K, Orime Y, Hata H, Yagi S, Tsukamoto S, Nakata K, Hata M, Negishi N, Sezai Y. Doppler echocardiographic evaluation of St. Jude Medical valves in the tricuspid position: criteria for normal and abnormal valve function. J Cardiovasc Surg (Torino) 2001;42:303-9.
- Blauwet LA, Danielson GK, Burkhart HM, Dearani JA, Malouf JF, Connolly HM, Hodge DO, Herges RM, Miller FA Jr. Comprehensive echocardiographic assessment of the hemodynamic parameters of 285 tricuspid valve bioprostheses early after implantation. J Am Soc Echocardiogr 2010;23:1045-1059, 1059.e1-2.
- Zhang X, Peng L, Fang L, Xu J, Wang J, Sun W, Gao T, Li Y, Zhang L, Lv Q, Xie M, Wu W. Transthoracic echocardiographic Doppler metrics in evaluating bioprosthetic tricuspid valve dysfunction. Echocardiography 2024;41:e15835. [Crossref] [PubMed]
- Chen M, Zhang X, Li G, Wang Y, Kong F, Ma C. Ventricular dysfunction consequences of mechanical dyssynchrony in isolated complete right bundle branch block versus left bundle branch block. Quant Imaging Med Surg 2024;14:5650-64. [Crossref] [PubMed]
- Maragiannis D, Aggeli C, Nagueh SF. Echocardiographic Evaluation of Tricuspid Prosthetic Valves: An Update. Hellenic J Cardiol 2016;57:145-51. [Crossref] [PubMed]
- Matsuda K, Hoshino M, Usui E, Hanyu Y, Sugiyama T, Kanaji Y, Hada M, Nagamine T, Nogami K, Ueno H, Sayama K, Sakamoto T, Yonetsu T, Sasano T, Kakuta T. Noninvasive transthoracic doppler flow velocity and invasive thermodilution to assess coronary flow reserve. Quant Imaging Med Surg 2024;14:421-31. [Crossref] [PubMed]
- Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155-63. [Crossref] [PubMed]
- Fadel BM, Alassas K, Clavel MA, Ayas MF, Kazzi BE, Pibarot P, Mohty D. Multiparametric Approach for the Assessment of Mechanical Prosthetic Tricuspid Leaflet Function. J Am Soc Echocardiogr 2022;35:206-16. [Crossref] [PubMed]
- Halkos ME, Puskas JD. Are all bileaflet mechanical valves equal? Curr Opin Cardiol 2009;24:136-41. [Crossref] [PubMed]
- Aoyagi S, Nishi Y, Kawara T, Oryoji A, Kosuga K, Ohishi K. Doppler echocardiographic evaluation of St. Jude Medical valves in the tricuspid position. J Heart Valve Dis 1993;2:279-86.
- Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:589-90. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Doppler echocardiographic evaluation of prosthetic valve function. Heart 2012;98:69-78. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000;36:1131-41. [Crossref] [PubMed]
- Fernandes V, Olmos L, Nagueh SF, Quiñones MA, Zoghbi WA. Peak early diastolic velocity rather than pressure half-time is the best index of mechanical prosthetic mitral valve function. Am J Cardiol 2002;89:704-10. [Crossref] [PubMed]


