
Association between deep gray matter iron deposition, white matter hyperintensity, and hypertensive cognitive impairment
Introduction
Hypertension is one of the most prevalent chronic diseases and can induce complex pathological changes in cerebral microvessels linked to cognitive impairment (1). Magnetic resonance imaging (MRI) can detect white matter hyperintensity (WMH)—a common imaging marker of microangiopathy visible on T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) sequences—which is highly prevalent in hypertensive patients (2). WMH can increase the risk of Alzheimer’s disease, vascular cognitive impairment, and affect many cognitive abilities such as executive function and attention (3-5). Meanwhile, iron deposition in deep gray matter (DGM) is a common pathological hallmark of the aging brain, and is thought to be related to neurodegeneration and cognitive impairment (6,7). There is increasing evidence that vascular abnormities may be an important mechanism of iron deposition in DGM (8-10). Research has shown that ferritin and hemosiderin in the striatum are distributed along arterioles (11). WMH and iron deposition often occur together in patients with hypertension (12). Therefore, further research needs to be conducted to determine whether WMH and iron deposition dependently or independently affect cognitive impairment.
Both WMH and iron deposition in DGM increase with age (4,6). Despite sharing overlapping pathophysiological mechanisms, such as disruption of the blood-brain barrier (BBB) (8,13), WMH and iron deposition are currently considered distinct pathological entities. A study of older adults showed that both WMH volume and iron deposition are associated with cognitive impairment (11). Another study of a normal-aged population showed that WMH volume did not mediate the association between iron and cognitive impairment (14). Together, these results suggest that WMH and iron deposition may independently deleteriously affect cognitive function. However, these studies only analyzed relatively healthy subjects, who might only have had mild iron deposition.
Previous studies have shown that iron deposition may be more obvious in hypertensive patients than healthy subjects (15,16). More importantly, previous studies have reported that the total volume or WMH rating is positively associated with some DGM iron deposition (12,17). Furthermore, a stronger association between WMH and DGM iron deposition has been observed in individuals with hypertension (12). However, it is unclear whether the association between iron deposition and cognitive impairment in hypertensive patients is affected by WMH.
Quantitative susceptibility mapping (QSM) is an advanced MRI technique that can be used to non-invasively quantify iron deposition, and its accuracy has been validated in autopsies and multiple clinical studies (18-20). In this study, QSM was used to quantify both global and regional iron content in major DGM. In addition, longitudinal studies have shown that vision-based scores of cerebral small vessel disease (CSVD) can effectively predict the risk of cognitive impairment and dementia (21). Thus, the severity of WMH was assessed using the Fazekas scale. This study aimed to (I) explore the distribution pattern and severity of DGM iron deposition and WMH in hypertensive patients; and (II) analyze the cognitive impairment resulting from WMH and iron deposition, as well as the potential interaction between these signs. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1610/rc).
Methods
Participants
This prospective observational study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. UHCT21811). All the participants in this study provided informed consent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
From September 2019 to October 2023, we prospectively recruited a total of 178 patients with primary hypertension, who were treated at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Following a 5-minute rest period, the patients’ blood pressure was measured three times using a portable monitor, and the mean value was calculated. Hypertension was diagnosed based on the following criteria: systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg at rest, or the use of antihypertensive medication (22).
Patients were excluded from the study if they met any of the following exclusion criteria: (I) had secondary hypertension caused by renal or endocrine disorders; (II) had obvious brain parenchymal lesions, including symptomatic infarcts, intracerebral hemorrhages >10 mm, brain tumors, or other discernible intracranial lesions, or a documented history of head surgery or trauma; (III) had multiple sclerosis, Parkinson’s disease, end-stage renal disease, or malignancies of other systems; (IV) failed to complete the MRI examination or cognitive function assessment; and/or (V) had missing or poor-quality MRI images that could not be effectively evaluated.
In total, 73 hypertensive patients were excluded from the study based on the aforementioned criteria. Ultimately, 105 patients (62 males, 43 females), who had been diagnosed with hypertension for at least 1 year and were taking antihypertensive medication regularly, were included in the analysis. Additionally, 31 age-, sex-, and education-matched healthy controls (HCs) were included in the analysis, and their clinical data were recorded.
MRI data acquisition
All the participants underwent brain imaging using a 3.0-T magnetic resonance scanner (Siemens Healthcare, Skyra, Erlangen, Germany) with a cranial 32-channel coil. The strategically acquired gradient echo (STAGE) comprised two fully flow compensated double-echo gradient echo acquisitions in 336 seconds, and the parameters of the sequence were as follows (23,24): (I) axial double-echo proton density weighted (PDW) imaging: repetition time (TR) =25 ms, echo time (TE) =7.5/17.5 ms, field of view (FOV) =256 mm × 256 mm, flip angle =6°, matrix =384×144, voxel size =0.67 mm × 1.33 mm × 2.0 mm, slice thickness =2 mm, number of slices =64; (II) axial double-echo T1-weighted (T1W) imaging: TR =25 ms, TE =8.75/18.75 ms, FOV =256 mm × 256 mm, flip angle =24°, matrix =384×144, voxel size =0.67 mm × 1.33 mm × 2.0 mm, slice thickness =2 mm, number of slices =64. Axial FLAIR images for WMH evaluation were acquired using the following parameters: TR =9,000 ms, TE =95 ms, FOV =220 mm × 220 mm, matrix =256×256, slice thickness =6 mm, number of slices =19, and scanning time =130 seconds. Additionally, axial T1W (TR =3,570 ms, TE =165 ms, FOV =220 mm × 220 mm, matrix =320×256, slice thickness =6 mm, number of slices =19, and scanning time =94 seconds), diffusion weighted (TR =4,400 ms, TE =83 ms, FOV =220 mm × 220 mm, matrix =160 mm × 160 mm, slice thickness =5 mm, number of slices =25, and scanning time =92 seconds), and sagittal T2W (TR =3,570 ms, TE =165 ms, FOV =220 mm × 220 mm, matrix =320×256, slice thickness =6 mm, number of slices =19, and scanning time =60 seconds) images were obtained to visualize the anatomy and other brain lesions.
MRI post-processing and measurement
The scanned original digital imaging and communications in medicine images were preprocessed to establish the STAGE pipeline using the SPIN software (SpinTech MRI, Bingham Farms, MI, USA), and the PDW and T1W images were used to reconstruct QSM using the STAGE software (version 2.1.4). The specific steps (25) comprised: (I) skull removal and phase unwrapping; (II) background field removal; and (III) QSM reconstruction using iterative susceptibility weighted imaging and mapping. Besides QSM, enhanced T1W contrast, T1-mapping, PD-mapping, true-susceptibility weighted imaging (tSWI), and other images were reconstructed using PDW and T1W original images.
In this study, three experienced neuroradiologists (i.e., W.W., Y.S., and J.K. with 7, 4, and 4 years of experience, respectively), identified the regions of interest (ROIs) using the SPIN software. When defining the ROIs, blood vessels and adjacent cerebral ventricles were excluded. Susceptibilities were reported in parts per billion (ppb) for the bilateral caudate nucleus (CN) head, putamen (PU), globus pallidus (GP), thalamus (TH), red nucleus (RN), substantia nigra (SN), dentate nucleus (DN), and frontal white matter (FWM), which was used as a reference. The final global region (RI) susceptibilities were obtained by taking the average of the measurements made by the three neuroradiologists. By setting the threshold for iron concentration in the ROIs, the high-iron content region (RII) susceptibilities were calculated automatically by incorporating the mean susceptibilities for a specific age group with one standard deviation (Figure 1) (19,26,27).
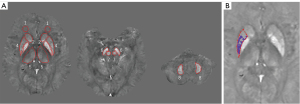
CSVD was evaluated by two veteran neuroradiologists (C.L. and W.W. with 10 and 7 years of experience in neuroradiology, respectively) using visual scales. For inconsistent results, a third senior neuroradiologist (L.W. with 15 years of experience) made a decision after re-evaluation. According to the Fazekas scale (28), WMH was divided into paraventricular WMH (PWMH; 0–3 points) and deep WMH (DWMH, 0–3 points), and the two scores were added to obtain the overall score (0–6 points).
tSWI was used to assess cerebral microbleeds, and the majority of lesions (2–5 mm diameter) exhibit low intensity, with a maximum diameter not exceeding 10 mm. The Microbleed Anatomical Rating Scale was used to record the location and number of the cerebral microbleeds (29). A recent small subcortical infarct was defined as a high signal <20 mm on diffusion-weighted imaging, and a lacune (of presumed vascular origin) was defined as a cerebrospinal fluid signal of 3–15 mm with a peripheral high FLAIR signal. Finally, the total burden of CSVD (scores of 0–3), including WMH, lacunes, and cerebral microbleeds, was calculated by referring to the simple CSVD scores as described by Amin Al Olama et al. (21).
Scores of cognitive function and grouping
All the participants completed the Montreal Cognitive Assessment (MoCA) (Beijing version). The scale comprises seven parts (i.e., visuospatial-executive function, naming, delayed recall, attention, language, abstraction, and orientation), and has a total possible score of 30 points. The scale was completed independently by the participants without the help of family members, and the test took about 10 minutes to complete. The MoCA serves as a rapid screening tool for cognitive impairment and has demonstrated great sensitivity in screening for mild cognitive impairment and early dementia (30). Given the important effect of cultural background on cognitive impairment, the optimal cut-off values in diagnosing cognitive impairment were 13/14 for illiterate participants, 19/20 for participants with 1–6 years of education, and 24/25 for participants with 7 or more years of education (31). Based on the diagnosis of hypertension and cognitive impairment, the participants were divided into the hypertension with cognitive impairment (HTN-CI), hypertension without cognitive impairment (HTN-NCI), and HC groups.
Statistical analysis
All the clinical data and susceptibilities were subjected to statistical analysis using SPSS (version 25.0; IBM Corp.; Chicago, IL, USA). The normality of the continuous data was tested using the Shapiro-Wilk method. The continuous data are presented as the mean ± standard deviation. The categorical variables are described as the frequency (%). Analyses of variance and Chi-squared tests were used to compare the clinical data and susceptibilities among the three groups, and multiple comparisons were performed using the Bonferroni method, and the P value was automatically adjusted. To assess the inter-observer agreement of susceptibilities, we calculated the intra-class correlation (ICC) coefficient. The t-test was used to compare the difference in the duration of hypertension between the HTN-CI and HTN-NCI groups. Age and education years were set as the covariates, and a partial correlation analysis was conducted to investigate the correlation between iron deposition and WMH scores in the HTN-CI group. Univariate analyses of cognitive score-associated factors were conducted using the t-test or a Spearman correlation analysis. The WMH scores and susceptibilities of different regions were set as the independent variables, and total MoCA scores were set as the dependent variables using multivariate linear regression models. Two models were constructed to control for possible confounding effects: Model A, which adjusted for age and education; Model B, which adjusted for age, education, cerebral microbleed, and lacune/recent small subcortical infarcts. Finally, an exploratory mediation analysis was conducted to investigate the potential mediating role of WMH scores in the relationship between susceptibilities and MoCA scores. To assess multicollinearity, we calculated the variance inflation factor (VIF). The P value of the ROI analysis results were corrected using the false discovery rate, and a P value <0.05 was considered statistically significant.
Results
Clinical data of patients
In total, 178 hypertensive patients were included in this study, and 73 patients were excluded due to incomplete clinical or cognitive data (n=5), intracerebral lesions (n=33), and Parkinson’s disease or end-stage renal disease (n=15), and incomplete or poor-quality images (n=20). Thus, ultimately, 105 hypertensive patients and 31 matched HCs were included in the study.
There were no statistically significant differences among the three groups in terms of age, sex, body mass index (BMI), DBP, and the proportion of smoking. Nor was there any statistically significant difference in the duration of hypertension between the HTN-CI and HTN-NCI groups. Compared to the HC group, the HTN-NCI group had higher SBP, and a higher proportion of patients with hyperlipidemia and diabetes (P<0.05). Compared to the HC group, the HTN-CI group had higher SBP and a higher proportion of patients with hyperlipidemia (P<0.05).
There were significant differences in the overall WMH, PWMH, and DWMH scores among the three groups. Further, pairwise comparisons showed that the HTN-CI and HTN-NCI groups had higher overall WMH, PWMH, and DWMH scores than the HC group (P<0.05). The HTN-CI group had a higher proportion of cerebral microbleeds than the HTN-NCI and HC groups (P<0.05). There were no statistically significant differences among the three groups in terms of lacune/recent small subcortical infarction. Compared to the HC group, the HTN-CI and HTN-NCI groups had more severe simple CSVD scores, but the difference was not statistically significant.
Neuropsychologically, the patients in the HTN-CI group had significantly lower scores than those in the HTN-NCI and HC groups in terms of the total MoCA score, and the visuospatial-executive, naming, attention, language, and orientation scores; while compared to the patients in the HTN-NCI group, those in the HTN-CI group had lower scores of abstraction (P<0.05) (Table 1).
Table 1
| Variables | HTN-CI group (n=55) | HTN-NCI group (n=50) | HC group (n=31) | P value |
|---|---|---|---|---|
| Age (years) | 60.45±8.18 | 57.02±8.64 | 57.29±7.83 | 0.073 |
| Male† | 32 (58.2) | 30 (60.0) | 23 (74.2) | 0.385 |
| BMI (kg/m2) | 24.29±3.68 | 24.96±3.29 | 24.62±2.44 | 0.588 |
| Smoking† | 41 (74.5) | 29 (58.0) | 9 (29.0) | 0.176 |
| SBP (mmHg) | 136.85±16.03 | 139.32±22.17 | 126.39±18.30 | 0.011*¶ǁ |
| DBP (mmHg) | 82.43±10.74 | 95.90±13.13 | 82.68±10.99 | 0.271 |
| Hyperlipidemia† | 16 (29.1) | 18 (36.0) | 1 (3.2) | 0.004*¶ǁ |
| Diabetes† | 10 (18.2) | 13 (26.0) | 1 (3.2) | 0.033*ǁ |
| Education (years) | 9.64±3.99 | 9.46±5.14 | 9.94±3.33 | 0.871 |
| Hypertension duration (years)‡ | 7.71±7.37 | 9.34±7.38 | – | 0.260 |
| Overall WMH scores | 2.65±1.18 | 2.23±1.08 | 1.32±0.75 | <0.001*¶ǁ |
| PWMH scores | 1.51±0.74 | 1.28±0.61 | 0.84±0.51 | <0.001*¶ǁ |
| DWMH scores | 1.14±0.58 | 0.95±0.59 | 0.47±0.50 | <0.001*¶ǁ |
| Cerebral microbleed† | 14 (25.5) | 4 (8.0) | 4 (12.9) | 0.020* |
| Lacune/recent small subcortical infarct† | 16 (29.1) | 13 (26.0) | 4 (12.9) | 0.228 |
| Simple CSVD scores† | 0.112 | |||
| 1 | 30 (54.5) | 34 (68.0) | 24 (77.4) | |
| 2 | 17 (30.9) | 14 (28.0) | 6 (19.4) | |
| 3 | 8 (14.5) | 2 (4.0) | 1 (3.2) | |
| Total MoCA scores | 18.33±3.87 | 25.26±3.08 | 27.00±1.29 | <0.001*§¶ |
| Visuospatial-executive | 2.76±1.37 | 3.82±1.12 | 4.39±0.76 | <0.001*§¶ |
| Naming | 2.42±0.92 | 2.84±0.37 | 2.84±0.45 | 0.002*§¶ |
| Attention | 4.20±1.37 | 5.54±0.84 | 5.45±0.72 | <0.001*§¶ |
| Language | 1.60±1.08 | 2.38±0.73 | 2.35±0.66 | <0.001*§¶ |
| Abstraction | 0.89±0.83 | 1.28±0.78 | 1.26±0.73 | 0.031*§ |
| Delayed recall | 1.07±1.45 | 3.50±1.23 | 3.29±0.82 | <0.001*§¶ |
| Orientation | 5.38±0.85 | 5.90±0.30 | 6.00±0.00 | <0.001*§¶ |
Data are presented as the mean ± standard deviation or n (%). †, P value was obtained using the Chi-squared test, and the Bonferroni method was used for pairwise comparisons. ‡, P value was obtained using the t-test. §, significant differences between the HTN-CI and HTN-NCI groups. ¶, significant differences between the HTN-CI and HC groups. ǁ, significant differences between the HTN-NCI and HC groups. *, P value <0.05. BMI, body mass index; CSVD, cerebral small vessel disease; DBP, diastolic blood pressure; DWMH, deep white matter hyperintensity; HC, healthy control; HTN-CI, hypertension with cognitive impairment; HTN-NCI, hypertension without cognitive impairment; MoCA, Montreal Cognitive Assessment; PWMH, paraventricular white matter hyperintensity; SBP, systolic blood pressure; WMH, white matter hyperintensity.
Comparison of susceptibilities
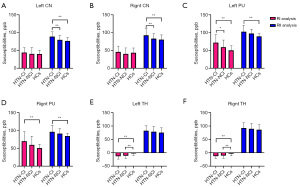
The ICC coefficients for susceptibilities in each brain region measured by the three radiologists were all >0.82 (Tables S1,S2). The RI analysis results showed significant differences in the susceptibilities of the bilateral PU and TH among the three groups. Post-hoc tests showed that the HTN-CI group exhibited significantly increased susceptibilities in the left PU compared to the HTN-NCI group (P<0.05). Compared to the HC group, the HTN-CI group showed significantly increased susceptibilities in the bilateral PU (P<0.05). While the HTN-CI and HTN-NCI groups showed significantly decreased susceptibilities in the bilateral TH (P<0.05).
The RII analysis results revealed significant differences in the susceptibilities of the bilateral CN head and PU among the three groups. Compared to the HTN-NCI group, the HTN-CI group exhibited significantly increased susceptibilities in the bilateral CN head (P<0.05). Compared to the HC group, the HTN-CI group showed significantly increased susceptibilities in the bilateral CN head and PU (P<0.05) (Table 2, Figure 2, and Figure S1). Integrated analysis of RI and RII data across brain regions showed that mean susceptibilities in the neostriatum (CN head and PU) were significantly elevated in HTN-CI patients versus HTN-NCI and HC groups (P<0.05) (Table S3).
Table 2
| ROI | RI analysis | RII analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HTN-CI group (n=55) | HTN-NCI group (n=50) | HC group (n=31) | Corrected P value | HTN-CI group (n=55) | HTN-NCI group (n=50) | HC group (n=31) | Corrected P value | ||
| Right CN head | 45.78±16.43 | 40.17±16.77 | 43.57±13.21 | 0.396 | 92.45±18.44 | 83.07±13.84 | 80.28±13.31 | 0.004†‡§ | |
| Left CN head | 44.11±13.72 | 40.16±18.08 | 40.12±10.44 | 0.493 | 88.99±13.29 | 79.76±12.38 | 76.58±9.73 | 0.004†‡§ | |
| Right GP | 147.78±48.33 | 129.95±36.05 | 140.75±30.21 | 0.206 | 177.98±18.32 | 177.18±21.93 | 173.73±16.89 | 0.697 | |
| Left GP | 141.86±48.72 | 130.10±40.88 | 139.89±31.50 | 0.493 | 179.90±19.99 | 180.01±23.92 | 176.92±18.45 | 0.835 | |
| Right PU | 69.94±27.17 | 59.69±24.15 | 50.45±10.75 | 0.004†§ | 96.26±18.01 | 91.27±13.69 | 84.40±7.60 | 0.004†§ | |
| Left PU | 71.77±22.59 | 59.09±20.04 | 50.19±12.91 | 0.004†‡§ | 102.72±18.29 | 97.42±12.44 | 89.60±7.47 | 0.004†§ | |
| Right TH | −12.56±9.28 | −10.53±9.64 | −3.19±7.35 | 0.004†§¶ | 92.71±19.74 | 89.55±20.31 | 86.18±18.84 | 0.469 | |
| Left TH | −13.06±10.54 | −11.11±9.27 | −2.77±6.95 | 0.004†§¶ | 82.55±18.19 | 88.87±19.62 | 75.56±17.65 | 0.469 | |
| Right RN | 134.54±33.70 | 125.90±33.39 | 131.10±34.90 | 0.569 | 175.70±19.01 | 173.61±18.45 | 169.88±14.47 | 0.469 | |
| Left RN | 130.94±32.34 | 129.73±33.06 | 127.40±35.00 | 0.893 | 172.65±17.67 | 170.94±17.64 | 166.77±13.73 | 0.469 | |
| Right SN | 147.67±44.37 | 131.27±41.88 | 148.43±27.98 | 0.173 | 175.18±18.60 | 173.15±21.25 | 170.15±16.59 | 0.624 | |
| Left SN | 141.30±39.15 | 129.01±42.67 | 149.76±28.92 | 0.173 | 180.41±19.67 | 177.80±19.64 | 173.94±15.05 | 0.469 | |
| Right DN | 125.78±38.49 | 118.83±36.39 | 118.54±32.68 | 0.667 | 159.40±16.95 | 157.64±17.44 | 153.11±12.41 | 0.469 | |
| Left DN | 124.95±38.21 | 120.71±37.77 | 121.14±32.21 | 0.883 | 159.63±16.76 | 157.93±17.64 | 151.89±11.18 | 0.256 | |
| Right FWM | −9.28±5.98 | −10.59±7.46 | −11.79±6.16 | 0.404 | 3.86±3.77 | 3.97±3.79 | 3.53±2.53 | 0.224 | |
| Left FWM | −10.12±6.77 | −9.37±6.47 | −10.08±7.27 | 0.883 | 4.75±3.16 | 4.63±3.69 | 3.01±3.95 | 0.857 | |
Data are presented as the mean ± standard deviation. †, significant after correction using the false discovery rate. ‡, significant differences between the HTN-CI and HTN-NCI groups. §, significant differences between the HTN-CI and HC groups. ¶, significant differences between the HTN-NCI and HC groups. CN, caudate nucleus; DN, dentate nucleus; FWM, frontal white matter; GP, globus pallidus; HC, healthy control; HTN-CI, hypertension with cognitive impairment; HTN-NCI, hypertension without cognitive impairment; ppb, parts per billion; PU, putamen; RI, global region; RII, high-iron content region; RN, red nucleus; ROI, region of interest; SN, substantia nigra; TH, thalamus.
Associations between WMH scores, susceptibilities, and cognitive scores
In the HTN-CI group, the bilateral susceptibilities of the SN were found to be positively correlated with the overall WMH scores based on the RI analysis (left SN, r=0.346, P=0.011; right SN, r=0.320, P=0.019); and the bilateral susceptibilities of the RN and SN were positively correlated with the overall WMH scores based on the RII analysis (left RN, r=0.312, P=0.023; right RN, r=0.336, P=0.014; left SN, r=0.347, P=0.011; right SN, r=0.316, P=0.021) (Figure 3).

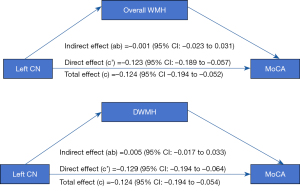
The univariate analysis identified the factors that may exert an influence on the MoCA scores, including age, education level, the overall WMH scores, DWMH scores, as well as the susceptibilities of the left CN head and right PU (P<0.05) (Table 3). The multivariate linear regression results indicated that both the overall WMH scores and susceptibilities of the bilateral CN head (especially the left CN head) exerted adverse effects on the total MoCA scores after excluding the effects of age, education, cerebral microbleeds, and lacune/recent small subcortical infarcts (β=−0.303 and β=−0.450, respectively). In addition, both higher DWMH scores and higher susceptibilities of the left CN head were significantly associated with lower total MoCA scores (β=−0.250, and β=−0.455, respectively). In both models, all VIFs were <3, indicating no significant multicollinearity between iron deposition and WMH (Table 4). Further, the mediation analysis showed that the relationship between left CN head susceptibilities and MoCA scores was not affected by both the overall WMH scores [95% confidence interval (CI): −0.023 to 0.031] and DWMH scores (95% CI: −0.017 to 0.033) (Figure 4).
Table 3
| Variables | MoCA | t/r | P |
|---|---|---|---|
| Sex | t=0.528 | 0.600 | |
| Male | 18.56±3.77 | ||
| Female | 18.00±4.08 | ||
| Smoking | t=−0.350 | 0.728 | |
| Yes | 18.22±4.21 | ||
| No | 18.64±2.76 | ||
| Diabetes | t=0.744 | 0.460 | |
| Yes | 18.51±3.89 | ||
| No | 17.50±3.87 | ||
| Hyperlipidemia | t=−0.823 | 0.414 | |
| Yes | 18.05±3.74 | ||
| No | 19.00±4.23 | ||
| Cerebral microbleed | t=1.260 | 0.213 | |
| Yes | 18.78±3.89 | ||
| No | 17.39±3.78 | ||
| Lacune/recent small subcortical infarct | t=1.172 | 0.246 | |
| Yes | 18.72±4.08 | ||
| No | 17.38±3.24 | ||
| Age (years) | r=−0.283 | 0.036* | |
| Education (years) | r=0.064 | <0.001* | |
| BMI (kg/m2) | r=−0.142 | 0.302 | |
| SBP (mmHg) | r=0.263 | 0.053 | |
| DBP (mmHg) | r=0.057 | 0.678 | |
| Hypertension duration (years) | r=−0.111 | 0.419 | |
| Overall WMH scores | r=−0.315 | 0.019* | |
| PWMH scores | r=−0.249 | 0.066 | |
| DWMH scores | r=−0.307 | 0.023* | |
| Right CN head† | r=−0.264 | 0.051 | |
| Left CN head† | r=−0.502 | <0.001* | |
| Right PU† | r=−0.286 | 0.034* | |
| Left PU† | r=−0.200 | 0.144 |
Data are presented as the mean ± standard deviation. †, RI susceptibilities of the CN head or PU. *, P value <0.05. BMI, body mass index; CN, caudate nucleus; DBP, diastolic blood pressure; DWMH, deep white matter hyperintensity; MoCA, Montreal Cognitive Assessment; PU, putamen; PWMH, paraventricular white matter hyperintensity; RI, global region; SBP, systolic blood pressure; WMH, white matter hyperintensity.
Table 4
| Factors | Model A | Model B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | P | Adjusted R2 of model | VIF | β | P | Adjusted R2 of model | VIF | ||
| Model 1 | 0.597 | 0.591 | |||||||
| Overall WMH scores | −0.209 | 0.045* | 1.4 | −0.251 | 0.049* | 2.0 | |||
| Right CN head† | −0.309 | 0.001* | 1.0 | −0.314 | 0.001* | 1.0 | |||
| Model 2 | 0.691 | 0.696 | |||||||
| Overall WMH scores | −0.243 | 0.009* | 1.4 | −0.303 | 0.007* | 2.1 | |||
| Left CN head† | −0.435 | <0.001* | 1.1 | −0.450 | <0.001* | 1.1 | |||
| Model 3 | 0.593 | 0.583 | |||||||
| DWMH scores | −0.182 | 0.062 | 1.2 | −0.179 | 0.086 | 1.4 | |||
| Right CN head† | −0.300 | 0.001* | 1.0 | −0.306 | 0.001* | 1.0 | |||
| Model 4 | 0.697 | 0.697 | |||||||
| DWMH scores | −0.243 | 0.005* | 1.2 | −0.250 | 0.007* | 1.4 | |||
| Left CN head† | −0.442 | <0.001* | 1.1 | −0.455 | <0.001* | 1.1 | |||
| Model 5 | 0.524 | 0.513 | |||||||
| Overall WMH scores | −0.172 | 0.128 | 1.4 | −0.233 | 0.091 | 2.0 | |||
| Right PU† | −0.182 | 0.091 | 1.3 | −0.187 | 0.090 | 1.3 | |||
| Model 6 | 0.530 | 0.513 | |||||||
| DWMH scores | −0.176 | 0.091 | 1.2 | −0.190 | 0.091 | 1.4 | |||
| Right PU† | −0.193 | 0.071 | 1.3 | −0.195 | 0.078 | 1.3 | |||
Model A comprises age and education as covariates; Model B comprises Model A plus an adjustment for cerebral microbleed and lacune/recent small subcortical infarcts. †, RI susceptibilities of the CN head or PU. *, P value <0.05. CN, caudate nucleus; DWMH, deep white matter hyperintensity; MoCA, Montreal Cognitive Assessment; PU, putamen; RI, global region; VIF, variance inflation factor; WMH, white matter hyperintensity.
Discussion
This study showed that more severe WMH and increased iron deposition in specific DGM are associated with hypertension. The CN head and PU were the DGM most prone to iron deposition in hypertension. The results showed that the iron deposition in the neostriatum was significantly higher in the HTN-CI patients than the HTN-NCI patients, and the iron deposition of the left CN head and right PU was negatively associated with cognitive scores, indicating that iron deposition is associated with poorer cognitive performance. Combined with the multivariate linear regression analysis and mediating analysis, the results identified both CN head iron deposition and WMH as inverse factors affecting cognitive function.
Hypertension, as a major risk factor for WMH, promotes WMH progression through microvascular damage and neuroinflammatory responses (1). A previous longitudinal study showed that controlling blood pressure can effectively improve WMH (32). As expected, this study showed the WMH lesions were more severe in the hypertensive patients than HCs. In addition to cerebral iron dysregulation and redistribution, vascular factors such as ischemia and hypoxia, BBB disruption, and local inflammatory responses can aggravate or promote cerebral iron deposition (13,33,34). Consistent with previous findings (15), we found that iron deposition in the CN head and PU was increased in the hypertensive patients. Additionally, a similar pattern of iron deposition was observed in hereditary microangiopathy, specifically cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (35). Increased iron deposition, accompanied by a decrease in local cerebral blood flow in the PU, has also been observed in hemodialysis patients (10). The lenticulostriate arteries, which vascularize the CN head and PU, are particularly susceptible to hypertension-induced microvascular injury. This vulnerability may explain the regional iron deposition observed in these structures. Further, this study found a positive correlation between WMH and DGM iron deposition. Therefore, we speculate that the emergence of WMH and iron deposition in hypertension shares a common vasculogenic pathogenesis.
WMH is often categorized into PWMH and DWMH subtypes, which are associated with distinct histopathological features, microstructural characteristics, and clinical manifestations (36). The degeneration of fiber tracts leads to thinning of the distal cerebral cortex (37), and the location of WMH affects different domains of cognition. Our results suggest that DWMH (rather than PWMH) significantly affect cognition; however, this is not completely consistent with previous findings (4,38), although this may be due to the different study design types (i.e., longitudinal vs. cross-sectional) and non-uniform cognitive scales. Additionally, histopathological evidence suggests that DWMH is more susceptible to ischemic damage than PWMH; notably, vessels in the watershed area are susceptible to hypertension. Conversely, mild PWMH may not be accompanied by arteriolosclerotic vessels and may be non-ischemic in nature (39,40). Studies based on diffusion tensor imaging and functional MRI have shown that WMH can cause the loss of fiber tracts and gray matter in non-connected brain regions, thereby reducing the efficiency of information transmission throughout the brain (41). Given the correlation between iron deposition and WMH, incorporating both factors into the assessment of cognitive function may provide novel insights into whole-brain damage.
Physiologically, iron in DGM is stored in the form of ferritin or hemosiderin, while excess iron deposition can produce reactive oxygen species, induce ferroptosis, and promote neuroinflammation (6). A previous partial correlation analysis showed multiple associations between iron deposition and cognitive impairment in hypertension, regardless of CSVD (15). Another study based on R2*-mapping showed that CSVD did not mediate the association between iron deposition and cognitive function (14).
In our univariate analysis, we found that iron deposition in the left CN head and right PU might affect overall cognition. After performing multiple linear regression analyses with clinical factors as covariates, we found that iron deposition in the bilateral CN head combined with WMH, especially in the left CN head and DWMH, adversely affected overall cognition. These results showed that the presence of WMH did not eliminate the association between iron deposition and cognitive function, and that iron deposition was not just an epiphenomenon of CSVD. A systematic review by Spence et al. (7) also reported that iron deposition in the CN head and PU is associated with poorer general cognitive performance, and this finding was largely consistent among all reports. The neostriatum receives information from the cortex, TH, and SN, and is associated with a variety of cognitive functions (42). Iron-mediated neuronal damage, as well as the disruption of connections, may lead to cognitive impairment (43). Therefore, we propose that iron deposition in DGM and WMH affect cognitive impairment in hypertension.
RII analysis is a novel QSM-based method for analyzing the inhomogeneous distribution of iron deposition in a single DGM. A study of HCs showed that RII analysis had a higher degree of linear fit between iron deposition and age than RI analysis (26), while other studies have shown that RII analysis can distinguish patients (with type 2 diabetes mellitus and Parkinson’s disease) from HCs more sensitively compared to RI analysis (18,27).
In this study, we found a significantly lower iron content in the TH of hypertensive patients than HCs using RI analysis, but no such difference was found using RII analysis. We hypothesize that the observed phenomenon may be attributed to the non-uniform distribution of iron in the TH or the cancellation of susceptibilities by diamagnetic myelin (44), or the age-related decrease of iron content in the TH (14). Moreover, unlike the RI analysis, the RII analysis showed that increased iron deposition in DGM was associated with WMH in hypertension, indicating the sensitivity of RII analysis in revealing subtle differences in iron deposition.
This study had some limitations. First, owing to the cross-sectional design and limited sample size, our study could not establish causal relationships or elucidate the mechanistic links between iron deposition, WMH, and cognitive decline. Larger sample-sized longitudinal studies need to be conducted to further track the dynamic changes of WMH and iron deposition parallelly in hypertensive patients. Second, this ROI-based QSM method only focused on iron deposition in specific DGM. Further voxel-based QSM analyses may aid in evaluating whole-brain iron deposition, and its association with WMH and cognitive impairment. Finally, the brief visual assessment scales for WMH used in this study were relatively subjective but were clinically practical and effective. Validated automatic or semi-automated quantitative analyses of WMH may provide more objective assessments in the future.
Conclusions
This study showed that increased WMH was associated with increased iron deposition in specific DGM in hypertensive patients. Based on the current results, both WMH and iron deposition in the CN head may independently affect cognitive function in hypertension. Notably, comprehensive evaluations and follow-up studies on iron deposition and WMH imaging markers may aid in the early identification of and interventions for hypertension-induced cognitive impairment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1610/rc
Funding: The research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1610/coif). E.M.H. was the founder and a board member of SpinTech MRI. E.M.H. has not been involved with SpinTech MRI for several years (since January, 2023); however, he does gold a very small percentage of shares in the company. His involvement in this study was strictly academic, and he did not receive any financial compensation or other benefits. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This prospective observational study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. UHCT21811). All the participants in this study provided informed consent. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 2021;17:639-54. [Crossref] [PubMed]
- Inoue Y, Shue F, Bu G, Kanekiyo T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer's disease. Mol Neurodegener 2023;18:46. [Crossref] [PubMed]
- Garnier-Crussard A, Bougacha S, Wirth M, Dautricourt S, Sherif S, Landeau B, Gonneaud J, De Flores R, de la Sayette V, Vivien D, Krolak-Salmon P, Chételat G. White matter hyperintensity topography in Alzheimer's disease and links to cognition. Alzheimers Dement 2022;18:422-33. [Crossref] [PubMed]
- Meng F, Yang Y, Jin G. Research Progress on MRI for White Matter Hyperintensity of Presumed Vascular Origin and Cognitive Impairment. Front Neurol 2022;13:865920. [Crossref] [PubMed]
- Zhao X, Zuo M, Zhan F, Fan P, Liu S, Taylor M, Ganau M, Hall WA, Ruan H, Wan L. Cognition mediates the relationship between white matter hyperintensity and motor function in patients with cerebral small vessel disease: a cross-sectional study. Quant Imaging Med Surg 2024;14:7306-17. [Crossref] [PubMed]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014;13:1045-60. [Crossref] [PubMed]
- Spence H, McNeil CJ, Waiter GD. The impact of brain iron accumulation on cognition: A systematic review. PLoS One 2020;15:e0240697. [Crossref] [PubMed]
- Uchida Y, Kan H, Sakurai K, Arai N, Inui S, Kobayashi S, Kato D, Ueki Y, Matsukawa N. Iron leakage owing to blood-brain barrier disruption in small vessel disease CADASIL. Neurology 2020;95:e1188-98. [Crossref] [PubMed]
- Wang H, Chai C, Wu G, Li J, Zhao C, Fu D, Zhang S, Wang H, Wang B, Zhu J, Shen W, Xia S. Cerebral blood flow regulates iron overload in the cerebral nuclei of hemodialysis patients with anemia. J Cereb Blood Flow Metab 2023;43:749-62. [Crossref] [PubMed]
- Wang H, Song L, Li M, Yang Z, Wang ZC. Association between susceptibility value and cerebral blood flow in the bilateral putamen in patients undergoing hemodialysis. J Cereb Blood Flow Metab 2023;43:433-45. [Crossref] [PubMed]
- Del C Valdés Hernández M. Ritchie S, Glatz A, Allerhand M, Muñoz Maniega S, Gow AJ, Royle NA, Bastin ME, Starr JM, Deary IJ, Wardlaw JM. Brain iron deposits and lifespan cognitive ability. Age (Dordr) 2015;37:100. [Crossref] [PubMed]
- Su Y, Wu W, Qin Z, Li C, Zhao J, Kang J, Wang Y, Zheng C, Haacke EM, Wang L. Deep gray matters iron deposition is positively associated with white matter hyperintensity in hypertension. J Clin Hypertens (Greenwich) 2023;25:768-77. [Crossref] [PubMed]
- Valdés Hernández M, Allerhand M, Glatz A, Clayson L, Muñoz Maniega S, Gow A, Royle N, Bastin M, Starr J, Deary I, Wardlaw J. Do white matter hyperintensities mediate the association between brain iron deposition and cognitive abilities in older people? Eur J Neurol 2016;23:1202-9. [Crossref] [PubMed]
- Ghadery C, Pirpamer L, Hofer E, Langkammer C, Petrovic K, Loitfelder M, Schwingenschuh P, Seiler S, Duering M, Jouvent E, Schmidt H, Fazekas F, Mangin JF, Chabriat H, Dichgans M, Ropele S, Schmidt R. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging 2015;36:925-32. [Crossref] [PubMed]
- Qin Z, Wu W, Liu D, Zheng C, Kang J, Zhou H, Meng X, Haacke EM, Wang L. Quantitative Susceptibility Mapping of Brain Iron Relating to Cognitive Impairment in Hypertension. J Magn Reson Imaging 2022;56:508-15. [Crossref] [PubMed]
- Li X, Jin D, Zhu Y, Liu L, Qiao Y, Qian Y, Tian J, Jiang B, Hou C, Geng J, Li X, Gao X, Ma Y, Wang S, Zong J, Qin Y. Quantitative susceptibility mapping to evaluate brain iron deposition and its correlation with physiological parameters in hypertensive patients. Ann Transl Med 2021;9:1582. [Crossref] [PubMed]
- Yan S, Sun J, Chen Y, Selim M, Lou M. Brain iron deposition in white matter hyperintensities: a 3-T MRI study. Age (Dordr) 2013;35:1927-36. [Crossref] [PubMed]
- Hu R, Gao B, Tian S, Liu Y, Jiang Y, Li W, Li Y, Song Q, Wang W, Miao Y. Regional high iron deposition on quantitative susceptibility mapping correlates with cognitive decline in type 2 diabetes mellitus. Front Neurosci 2023;17:1061156. [Crossref] [PubMed]
- Fushimi Y, Nakajima S, Sakata A, Okuchi S, Otani S, Nakamoto Y. Value of Quantitative Susceptibility Mapping in Clinical Neuroradiology. J Magn Reson Imaging 2024;59:1914-29. [Crossref] [PubMed]
- Yan Y, Wang Z, Wei W, Yang Z, Guo L, Wang Z, Wei X. Correlation of brain iron deposition and freezing of gait in Parkinson's disease: a cross-sectional study. Quant Imaging Med Surg 2023;13:7961-72. [Crossref] [PubMed]
- Amin Al Olama A, Wason JMS, Tuladhar AM, van Leijsen EMC, Koini M, Hofer E, Morris RG, Schmidt R, de Leeuw FE, Markus HS. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology 2020;94:e1294-302. [Crossref] [PubMed]
- Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao RChina Hypertension Survey Investigators. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation 2018;137:2344-56. [Crossref] [PubMed]
- He N, Wu B, Liu Y, Zhang C, Cheng J, Gao B, Miao Y, Wu W, Wang L, Sun R, Sun W, Xu H, Bai Y, Wang M, Chai C, Xia S, Zheng Q, Li Y, Qin Y, Liao W, Chen Y, Jokar M, Wang Y, Yan F, Haacke EM. Stage as a multicenter, multivendor protocol for imaging Parkinson’s disease: A validation study on healthy controls. Chin J Acad Radiol 2022;5:47-60.
- Chen Y, Liu S, Wang Y, Kang Y, Haacke EM. STrategically Acquired Gradient Echo (STAGE) imaging, part I: Creating enhanced T1 contrast and standardized susceptibility weighted imaging and quantitative susceptibility mapping. Magn Reson Imaging 2018;46:130-9. [Crossref] [PubMed]
- Zhou L, Chen Y, Li Y, Gharabaghi S, Chen Y, Sethi SK, Wu Y, Haacke EM. Intracranial iron distribution and quantification in aceruloplasminemia: A case study. Magn Reson Imaging 2020;70:29-35. [Crossref] [PubMed]
- Liu M, Liu S, Ghassaban K, Zheng W, Dicicco D, Miao Y, Habib C, Jazmati T, Haacke EM. Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. J Magn Reson Imaging 2016;44:59-71. [Crossref] [PubMed]
- Ghassaban K, He N, Sethi SK, Huang P, Chen S, Yan F, Haacke EM. Regional High Iron in the Substantia Nigra Differentiates Parkinson's Disease Patients From Healthy Controls. Front Aging Neurosci 2019;11:106. [Crossref] [PubMed]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351-6. [Crossref] [PubMed]
- Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, Werring DJ. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 2009;73:1759-66. [Crossref] [PubMed]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. [Crossref] [PubMed]
- Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, Jia XF, Song H, Jia J. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol 2011;24:184-90. [Crossref] [PubMed]
- Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation 2011;123:266-73. [Crossref] [PubMed]
- Petrova J, Manolov V, Vasilev V, Tzatchev K, Marinov B. Ischemic stroke, inflammation, iron overload - Connection to a hepcidin. Int J Stroke 2016;11:NP16-7. [Crossref] [PubMed]
- Li J, Nguyen TD, Zhang Q, Guo L, Wang Y. Cerebral Microbleeds Are Associated With Increased Brain Iron and Cognitive Impairment in Patients With Cerebral Small Vessel Disease: A Quantitative Susceptibility Mapping Study. J Magn Reson Imaging 2022;56:904-14. [Crossref] [PubMed]
- Hong H, Wang S, Yu X, Jiaerken Y, Guan X, Zeng Q, Yin X, Zhang R, Zhang Y, Zhu Z, Huang P, Zhang M. White Matter Tract Injury by MRI in CADASIL Patients is Associated With Iron Accumulation. J Magn Reson Imaging 2023;57:238-45. [Crossref] [PubMed]
- Griffanti L, Jenkinson M, Suri S, Zsoldos E, Mahmood A, Filippini N, Sexton CE, Topiwala A, Allan C, Kivimäki M, Singh-Manoux A, Ebmeier KP, Mackay CE, Zamboni G. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: A study in older adults. Neuroimage 2018;170:174-81. [Crossref] [PubMed]
- Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology 2015;84:1685-92. [Crossref] [PubMed]
- Jiménez-Balado J, Riba-Llena I, Abril O, Garde E, Penalba A, Ostos E, Maisterra O, Montaner J, Noviembre M, Mundet X, Ventura O, Pizarro J, Delgado P. Cognitive Impact of Cerebral Small Vessel Disease Changes in Patients With Hypertension. Hypertension 2019;73:342-9. [Crossref] [PubMed]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126-35. [Crossref] [PubMed]
- Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008;64:273-80. [Crossref] [PubMed]
- Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019;18:684-96. [Crossref] [PubMed]
- Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2012;2:a009621. [Crossref] [PubMed]
- Salami A, Avelar-Pereira B, Garzón B, Sitnikov R, Kalpouzos G. Functional coherence of striatal resting-state networks is modulated by striatal iron content. Neuroimage 2018;183:495-503. [Crossref] [PubMed]
- Hametner S, Endmayr V, Deistung A, Palmrich P, Prihoda M, Haimburger E, Menard C, Feng X, Haider T, Leisser M, Köck U, Kaider A, Höftberger R, Robinson S, Reichenbach JR, Lassmann H, Traxler H, Trattnig S, Grabner G. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation - A biochemical and histological validation study. Neuroimage 2018;179:117-33. [Crossref] [PubMed]


