
Echocardiography derived-left atrial stiffness index as a more cost-effective and powerful approach compared to brain natriuretic peptide for predicting left atrioventricular uncoupling in patients with acute ischemic stroke
Introduction
Acute ischemic stroke (AIS), as one of the most common cerebrovascular diseases, can be life-threatening or severely debilitating to individuals and imposes a heavy burden on families and society. Although neurological injury remains a primary cause of stroke-induced mortality, cardiovascular complications also constitute a substantial additional contributor to poststroke death. Following the occurrence of AIS, patients exhibit a spectrum of cardiovascular complications including ischemic and nonischemic myocardial injury, acute coronary syndrome, systolic or diastolic dysfunction, heart failure, arrhythmia, hemodynamic instability, and cardiac arrest, among others (1,2). Research indicates a bidirectional neurocardiac pathophysiology for AIS, wherein stroke-induced mechanisms—particularly dysregulated neuroendocrine activation (hypothalamic-pituitary-adrenal axis), autonomic nervous system dysregulation (sympathetic-parasympathetic imbalance), systemic inflammation, and gut-brain axis perturbations—collectively constitute the core pathophysiological framework of the brain-heart interaction continuum for poststroke complications (1,2).
Barnes et al. reported left atrial (LA) volume to be independently predictive of first ischemic stroke, incremental to age, diabetes, myocardial infarction, and hyperlipidemia (3). Mendez et al. found that patients with severe LA enlargement who were 75 years old or older had a significantly higher probability of experiencing stroke recurrence (4). Meanwhile, in Xue et al.’s study, LA enlargement was associated with a greater severity of initial neurologic deficit among patients with AIS and different embolic subtypes (5). Pathophysiologically, morphological and functional changes in the atria tend to occur earlier than do those in the ventricles. This is because the atria is more prone to early functional and structural changes due to a relatively thin atrial wall and a greater sensitivity to fluctuation in load and pressure. Studies on atrial structure and function can detect AIS-induced cardiac complications relatively early and thus facilitate early intervention.
The left atrioventricular coupling index (LACI), defined as the ratio of the LA volume to the left ventricular (LV) end-diastolic volume (LVEDV) (6-8), is a strong and independent predictor of cardiovascular events such as heart failure, atrial fibrillation, hard cardiovascular disease, and coronary heart disease-related death. Compared with individual LA parameters, LACI has incremental prognostic value for predict cardiovascular events over traditional risk factors including age, male sex, body mass index, diabetes, hypertension, smoking, dyslipidemia, coronary artery disease, and a history of heart failure (6,7). However, the prevalence of left atrioventricular uncoupling (LAU) (i.e., a higher LACI) and its risk factors in patients with AIS remains unknown.
Echocardiography and blood biomarker assays are common methods for evaluating cardiac involvement in stroke. LA volume, stiffness, strain, and emptying fraction can be evaluated quantitatively via real-time four-dimensional (4D) echocardiography. Myocardial injury can be assessed through biomarkers such as high-sensitivity cardiac troponin-I (hs-cTnI), soluble growth stimulation expressed gene 2 (sST2), and brain natriuretic peptide (BNP); however, it is not clear which of these is associated with LAU.
Therefore, the aims of this study were as follows: (I) to investigate the incidence of LAU in patients with AIS and determine its associated risk factors; and (II) to compare the performance and cost-effectiveness of the risk factors in predicting LAU in patients with AIS. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1243/rc).
Methods
Study population
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by institutional ethics board of Human Research Ethics Committee of Yangpu Hospital, School of Medicine, Tongji University (No. LL-2023-SCI-018). Informed consent was obtained from all patients. From February to November 2023, 224 patients with a diagnosis of AIS, irrespective of thrombolytic or endovascular treatment, were consecutively enrolled. The exclusion criteria were transient ischemic attack, cerebellar infarcts, hemorrhagic stroke, traumatic brain injury, coronary heart disease with ≥50% stenosis as confirmed by angiography (9,10), and structural cardiac abnormalities (congenital, valvular, or cardiomyopathic disorders) (11,12).
Data collection
The demographic data and cardiovascular risk factors of patients were documented at admission. Analyzed blood biomarkers included creatine kinase (CK), CK-myocardial band (CK-MB), hs-cTnI, high-sensitivity C-reactive protein (hsCRP), BNP, and sST2.
4D echocardiography
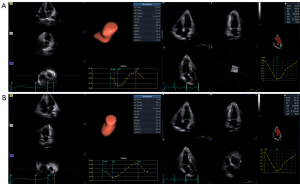
All participants underwent standardized 4D transthoracic echocardiography within the first 48 hours after symptom onset via a Vivid E95 system (GE HealthCare, Chicago, IL, USA) with a 4VC transducer. Full-volume datasets of the LV and LA were captured in an apical four-chamber orientation (90° × 90° sector angle), with a ≥40% frame rate relative to the heart rate being maintained across four cardiac cycles. Imaging protocols prioritized a clear visualization of ventricular/apical walls, mitral annulus, and atrial structures. Patients were instructed to perform end-expiration breath-holding to minimize motion artifacts. Raw digital data were archived in the EchoPAC v. 203 workstation (GE HealthCare) for blinded offline analysis by a single cardiology imaging specialist. As shown in Figure 1, LVEDV, LV end-systolic volume (LVESV), and LV ejection fraction (LVEF) were obtained with the 4D auto LV quantification (LVQ) tool (13,14). Meanwhile, LA minimal volume (LAVmin), LA maximum volume (LAVmax), LA maximum volume index (LAVImax), LA ejection volume (LAEV), LA ejection fraction (LAEF), LA peak longitudinal strain during the reservoir phase (LASr), LA peak longitudinal strain during the conduit phase (LAScd), and LA peak longitudinal strain during the contractile phase (LASct) were obtained with the 4D auto LA quantification (LAQ) tool (15,16). The ratio of peak early diastolic transmitral filling velocity to peak early diastolic septal mitral annulus tissue velocity (E/e’) and tricuspid annular plane systolic excursion (TAPSE) values were obtained according to the American Society of Echocardiography (ASE) guidelines (17,18). Finally, LACI and the LA stiffness index (LASI) were calculated according to the following formulae: LACI = LAVmin/LVEDV (10-12); LASI = E/e’/LASr (19-22). LAU was defined as a LACI >0.25 (6,7).
Statistical analysis
Continuous variables with a normal distribution are expressed as the mean ± standard deviation (SD) and were compared via the independent-samples t-test. Nonparametric continuous variables are expressed as median and interquartile range (IQR) and were assessed with the Mann-Whitney test. Categorical data are presented as counts (%) and were compared with the Chi-squared or Fisher exact test as appropriate. Multivariable logistic regression analyses were constructed to identify the independent risk factors for LAU in patients with AIS. The predictive ability of the risk factors was assessed via the area under the receiver operating characteristic curve (AUC). Statistical significance was defined as a P value <0.05. Analyses were performed with SPSS 19.0 (IBM Corp., Armonk, NY, USA) and MedCalc 16.8.4 (MedCalc Software, Ostend, Belgium).
Cost-effectiveness analysis
Decision model
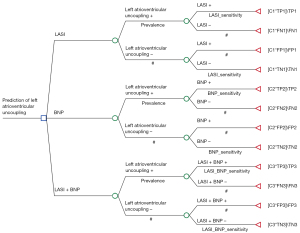
A decision-analytic model was developed in TreeAge Pro 2022 software (TreeAge Software, Williamstown, MA, USA) to assess the cost-effectiveness of algorithms combining ≥2 independent echocardiographic and/or biomarker-derived risk factors in predicting LAU in patients with AIS (Figure 2). The model involved 10,000 patients with AIS with presumptive LAU. We used the pooled values from our current research and studies in the literature (6,8,23-27) as the base estimates for the sensitivity and specificity of echocardiography or/and blood biomarker assay. The outcome measure of this model was cost per LAU patient detected when echocardiography or/and blood biomarker assay were used. We also determined the incremental cost-effectiveness ratios (ICERs), which represent the incremental costs per additional correct prediction in our study. The ICER was calculated according to the following formula: ICER = (CostA − CostB)/(EffectivenessA − EffectivenessB), where CostA and CostB are the costs of strategy A and B, respectively; and EffectivenessA and EffectivenessB are the effectiveness of strategy A and B, respectively. In order to evaluate the economic advantages or disadvantages of the two strategies represented by the ICER, we compared them with a cost-effectiveness threshold [one to three times the gross domestic product (GDP) per capita], as recommended by the World Health Organization (WHO) for developing countries. If the ICER was less than the threshold, this indicated that strategy A is more economical than strategy B.
Cost
The costs of the LAU prediction methods were assessed from the service provider’s perspective. All costs were estimated in Chinese yuan (CNY) based on 2023 values. Direct medical costs that resulted from the materials and equipment, capital costs, maintenance, and time spent by the service provider were considered. The staff salary was based on the time required to process the echocardiography (5–10 min), blood biomarker assay (10–240 min), and the combined echocardiography and blood laboratory tests (15–250 min). We assumed a maximum of 48 tests per day for echocardiography, 2–48 tests per day for blood biomarker assay, 2–24 tests per day for the combined echocardiography and blood biomarker assay, a total of 254 working days per year, 15 days for echocardiography training, 10 days for blood biomarker assay training, and 25 days of training for the combined echocardiography and blood biomarker assay. The cost of a unit of echocardiography or/and blood biomarker assay was estimated. Indirect medical costs such as water, space, and overhead were automatically cancelled out in the calculation since they were the same for the echocardiography or/and blood biomarker assay.
Effectiveness
The number of true positives, false positives, true negatives, and false negatives in a cohort of 10,000 patients with suspected LAU was used to determine the effectiveness of the prediction strategies for LAU. These metrics were calculated according to the prevalence, sensitivity, and specificity of each LAU prediction strategy and through the method outlined in Rautenberg et al.’s study (Figure 2) (28).
Sensitivity analysis
The sensitivity analysis of the developed model was based on adjustments of (I) LAU prevalence from our current research and studies in the literature (6,8,23-27); (II) the diagnostic accuracy of echocardiography or/and blood biomarker assay from our current research and studies in the literature (6,8,23-27); (III) the useful life of the capital equipment of echocardiography or/and blood biomarker assay between 5 and 10 years; (IV) the average number of tests performed per day between 2 and 48; (V) the different prices of the equipment for echocardiography; (VI) the different prices of the reagent cartridges for blood biomarker assay; and (VII) the percentage allocated for shared equipment or staff time.
Results
Characteristics of the included patients
Of the 224 patients with AIS initially enrolled, 17 were excluded due to poor image quality, and thus 207 patients were eventually included, who had a median National Institutes of Health Stroke Scale (NIHSS) score of 5 (IQR, 3–7) (29,30). According to the diagnostic criteria of 4D echocardiography, 146 patients had LAU and 61 did not, representing an LAU prevalence of 70.53%. The patients with LAU had a higher NIHSS score (median, 7; IQR, 4.75–10) than did those without (median, 3; IQR, 2–5) (P=0.016). Table 1 summarizes the baseline characteristics at admission. No significant differences were observed between the two groups in terms of cardiovascular risk factors (P>0.05). Compared to those without LAU, the patients with AIS and LAU were older and had higher BNP levels and greater LA volume, stiffness, and functional impairment (P<0.05).
Table 1
| Variables | LAU− (n=61) | LAU+ (n=146) | P value |
|---|---|---|---|
| Age (years) | 64.44±9.92 | 71.36±8.89 | 0.003 |
| Male | 27 (44.26) | 74 (50.68) | 0.134 |
| Body mass index (kg/m2) | 26.34±6.01 | 27.18±5.83 | 0.654 |
| Hypertension | 42 (68.85) | 107 (73.29) | 0.553 |
| Smoker | 41 (67.21) | 95 (65.07) | 0.622 |
| Diabetes mellitus | 22 (36.07) | 61 (41.78) | 0.416 |
| Hypercholesterolemia | 42 (68.85) | 109 (74.66) | 0.528 |
| CK (μ/L) | 87.23±85.77 | 97.31±75.76 | 0.477 |
| CK-MB (ng/mL) | 1.76±0.78 | 2.26±0.86 | 0.159 |
| BNP (pg/mL) | 93.86±64.52 | 126.21±98.88 | 0.025 |
| hs-cTnI (ng/mL) | 13.21±12.16 | 15.73±13.41 | 0.172 |
| hsCRP (mg/L) | 14.03±31.11 | 12.66±24.82 | 0.859 |
| sST2 (ng/mL) | 19.91±16.56 | 23.12±19.78 | 0.374 |
| LVEDV (mL) | 86.69±12.45 | 79.11±15.74 | 0.226 |
| LVESV (mL) | 30.09±7.63 | 32.30±8.96 | 0.197 |
| LVEF (%) | 58.34±6.46 | 59.20±6.98 | 0.401 |
| TAPSE (mm) | 21.76±2.64 | 21.92±3.21 | 0.795 |
| E/e’ | 6.75±1.96 | 8.88±2.82 | <0.0001 |
| LAVmin (mL) | 18.95±4.12 | 30.75±11.97 | <0.0001 |
| LAVmax (mL) | 45.77±9.05 | 59.16±15.26 | <0.0001 |
| LAVImax (mL/m2) | 26.05±4.87 | 34.79±8.09 | <0.0001 |
| LAEV (mL) | 26.78±6.67 | 28.42±8.37 | 0.167 |
| LAEF (%) | 58.28±5.87 | 48.62±9.28 | <0.0001 |
| LASr (%) | 23.73±6.92 | 18.95±6.63 | <0.0001 |
| LAScd (%) | −12.17±5.76 | −8.54±4.70 | <0.0001 |
| LASct (%) | −11.51±6.14 | −10.36±5.35 | 0.173 |
| LACI | 0.22±0.04 | 0.40±0.18 | <0.0001 |
| LASI | 0.31±0.16 | 0.56±0.34 | <0.0001 |
Data are expressed as the mean ± SD or n (%). AIS, acute ischemic stroke; BNP, brain natriuretic peptide; CK, creatine kinase; CK-MB, creatine kinase myocardia band; E/e’, ratio of peak early diastolic transmitral filling velocity to peak early diastolic septal mitral annulus tissue velocity; hs-cTnI, high-sensitivity cardiac troponin-I; hsCRP, high-sensitivity C-reactive protein; LACI, left atrioventricular coupling index; LAEF, left atrial ejection fraction; LAEV, left atrial ejection volume; LAScd, left atrial peak longitudinal strain during the conduit phase; LASct, left atrial peak longitudinal strain during contractile phase; LASI, left atrial stiffness index; LASr, left atrial peak longitudinal strain during the reservoir phase; LAU, left atrioventricular uncoupling; LAU+, with left atrioventricular uncoupling; LAU−, without left atrioventricular uncoupling; LAVImax, left atrial maximum volume index; LAVmax, left atrial maximum volume; LAVmin, left atrial minimal volume; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; SD, standard deviation; sST2, soluble growth stimulation expressed gene 2; TAPSE, tricuspid annular plane systolic excursion.
Multivariate logistic regression analysis
Multivariate logistic regression analysis of age, BNP, E/e’, LAVmin, LAVmax, LAVImax, LAEF, LASr, LAScd, and LASI revealed that LASI [odds ratio (OR) =822.286; 95% confidence interval (CI): 25.487–182,900.741; P<0.0001] and BNP (OR =1.115; 95% CI: 0.998–1.246; P=0.031) were the two independent risk factors for LAU in patients with AIS.
Receiver operating characteristic curve analysis
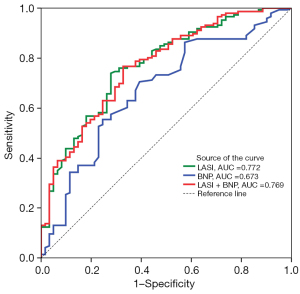
LASI demonstrated a superior ability to predict LAU in patients with AIS (AUC =0.772; 95% CI: 0.708–0.827) than did BNP (AUC =0.673; 95% CI: 0.604–0.736) (P<0.05). When these factors were combined, the AUC was 0.769 (95% CI 0.705–0.824), which was similar to that of LASI (P>0.05) but larger than that of BNP (P<0.05) (Figure 3). At a cutoff value of 0.34, LASI yielded a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 74.15%, 73.02%, 87.09%, 54.22%, and 73.91%, respectively; BNP at a cutoff value of 39 pg/mL yielded values of 70.55%, 60.66%, 81.10%, 46.25%, and 67.63%; respectively; and the combination of LASI and BNP at a cutoff value of 0.63 mL yielded values of 76.71%, 67.21%, 84.85%, 54.67%, and 73.89%, respectively.
Cost-effectiveness analysis
The average cost of each LASI echocardiographic measurement was approximately CNY ¥62.50 while that for the BNP assay was CNY ¥280.00. The average cost of the combined LASI echocardiographic measurement and BNP assay was CNY ¥352. Compared to the ICER of the algorithm employing LASI echocardiographic measurement, those for the algorithm composed of the BNP assay and the combination of the LASI echocardiographic measurement and BNP assay were −2,106.93 and −27,210.91, respectively. This indicated that the algorithm consisting of LASI echocardiographic measurement detected more patients at lower costs, identifying it as the superior option to the other two models (Table 2).
Table 2
| Category | Strategy | Cost | Incr cost | Eff | Incr Eff | ICER (IC/IE) | NMB |
|---|---|---|---|---|---|---|---|
| Excluding dominated | |||||||
| Undominated | LASI | 195,878.10 | 3,109.18 | −195,878.10 | |||
| All | |||||||
| Undominated | LASI | 195,878.10 | 3,109.18 | −195,878.10 | |||
| Abs. dominance | BNP | 791,424.32 | 595,546.22 | 2,826.52 | −282.66 | −2,106.93 | −791,424.32 |
| Abs. dominance | LASI + BNP | 1,082,954.80 | 887,076.70 | 3,076.58 | −32.60 | −27,210.91 | −1,082,954.80 |
Abs., absolute; AIS, acute ischemic stroke; BNP, brain natriuretic peptide; Eff, effectiveness; IC, incremental cost; ICER, incremental cost-effectiveness ratio; IE, incremental effectiveness; Incr, incremental; LASI, left atrial stiffness index; LAU, left atrioventricular uncoupling; NMB, net monetary benefit.
Sensitivity analysis
In one-way sensitivity analyses, the superiority of the algorithm consisting of the LASI echocardiographic measurement maintained across various values (LAU prevalence, 10–90%; sensitivity of LASI, 10–90%; specificity of LASI, 10–90%; sensitivity of BNP: 10–90%; specificity of BNP, 10–90%; sensitivity of the LASI-BNP combination, 10–90%; specificity of the LASI-BNP combination, 10–90%; cost of LASI echocardiographic measurement, CNY ¥50–150; cost of BNP assay, CNY ¥150–400; cost the LASI-BNP combination, CNY ¥200–600). The prevalence of LAU in patients with AIS, the cost of the BNP assay, and the specificity of the LASI had the most influence on the ICER.
Discussion
To our knowledge, this is the first study to compare the performance and cost-effectiveness of LASI echocardiographic measurement and BNP assay for predicting LAU in patients with AIS. LASI echocardiographic measurement was found to be a cost-effective approach for detecting LAU.
LA abnormalities have been associated with increased stroke risk, and stroke, in turn, can lead to abnormalities in the LA. This neurocardiac interaction was first documented in the 1940s (31); the then termed post-cerebral injury cardiac dysfunction is now known as brain-heart syndrome or stroke-heart syndrome (1,2,32,33). Poststroke systemic (inflammation, central autonomic dysregulation, catecholamine release, and cell death signal release) and local injury (inflammation, sympathetic nerve sprouting, myocardial necrosis, hemorrhage, fibrosis, vascular endothelial dysfunction, atherogenesis, and plaque rupture) collectively drive metabolic disturbances, hemodynamic instability, and tissue-level alterations in both atrial and ventricular cardiac tissue through the above-described pathophysiological pathways. Daccarett et al. found there to be independent association between LA fibrosis and stroke (34), with patients with a history of stroke exhibiting significantly more LA fibrosis as compared to patients without stroke.
LA stiffness is a measurement of atrial myocardial deformation. In our study, the LASI, as an index for quantifying LA stiffness, was derived from the conjunction of E/e’ (a widely used noninvasive measurement of pulmonary artery wedge pressure) and LASr (the peak atrial longitudinal strain and a widely used noninvasive measurement of myocardial stretch). Previous studies have confirmed that LA strain is inversely correlated with the extent of atrial fibrosis (35-37). Therefore, atrial fibrosis results in an increased LASI. It has also been demonstrated that LASI is an early sign of subclinical myocardial dysfunction (19), and as a higher LASI precedes LA enlargement and LV hypertrophy, it can be used as a marker of early target organ damage in hypertension (20). Moreover, the LASI is independently associated with impaired exercise tolerance and quality of life in obese patients with heart failure and preserved ejection fraction (HFpEF) (21) and has also been associated with increased risk for all-cause mortality and hospitalization caused by heart failure in patients with HFpEF (22).
Atrioventricular chamber filling, emptying, and active contraction are not synchronous. Normally, the atria fill as the ventricles contract and eject blood into the pulmonary and systemic circulations, and they empty as the ventricles relax and contract at the end of ventricular diastole. LA and LV are only directly in sync during diastole, with their function and filling pressures being tightly coupled (38,39). The LACI measured at this moment can capture the combined LA and LV performance and has better prognostic value for the early prediction of cardiovascular outcomes than do LA or LV parameters used alone (6,40,41). A high LACI is independently associated with an impaired cardiac function, including larger LA and LV volume, lower LVEF and LAEF, higher pulmonary artery wedge pressure, higher LA pressure, increased LA wall tension and fibrosis, higher pulmonary artery systolic pressure, worse right ventricular dysfunction, higher degrees of mitral and tricuspid regurgitation, and more severe heart failure symptoms. Conversely, a lower LACI can effectively rule out the risk of event occurrence and provide further risk stratification in patients at relatively low risk. In our study, we used LACI >0.25 as the criterion of LAU because, as indicated by Pezel et al.’s study, it is the optimal cutoff for the composite of the four cardiovascular outcomes: incident atrial fibrillation, incident heart failure, coronary heart disease death, and hard cardiovascular disease according to (6).
BNP is mainly secreted by myocytes in the LV in response to volume and pressure overload and can be used as a cardiovascular biomarker for the diagnosis and monitoring of heart failure and stroke. BNP is further associated with increased mortality in stroke, the occurrence of cardioembolic stroke, and the recurrence of stroke (42). Moreover, among patients with acute heart failure and atrial fibrillation, it is associated with LA structural remodeling and poor prognosis (43). In our study, a higher level of BNP in patients with AIS and LAU indicated the presence of impaired atrioventricular structure and function. Consequently, it is possible that LASI and BNP are independent risk factors for LAU, which is line with the study by Pezel et al., in which LACI was independently associated with the markers of myocardial fibrosis and the concentration of N-terminal pro B-type natriuretic peptide (8). However, inconsistent with our findings, Perez et al. also reported age, sex, ethnicity, diabetes, and body mass index to be independent determinants of LACI (8). This discrepancy may be attributable to the differences in imaging protocols, study populations, and samples used in the studies.
In 2020, the Chinese government developed and implemented an innovative case-based payment method under the regional global budget, referred to as the diagnosis-intervention packet (DIP) payment to pay for inpatient care. Compared to the preintervention period, the postintervention period had significantly decreases in inpatient medical costs per case and the proportion of the out-of-pocket expenditure in inpatient medical costs both in tertiary and secondary hospitals. The DIP payment reform demonstrated a dual effectiveness in the regulation of hospital inpatient care practices and optimization of regional healthcare resource allocation (44). As far as service providers are concerned, it is necessary to select the appropriate diagnosis and treatment strategies to achieve a cost-effective balance. In our study, the LASI echocardiographic measurement demonstrated good performance in predicting LAU and was also relatively economical, indicating its obvious superiority.
However, certain limitations to our should be acknowledged. First, a cardiac magnetic resonance (CMR)-derived LACI (>0.25) was used as the LAU criterion, and the results of echocardiography and CMR differed to an extent. Second, patients with severe stroke often experienced delirium, did not cooperate fully during echocardiograms, and required life-support interventions (intubation, mechanical ventilation, and cardiac monitoring), hindering standardized cardiac imaging. Third, arrhythmia, especially atrial fibrillation, inhibited the accurate and reliable assessment of LA volume and strain via the 4D auto LAQ tool in these patients. Fourth, all of the study participants had lesions located in the anterior circulation, and there were no patients with cerebellar infarcts, transient ischemic attack, acute hemorrhagic stroke, or traumatic brain injury. Finally, the sample size was small, and there may thus be a deviation in the statistical results. Studies based on larger populations would provide greater statistical weight.
Conclusions
This study assessed the performance and cost-effectiveness of echocardiographic cardiac function measurement and blood biomarker assay on admission for predicting LAU in patients with AIS. Despite certain limitations, the findings have clinical value in informing the risk assessment and monitoring of patients with AIS.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1243/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1243/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by institutional ethics board of Human Research Ethics Committee of Yangpu Hospital, School of Medicine, Tongji University (No. LL-2023-SCI-018). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sposato LA, Hilz MJ, Aspberg S, Murthy SB, Bahit MC, Hsieh CY, Sheppard MN, Scheitz JF. World Stroke Organisation Brain & Heart Task Force. Post-Stroke Cardiovascular Complications and Neurogenic Cardiac Injury: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:2768-85. [Crossref] [PubMed]
- Fan X, Cao J, Li M, Zhang D, El-Battrawy I, Chen G, Zhou X, Yang G, Akin I. Stroke Related Brain-Heart Crosstalk: Pathophysiology, Clinical Implications, and Underlying Mechanisms. Adv Sci (Weinh) 2024;11:e2307698. [Crossref] [PubMed]
- Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc 2004;79:1008-14. [Crossref] [PubMed]
- Mendez B, Ramos-Ventura C, Zapata C, Arteaga C, Soriano-Navarro E, Espinosa-Lira F, González-Oscoy R, Barboza M, Roldán FJ, Arauz A. The role of left atrial enlargement and age in the prediction of recurrence in embolic strokes of undetermined source. Arch Cardiol Mex 2020;90:498-502. [Crossref] [PubMed]
- Xue J, Xu XS, Zhu XQ, Li ZZ, Zhang XG, Ma YT, Yang WH, Liu LY, Yue YH. Left Atrial Enlargement is Associated with Stroke Severity with Cardioembolic and Cryptogenic Subtypes in a Chinese Population. J Stroke Cerebrovasc Dis 2020;29:104767. [Crossref] [PubMed]
- Pezel T, Venkatesh BA, De Vasconcellos HD, Kato Y, Shabani M, Xie E, Heckbert SR, Post WS, Shea SJ, Allen NB, Watson KE, Wu CO, Bluemke DA, Lima JAC. Left Atrioventricular Coupling Index as a Prognostic Marker of Cardiovascular Events: The MESA Study. Hypertension 2021;78:661-71. [Crossref] [PubMed]
- Pezel T, Garot P, Toupin S, Sanguineti F, Hovasse T, Unterseeh T, Champagne S, Morisset S, Chitiboi T, Jacob AJ, Sharma P, Venkatesh BA, Lima JAC, Garot J. AI-Based Fully Automated Left Atrioventricular Coupling Index as a Prognostic Marker in Patients Undergoing Stress CMR. JACC Cardiovasc Imaging 2023;16:1288-302. [Crossref] [PubMed]
- Pezel T, Venkatesh BA, Vasconcellos HD, Kato Y, Post WS, Wu CO, Heckbert SR, Bluemke DA, Cohen-Solal A, Logeart D, Henry P, Lima JAC. Determinants of left atrioventricular coupling index: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Cardiovasc Dis 2022;115:414-25. [Crossref] [PubMed]
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Kardiol Pol 2014;72:1253-379. [Crossref] [PubMed]
- Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;64:1929-49. [Crossref] [PubMed]
- Ibrahim H, Lowenstern A, Goldsweig AM, Rao SV. Integrating Structural Heart Disease Trainees within the Dynamics of the Heart Team: The Case for Multimodality Training. Struct Heart 2023;7:100167. [Crossref] [PubMed]
- Izumo M, Akashi YJ. Exercise echocardiography for structural heart disease. J Echocardiogr 2016;14:21-9. [Crossref] [PubMed]
- Aurich M, André F, Keller M, Greiner S, Hess A, Buss SJ, Katus HA, Mereles D. Assessment of left ventricular volumes with echocardiography and cardiac magnetic resonance imaging: real-life evaluation of standard versus new semiautomatic methods. J Am Soc Echocardiogr 2014;27:1017-24. [Crossref] [PubMed]
- Kuusisto JK, Järvinen VM, Sinisalo JP. Validation of 3D echocardiographic volume detection of left atrium by human cadaveric casts. BMC Med Imaging 2018;18:43. [Crossref] [PubMed]
- Chen L, Zhang C, Wang J, Guo L, Wang X, Liu F, Li X, Zhao Y. Left atrial strain measured by 4D Auto LAQ echocardiography is significantly correlated with high risk of thromboembolism in patients with non-valvular atrial fibrillation. Quant Imaging Med Surg 2021;11:3920-31. [Crossref] [PubMed]
- Xing Y, Zhang Y, Zhao R, Shi J, Chen Y, Chen L, Pan C. Changes of left atrial morphology and function evaluated with four-dimensional automated left atrial quantification echocardiography in patients with coronary slow flow phenomenon and preserved left ventricular ejection fraction. Int J Cardiol 2023;393:131351. [Crossref] [PubMed]
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32:1-64. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Mahfouz RA, Gomma A, Goda M, Safwat M. Relation of left atrial stiffness to insulin resistance in obese children: Doppler strain imaging study. Echocardiography 2015;32:1157-63. [Crossref] [PubMed]
- Zhao Y, Sun Q, Han J, Lu Y, Zhang Y, Song W, Cheng Y, Cong T, Liu Y, Jiang Y. Left atrial stiffness index as a marker of early target organ damage in hypertension. Hypertens Res 2021;44:299-309. [Crossref] [PubMed]
- Singleton MJ, Nelson MB, Samuel TJ, Kitzman DW, Brubaker P, Haykowsky MJ, Upadhya B, Chen H, Nelson MD. Left Atrial Stiffness Index Independently Predicts Exercise Intolerance and Quality of Life in Older, Obese Patients With Heart Failure With Preserved Ejection Fraction. J Card Fail 2022;28:567-75. [Crossref] [PubMed]
- Kim D, Seo JH, Choi KH, Lee SH, Choi JO, Jeon ES, Yang JH. Prognostic Implications of Left Atrial Stiffness Index in Heart Failure Patients With Preserved Ejection Fraction. JACC Cardiovasc Imaging 2023;16:435-45. [Crossref] [PubMed]
- Meng F, Li J, Zhao R, Wu Y, Liu Y, Yang Y, Yang Y, Zhou N, Dong L, Kong D, Chen H, Shu X, Liu P, Pan C. Left atrioventricular coupling index assessed with three-dimensional echocardiography: a prognostic marker of short-term outcomes in light-chain cardiac amyloidosis. Amyloid 2025;32:63-71. [Crossref] [PubMed]
- Fan J, Wang H, Ma C, Zhou B. Characteristics of atrial ventricular coupling and left atrial function impairment in early Fabry disease patients using two-dimensional speckle tracking echocardiography. Int J Cardiol 2025;422:132967. [Crossref] [PubMed]
- Francesca P, Eluisa F, Lorenzo P, Giovanni G, Diego B, Vincenzo N, Paolo M, Mulè M, Concetta Z, Gianluca DB, Carerj S, Manlio C, Faletra FF. The role of left atrio-ventricular coupling index and left atrial ejection fraction in predicting onset of atrial fibrillation and adverse cardiac events in hypertrophic cardiomyopathy. Cardiovasc Ultrasound 2025;23:10. [Crossref] [PubMed]
- Meucci MC, Fortuni F, Galloo X, Bootsma M, Crea F, Bax JJ, Marsan NA, Delgado V. Left atrioventricular coupling index in hypertrophic cardiomyopathy and risk of new-onset atrial fibrillation. Int J Cardiol 2022;363:87-93. [Crossref] [PubMed]
- Fortuni F, Biagioli P, Myagmardorj R, Mengoni A, Chua AP, Zuchi C, Sforna S, Bax J, Ajmone Marsan N, Ambrosio G, Carluccio E. Left Atrioventricular Coupling Index: A Novel Diastolic Parameter to Refine Prognosis in Heart Failure. J Am Soc Echocardiogr 2024;37:1038-46. [Crossref] [PubMed]
- Rautenberg T, Gerritsen A, Downes M. Health Economic Decision Tree Models of Diagnostics for Dummies: A Pictorial Primer. Diagnostics (Basel) 2020.
- Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. [Crossref] [PubMed]
- Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994;25:2220-6. [Crossref] [PubMed]
- BYER E. ASHMAN R, TOTH LA. Electrocardiograms with large, upright T waves and long Q-T intervals. Am Heart J 1947;33:796-806. [Crossref] [PubMed]
- Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ Res 2017;121:451-68. [Crossref] [PubMed]
- Samuels MA. The brain-heart connection. Circulation 2007;116:77-84. [Crossref] [PubMed]
- Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol 2011;57:831-8. [Crossref] [PubMed]
- Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231-9. [Crossref] [PubMed]
- Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498-506. [Crossref] [PubMed]
- Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, Lisi G, Maccherini M, Chiavarelli M, Massetti M, Tanganelli P, Mondillo S. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 2013;111:595-601. [Crossref] [PubMed]
- Sengupta PP, Narula J À. LA mode atrioventricular mechanical coupling. JACC Cardiovasc Imaging 2014;7:109-11. [Crossref] [PubMed]
- Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol 2008;101:1626-9.
- Pezel T, Dillinger JG, Toupin S, Mirailles R, Logeart D, Cohen-Solal A, Unger A, Canuti ES, Beauvais F, Lafont A, Gonçalves T, Lequipar A, Gall E, Boutigny A, Ah-Sing T, Hamzi L, Lima JAC, Bousson V, Henry P. Left atrioventricular coupling index assessed using cardiac CT as a prognostic marker of cardiovascular death. Diagn Interv Imaging 2023;104:594-604. [Crossref] [PubMed]
- Lange T, Backhaus SJ, Schulz A, Evertz R, Kowallick JT, Bigalke B, Hasenfuß G, Thiele H, Stiermaier T, Eitel I, Schuster A. Cardiovascular magnetic resonance-derived left atrioventricular coupling index and major adverse cardiac events in patients following acute myocardial infarction. J Cardiovasc Magn Reson 2023;25:24. [Crossref] [PubMed]
- Harpaz D, Seet RCS, Marks RS, Tok AIY. B-Type Natriuretic Peptide as a Significant Brain Biomarker for Stroke Triaging Using a Bedside Point-of-Care Monitoring Biosensor. Biosensors (Basel) 2020;10:107. [Crossref] [PubMed]
- Sakane K, Kanzaki Y, Okuno T, Nakayama S, Hasegawa H, Tokura D, Horai R, Tsuda K, Maeda D, Sakatani Y, Hoshiga M. Left Atrial Remodeling Related to Disproportionately Low B-Type Natriuretic Peptide in Acute Heart Failure Patients with Atrial Fibrillation. Am J Cardiol 2023;209:128-37. [Crossref] [PubMed]
- Ding Y, Yin J, Zheng C, Dixon S, Sun Q. The impacts of diagnosis-intervention packet payment on the providers' behavior of inpatient care-evidence from a national pilot city in China. Front Public Health 2023;11:1069131. [Crossref] [PubMed]


