
Diagnostic value of different contrast-enhanced ultrasound (CEUS) methods for sentinel lymph node metastasis in patients with breast neoplasms: a meta-analysis and indirect comparison
Introduction
Breast cancer is the most common malignant tumor in female patients, and its incidence is increasing year by year (1). According to the American Cancer Society’s update of breast cancer statistics for women in the United States, the incidence of breast cancer among women has increased by 0.5% annually in the last decade; however, the mortality rate has declined by 1.3% annually due to advances in diagnostic technology (2,3). Axillary lymph node dissection must be performed in patients with axillary lymph node metastasis; however, axillary lymph node dissection may cause complications such as upper limb swelling, limb movement disturbance, and arm numbness, and can seriously affect the quality of life of patients (4). It is important to evaluate axillary lymph node metastasis in breast cancer before surgery to select the most appropriate treatment and evaluate patient prognosis.
In breast cancer, the sentinel lymph node (SLN) is the first lymph node to which tumor cells metastasize, and thus can indicate the status of axillary lymph nodes (4,5). In recent years, SLN has gradually been applied in the diagnosis of axillary lymph node metastasis, and has attracted increasing attention since the publication of the ACOSOG Z0011 trial (4). Usually, SLNs can be displayed using blue dye, indocyanine green, or intraoperative nuclide tracking, and qualitative diagnosis can be combined with SLN biopsy (6). However, SLN biopsy can still cause unnecessary harm to patients. Therefore, determining the diagnostic status of SLNs before surgery could reduce the need for biopsy and clearance, and contribute to the staging and clinical treatment of patients with axillary lymph node metastasis.
Traditional preoperative imaging methods can provide diagnostic information on axillary lymph nodes; however, such methods are not ideal for the location and qualitative evaluation of SLNs (7,8). With the rapid development of contrast-enhanced ultrasound (CEUS) technology, the contrast medium collected in lymph nodes through lymphatic vessels and SLNs can be detected by CEUS (9,10). A number of studies have shown that CEUS is valuable in the diagnosis of SLN breast cancer; using an intraoperative blue dye, CEUS can be used to locate SLNs, and assess SLN status based on the enhancement patterns (11,12). At present, the usual CEUS injection methods in breast cancer include elbow vein injection and four-point subcutaneous injection around the areola (13,14). Recent studies have shown that the diagnostic value of ultrasound contrast agents injected at different sites for breast cancer SLN metastasis varies (11,13), and to date, no systematic review has been conducted.
Therefore, this study sought to summarize the findings of published original studies on the CEUS diagnosis of SLN metastasis in breast cancer patients and compare the value of the four-point subcutaneous injection around the areola and injection via the cubital vein in predicting SLN metastasis in breast cancer patients. Our findings could aid in clinical staging, reduce unnecessary biopsies, and improve patient prognosis. We present this article in accordance with the PRISMA-DTA reporting checklist (available at: https://qims.amegroups.com/article/view/10.21037/qims-24-317/rc).
Methods
Before commencing the literature search, the protocol for this review was registered with the PROSPERO registry (No. CRD42023475494).
Literature search
The PubMed, Web of Science, Embase, OVID, and Cochrane Library databases were searched to retrieve relevant articles on breast cancer patients with SLN metastasis from database establishment to October 1, 2023. The language was limited to English. The keywords included the following subject words and free words: “Breast Neoplasm” [Medical Subject Headings (MeSH)], “Sentinel Lymph Node”, and “contrast-enhanced ultrasound”. The keywords were combined to generate a retrieval strategy. Details of the literature search strategy are provided in Appendix 1. Two reviewers independently screened the articles.
Selection criteria
The full text of each article was rigorously evaluated based on the following inclusion criteria: (I) population: patients diagnosed with breast cancer; (II) intervention/exposure: CEUS of SLN was performed, and the injection modality was clearly documented; (III) gold standard: pathological results of intraoperative SLN; (IV) results: evaluation of the diagnostic accuracy of preoperative CEUS for SLN; all the data were organized into a 2×2 contingency table, and the results included true positive (TP), false positive (FP), false negative (FN), and true negative (TN) data; and (V) number of patients: ≥30. Articles were excluded if they met any of the following exclusion criteria: (I) were not directly related to this meta-analysis; (II) included patients who had previously received radiotherapy/chemotherapy; (III) had inadequate data (i.e., the data only included the differential rate or diagnostic value of CEUS-guided core biopsy, and did not include the corresponding pathologic results, and the TP, FP, FN, and TN were are not available); (IV) the data were derived from subsequent articles or duplicate reports, in which case the most recently published or sampled article was selected; and/or (V) the article comprised a conference abstract, review, case report, animal experiment, or graduation thesis publication. In this study, two independent reviewers screened the title, abstract, and full text of the articles, and any disagreements were resolved through discussion.
Data extraction
The selection of the titles, abstracts, and full-text articles, as well as the evaluation of the quality of the research, was independently completed by two researchers, and consensus was reached on all issues through discussion and review. The following data were extracted from articles that met the study criteria: first author, year of publication, country, sample size, contrast agent, injection dose, and equipment, as well as the TP, FP, FN and TN data, which were published in a four-cell (2×2) table.
Quality assessment
The methodological quality was independently evaluated by two researchers using the quality assessment tool QUADAS-2, which includes 14 evaluation items. Each item was rated as “yes” (2 points), “no” (0 points), or “unclear” (1 points). The total QUADAS score can range from 0 (points) to 20 (points). A score ≥18 points indicates good quality, a score ≥16 points but <18 points indicates average quality, and a score <16 points indicates poor quality.
Data analysis
The diagnostic four-grid table data were extracted from the literature. Stata 14.0 (StataCorp LLC, College Station, Texas, USA) was used to calculate the combined sensitivity (SEN), combined specificity (SPE), combined positive likelihood ratio (PLR), combined negative likelihood ratio (NLR), diagnostic score, diagnostic odds ratio (DOR), and area under the curve (AUC) of the receiver operating characteristic (ROC) curve of SLN metastasis of breast cancer diagnosed by ultrasound contrast agent via four-point subcutaneous injection (i.e., into the skin around the areola at 3, 6, 9, and 12 o’clock) or intravenous injection. The relative SEN, relative SPE, relative positive likelihood ratio (RPLR), relative negative likelihood ratio (RNLR), and relative diagnostic odds ratio (RDOR) of the four-point subcutaneous injection method and the intravenous injection method were calculated. When the confidence interval (CI) for the relative odds ratio (OR) of the diagnostic index contained 1, it indicated that there was no statistically significant difference between the two injection methods.
The potential heterogeneity between studies was evaluated using Cochran’s Q-statistic and the I2 test. If the Q-test showed a P value <0.05 or the I2 test showed a value >50%, indicating significant heterogeneity, then a random-effects model was used; otherwise, a fixed-effects model was used. If there was heterogeneity, a meta-regression analysis on the source of heterogeneity was first performed, and if the regression revealed the source of heterogeneity, a subgroup analysis was performed. Stata 14.0 (StataCorp LLC, College Station, Texas, USA) was used to draw Deek’s funnel plots to identify publication bias in the included studies.
Results
Included research articles and quality assessment
A total of 867 articles were retrieved from the database search, and an additional 21 articles were identified through a review of the relevant literature or reference lists. After excluding duplicate articles, 532 articles remained, of which 330, including reviews, case reports, guidelines and animal experiments, were excluded. An additional 109 articles were excluded after the title and abstract reading because they were inconsistent with the direction of the study. Of the remaining 93 articles, 73 were excluded because they were not related to SLN, or the target values could not be obtained, or they were not published in English. Additionally, three articles were excluded because they did not include the final pathology results. Thus, ultimately, this meta-analysis included 17 articles (with 19 datasets). The study of Zhuang et al. subcutaneously injected contrast medium into the skin around the areola at 3, 6, 9, and 12 o’clock (13); the study of Sun et al. used four-point subcutaneous injection around the areola, but used two contrast agents SonoVue and Sonazoid (15). The flowchart is shown in Figure 1.
In the included studies, metastatic lymph nodes were defined as positive when confirmed by SLN biopsy or axillary lymph node dissection, and negative when no metastasis was found by SLN biopsy or total axillary lymph node dissection. Of the datasets, seven comprised patients who were injected intravenously and 12 comprised patients who were injected subcutaneously at 3, 6, 9, and 12 o’clock around the areola. All studies used the pathological diagnosis of SLN as the gold standard, and the basic information of all included studies is summarized in Table 1.
Table 1
| No. | Study | Country | Year | No. of patients | Age (years) | Contrast agent | Injection dose (mL) | Instrument | 2×2 table | QUADAS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | ||||||||||
| 1 | Dellaportas, D. (16) | Greece | 2015 | 50 | 60±12.5 | SNV | 5 | – | 15 | 5 | 3 | 27 | 18 |
| 2 | Agliata, G. (17) | Italy | 2017 | 50 | 55±10.8 | SNV | 2.4 | Philips | 28 | 4 | 0 | 18 | 15 |
| 3 | Zhuang, L. (13) | China | 2022 | 60 | 55±14.6 | SNV | 5 | GE | 24 | 5 | 3 | 37 | 18 |
| 4 | Liu, H. (18) | China | 2008 | 104 | 50±13.4 | SNV | 2.4 | Philips | 17 | 1 | 26 | 59 | 20 |
| 5 | Matsuzawa, F. (19) | Japan | 2015 | 32 | – | SNZ | 0.015 | Aplio | 9 | 1 | 7 | 20 | 18 |
| 6 | Yang, S. L. (20) | China | 2016 | 98 | 45±12.3 | SNV | 2.5 | Esaote | 12 | 1 | 16 | 3 | 16 |
| 7 | Sun, Y. J. (21) | China | 2012 | 52 | 46±10.6 | SNV | 2.5 | Siemens | 7 | 0 | 25 | 25 | 19 |
| 8 | Qiao, J. (22) | China | 2021 | 212 | 47±11.8 | SNV | 0.5 | Philips | 61 | 17 | 6 | 124 | 18 |
| 9 | Li, J. (23) | China | 2019 | 453 | 49±3.2 | SNV | 0.6 | Philips | 274 | 39 | 9 | 443 | 13 |
| 10 | Li, J. T. (24) | China | 2019 | 83 | 47±9.5 | SNV | 2 | Philips | 21 | 8 | 5 | 49 | 19 |
| 11 | Zhuang, L. (13) | China | 2022 | 60 | 55±14.6 | SNV | 1.2 | GE | 25 | 2 | 4 | 38 | 18 |
| 12 | Sun, Y. (15) | China | 2021 | 205 | 53±12.3 | SNV | 2 | GE | 30 | 9 | 8 | 163 | 18 |
| 13 | Sun, Y. (15) | China | 2021 | 205 | 53±12.3 | SNZ | 0.4 | GE | 23 | 7 | 3 | 69 | 18 |
| 14 | Zheng, Y. (25) | China | 2023 | 163 | 54±13.3 | SNV | 4 | Esaote | 41 | 29 | 13 | 82 | 16 |
| 15 | Liu, Y. B. (26) | China | 2021 | 144 | – | SNV | 2.4 | Esaote | 29 | 0 | 8 | 84 | 17 |
| 16 | Zhu, Y. (27) | China | 2021 | 201 | 52±10.6 | SNV | 0.5 | Philips | 62 | 22 | 15 | 90 | 18 |
| 17 | Niu, Z. (9) | China | 2023 | 78 | 52±12.1 | SNZ | 0.5 | GE | 19 | 11 | 4 | 44 | 20 |
| 18 | Zhang, Q. (28) | China | 2021 | 120 | 51±9.6 | SNV | 2 | Philips | 33 | 7 | 2 | 78 | 17 |
| 19 | Ma, S. (29) | China | 2021 | 108 | 48±4.0 | SNV | 0.5 | Philips | 88 | 26 | 20 | 114 | 18 |
Data of age are presented as mean ± standard deviation. Studies 1–7: contrast medium was intravenously injected; studies 8–19 contrast medium was subcutaneously injected into the skin around the areola at 3, 6, 9, and 12 o’clock. FN, false negative; FP, false positive; QUADAS, the quality assessment tool of diagnostic accuracy studies; SNV, SonoVue; SNZ, Sonazoid; TN, true negative; TP, true positive.
Quality evaluation
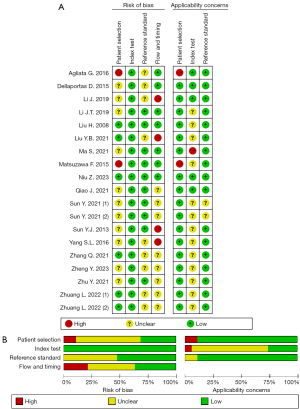
Quality evaluation of diagnostic experimental accuracy study tool (QUADAS-2) was used to assess the quality of the literature. Overall, in four fields (i.e., patient sampling, index tests, reference standard, and flow and timing), the number of articles with a low bias risk and an unclear bias risk was 10 and 2, respectively, and the number of articles with a high bias risk was 7; while in three fields, the number of articles with high, unclear, and low risks that the included patients and setting did not match the review question was 3, 1, and 15, respectively. Among the datasets, two were low quality, seven were average quality, and 10 were good quality. The results are shown in Figure 2.
Diagnosis of benign and malignant SLNs
Comparison of the four-point subcutaneous injection and intravenous injection of contrast agent
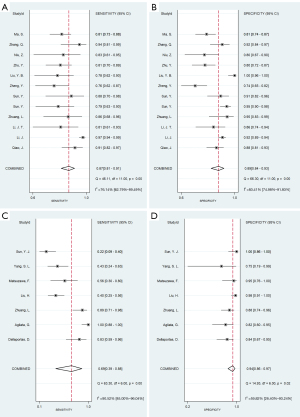
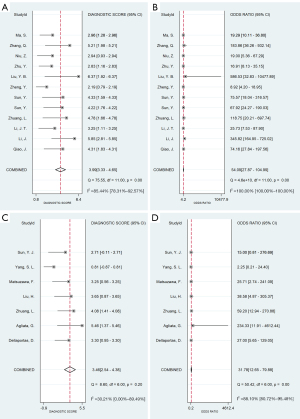
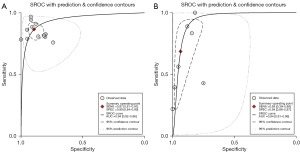
Statistical heterogeneity was found in both the ultrasound contrast agent methods used in the studies; that is, the four-point subcutaneous injection method (11 articles (9,13,15,22-29), comprising 12 groups of data; P<0.001, I2>50%) and the intravenous injection method [seven articles (13,16-21), comprising seven groups of data; P<0.001, I2>50%]. A random-effects model was used for the meta-analysis. The results showed that the SEN and SPE of the four-point subcutaneous injection method were 0.87 [95% confidence interval (CI): 0.81–0.91] and 0.89 (95% CI: 0.84–0.93), respectively (Figure 3A,3B), while the SEN and SPE of the intravenous injection method were 0.69 (95% CI: 0.39–0.88) and 0.94 (95% CI: 0.86–0.97), respectively (Figure 3C,3D); the PLR and NLR of the four-point subcutaneous injection method were 8.13 (95% CI: 5.39–12.28) and 0.15 (95% CI: 0.10–0.22), respectively (Figure 4A,4B), while the PLR and NLR of the intravenous injection method were 10.65 (95% CI: 5.60–20.28) and 0.34 (95% CI: 0.15–0.75), respectively (Figure 4C,4D); the diagnostic score and DOR of the four-point subcutaneous injection method were 3.99 (95% CI: 3.33–4.65) and 54.09 (95% CI: 27.87–104.99), respectively (Figure 5A,5B), while the diagnostic score and DOR of the intravenous injection method were 3.46 (95% CI: 2.54–4.38) and 31.78 (95% CI: 12.65–79.88), respectively (Figure 5C,5D); the area under the ROC curve of the four-point subcutaneous injection method was 0.94 (95% CI: 0.92–0.96) (Figure 6A) and that of the intravenous injection method was 0.94 (95% CI: 0.91–0.96) (Figure 6B); the missed diagnosis rate of the four-point subcutaneous injection method was 12%, and that of the intravenous injection method was 42%.

To analyze the source of heterogeneity, a meta-regression analysis was conducted, and age, sample size, contrast agent, and contrast agent dose were taken as the variables. The results of the meta-analysis of the four-point subcutaneous injection method showed that age (P=0.569), sample size (P=0.876), contrast agent (P=0.726), and contrast agent dose (P=0.959) all had P values >0.05, indicating that these variables were not sources of heterogeneity (Table 2). Despite the heterogeneity among the seven studies, no subgroup analysis was performed because the included data did not meet the subgroup requirements.
Table 2
| LogES | Coef. | SE | t | P | 95% CI |
|---|---|---|---|---|---|
| Age (years) | –0.55 | 0.92 | –0.61 | 0.57 | –2.72, 1.63 |
| Sample | 0.19 | 1.21 | 0.16 | 0.88 | –2.66, 3.04 |
| Agent | 0.72 | 1.99 | 0.36 | 0.73 | –3.99, 5.44 |
| Injection dose (mL) | –0.05 | 0.92 | –0.05 | 0.96 | –2.24, 2.14 |
ES, effect size; Coef., coefficient; SE, standard error; CI, confidence interval.
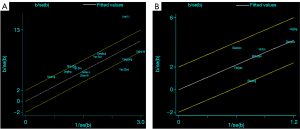
To further explore the sources of heterogeneity, a Galbraith radial plot was used. In terms of the four-point subcutaneous injection method, the distribution of Li et al.’s article was relatively scattered (Figure 7A), and was thus considered a source of heterogeneity (23). The reason for heterogeneity in Li et al.’s study is that the sample size was relatively large, with 453 cases accounting for a high proportion of all included patients. Further, the selection of patients was not continuous, which also had a significant effect on the heterogeneity of the included literature. In terms of the intravenous injection method, the distribution of two articles by Agliata et al. and Yang et al. was relatively scattered (Figure 7B), and was thus considered source of heterogeneity (17,20). Notably, these two articles did not strictly follow the patient selection criteria, and the selection of patients was not continuous; thus, the sources of heterogeneity were obvious.
Indirect comparison of the four-point subcutaneous injection and intravenous injection
Taking the final pathological results as the common diagnostic gold standard, the relative ratio of the diagnostic index of SLN metastasis of breast cancer using the four-point subcutaneous injection method and the intravenous injection method was calculated. Compared to the intravenous injection method, the four-point subcutaneous injection method had a relative ratio of 1.26 (95% CI: 0.88–1.81), a relative SPE of 0.95 (95% CI: 0.88–1.00), a RPLR of 0.76 (95% CI: 0.34–1.71), a RNLR of 0.44 (95% CI: 0.17–1.16), a relative diagnostic score of 1.15 (95% CI: 0.84–1.58), and a RDOR of 1.61 (95% CI: 0.44–5.90). The relative ratio of the diagnostic indexes suggests that the original diagnostic performance was not reduced, and the four-point subcutaneous injection of ultrasound contrast agent improved the SEN and reduced the rate of missed diagnosis. The area under the ROC curve of the four-point subcutaneous injection method in the diagnosis of SLN metastasis of breast cancer was larger than that of the intravenous injection method, suggesting that the four-point subcutaneous injection method was better than the intravenous injection method in the diagnosis of SLN metastasis of breast cancer.
Evaluation of publication bias
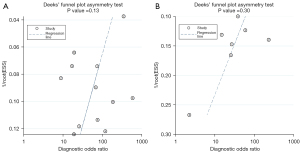
We generated a Deek’s funnel plot using Stata 14.0 (StataCorp LLC, College Station, Texas, USA) and the following input instructions: midas, TP, FP, FN, TN, and publication bias. The results suggested that compared to intravenous injection there was less publication bias in the diagnosis of breast cancer SLN metastasis by four-point subcutaneous injection (t=−0.86, P=0.13; Figure 8A); The results also suggested that compared to intravenous injection there was less possibility of publication bias in the diagnosis of SLN metastasis of breast cancer via the intravenous injection of the ultrasound contrast agent (t=−1.16, P=0.30; Figure 8B).
Discussion
Previous studies have shown that CEUS has good value in predicting SLN metastasis in breast cancer (30). Both intravenous injection and four-point subcutaneous injection have been shown to improve the detection of preoperative SLN metastasis in breast cancer (9,22,29). Liu et al. reported that four-point subcutaneous injection around the areola had a higher value in predicting SLN metastasis than intravenous injection (31). The conclusions reached in this study are consistent with those of Liu et al. Compared with the intravenous injection method, the four-point subcutaneous injection method improved the SEN of CEUS in the diagnosis of SLN metastasis of breast cancer, reduced the rate of missed diagnosis, and was better able to diagnose the status of SLN. This may be related to the way in which breast lymph nodes drain; the micro-lymphatic vessels between breast glands radiate like the lymphatic plexus in the areola area, and then from one to two collecting lymphatic vessels through the areola area lymphatic plexus to drain to the SLN. The course of the lymphatic vessels and the SLN can be clearly displayed by CEUS (30,32,33).
Axillary lymph node metastasis is a necessary path for lymphatic metastasis in breast cancer patients, and the choice of treatment plan and the prognosis of breast cancer patients are closely related to axillary lymph node metastasis. The SLN is the first site of axillary lymph node metastasis in breast cancer, and predicts axillary lymph node metastasis. Axillary lymph node dissection is not feasible when there are less than three SLN metastases (4,34). Excessive lymph node dissection may cause complications such as swelling of the upper limb on the operated side, impaired limb mobility, and numbness of the arm, which may seriously affect the quality of life of patients (35). In addition, because ultrasonography is used as an alternative to SLN biopsy, subsequent ultrasound clinical trials on axillary SLN metastasis may allow operators to omit the biopsy step and reduce the complexity of ultrasound clinical trials on axillary SLN. The preoperative prediction of SLN metastasis is very important, and accurate prediction could help clinicians to adjust surgical plans in a timely manner and improve patient prognosis.
CEUS has a number of advantages, including real-time visualization, simplicity, low costs, and ease of dissemination. Previous studies have shown that sentinel ultrasonography can be used as a SLN tracer with high accuracy in the diagnosis of SLN metastases in breast cancer (23). Compared with magnetic resonance imaging (MRI), ultrasonography is simpler, faster, and easier to promote, and it can be applied to some patients for whom MRI is contraindicated, such as those who are post-cardiac stenting, post-pacemaker surgery, and post-fracture nail placement. Compared with conventional ultrasound, ultrasonography has high SEN and SPE, and its AUC for the diagnosis of SLN metastasis of breast cancer was significantly higher than that of conventional ultrasound (9). Compared to positron emission tomography-computed tomography, CEUS is radiation-free and affordable. Therefore, CEUS can more easily help clinicians diagnose SLN metastasis.
The disadvantage of all the included studies is that they were small. Further, we searched databases and found that most studies related to the use of CEUS for breast cancer used only one method, and relatively few studies compared transcubital veins with subcutaneous four-point injection around the areola. Moreover, patients with a history of surgery and trauma to the axilla and upper outer quadrant of the breast were excluded from the meta-analysis, which created an unavoidable selection bias.
The advantage of this study is that four-point subcutaneous injection around the areola can clearly display lymphatic vessels and SLNs. Conversely, cubital venography cannot clearly display the course of lymphatic vessels. The disadvantage of this study is that it only compared four-point subcutaneous injection around the areola with intravenous injection. Currently, the injection methods used include single-point injection through the outer upper quadrant of the breast, subcutaneous injection around the tumor, and gland injection around the tumor. If the best injection method could be identified, the pain of patients could be reduced. This study did not calculate the SLN detection rates of the two methods, as only a few articles provided detection rates; however, if the detection rates of the two methods could be quantitatively analyzed, a reference for the staging of axillary lymph nodes in breast cancer could be provided. This study can be followed up by expanding the sample size and comparing more injection methods.
Conclusions
Compared with intravenous injection, the four-point subcutaneous injection around the areola at 3, 6, 9, and 12 o’clock improved the SEN of CEUS in the diagnosis of the SLN metastasis of breast cancer, reduced the rate of missed diagnosis, was better able to diagnose the status of SLNs, and provided more accurate axillary lymph node staging for breast cancer patients.
Acknowledgments
We would like to thank AME Editing Service (https://editing.amegroups.com/) for the English-language editing of the manuscript.
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-317/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-317/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134-50. [Crossref] [PubMed]
- Bundred NJ, Barnes NL, Rutgers E, Donker M. Is axillary lymph node clearance required in node-positive breast cancer? Nat Rev Clin Oncol 2015;12:55-61. [Crossref] [PubMed]
- Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. Axillary Nodal Evaluation in Breast Cancer: State of the Art. Radiology 2020;295:500-15. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Huang TW, Su CM, Tam KW. Axillary Management in Women with Early Breast Cancer and Limited Sentinel Node Metastasis: A Systematic Review and Metaanalysis of Real-World Evidence in the Post-ACOSOG Z0011 Era. Ann Surg Oncol 2021;28:920-9. [Crossref] [PubMed]
- Hounschell CA, Kilgore LJ, Pruitt P, Wilder C, Balanoff CR, Wagner JL, Baker J, Chollet-Hinton L, Larson KE. Evaluation of learning curve with Indocyanine Green (IcG) versus blue dye for sentinel lymph node biopsy in breast cancer. Am J Surg 2024;227:218-23. [Crossref] [PubMed]
- Bargon CA, Huibers A, Young-Afat DA, Jansen BAM, Borel-Rinkes IHM, Lavalaye J, van Slooten HJ, Verkooijen HM, van Swol CFP, Doeksen A. Sentinel Lymph Node Mapping in Breast Cancer Patients Through Fluorescent Imaging Using Indocyanine Green: The INFLUENCE Trial. Ann Surg 2022;276:913-20. [Crossref] [PubMed]
- Giammarile F, Vidal-Sicart S, Paez D, Pellet O, Enrique EL, Mikhail-Lette M, Morozova O, Maria Camila NM, Diana Ivonne RS, Delgado Bolton RC, Valdés Olmos RA, Mariani G. Sentinel Lymph Node Methods in Breast Cancer. Semin Nucl Med 2022;52:551-60. [Crossref] [PubMed]
- Niu Z, Gao Y, Xiao M, Mao F, Zhou Y, Zhu Q, Jiang Y. Contrast-enhanced lymphatic US can improve the preoperative diagnostic performance for sentinel lymph nodes in early breast cancer. Eur Radiol 2023;33:1593-602. [Crossref] [PubMed]
- Buonomo OC, Materazzo M, Pellicciaro M, Iafrate G, Ielpo B, Rizza S, Pistolese CA, Perretta T, Meucci R, Longo B, Cervelli V, Vanni G. Contrast-enhanced Ultrasound Using Intradermal Microbubble Sulfur Hexafluoride for Identification of Sentinel Lymph Nodes During Breast Cancer Surgery: A Clinical Trial. Anticancer Res 2023;43:557-67. [Crossref] [PubMed]
- Huang C, Luo J, Shan Z, Zhen T, Li J, Ma Q, Wei L, Liang J, Xie X, Zheng Y. The value of the improved percutaneous and intravenous contrast-enhanced ultrasound diagnostic classification in sentinel lymph nodes of breast cancer. Quant Imaging Med Surg 2024;14:2391-404. [Crossref] [PubMed]
- Fan Y, Luo J, Lu Y, Huang C, Li M, Zhang Y, Shao N, Wang S, Zheng Y, Lin Y, Shan Z. The application of contrast-enhanced ultrasound for sentinel lymph node evaluation and mapping in breast cancer patients. Quant Imaging Med Surg 2023;13:4392-404. [Crossref] [PubMed]
- Zhuang L, Ming X, Liu J, Jia C, Jin Y, Wang J, Shi Q, Wu R, Jin L, Du L. Comparison of lymphatic contrast-enhanced ultrasound and intravenous contrast-enhanced ultrasound in the preoperative diagnosis of axillary sentinel lymph node metastasis in patients with breast cancer. Br J Radiol 2022;95:20210897. [Crossref] [PubMed]
- Huang D, Cao W, Luo Y, Guan C, Liu Y, Li C, Chen J, Luo J, Luo J. Can preoperative percutaneous injection of ultrasound contrast agent locate sentinel lymph nodes of breast cancer? Front Oncol 2024;14:1471443. [Crossref] [PubMed]
- Sun Y, Cui L, Wang S, Shi T, Hao Y, Lei Y. Comparative study of two contrast agents for intraoperative identification of sentinel lymph nodes in patients with early breast cancer. Gland Surg 2021;10:1638-45. [Crossref] [PubMed]
- Dellaportas D, Koureas A, Contis J, Lykoudis PM, Vraka I, Psychogios D, Kondi-Pafiti A, Voros DK. Contrast-Enhanced Color Doppler Ultrasonography for Preoperative Evaluation of Sentinel Lymph Node in Breast Cancer Patients. Breast Care (Basel) 2015;10:331-5. [Crossref] [PubMed]
- Agliata G, Valeri G, Argalia G, Tarabelli E, Giuseppetti GM. Role of Contrast-Enhanced Sonography in the Evaluation of Axillary Lymph Nodes in Breast Carcinoma: A Monocentric Study. J Ultrasound Med 2017;36:505-11. [Crossref] [PubMed]
- Liu H, Jiang YX, Liu JB, Zhu QL, Sun Q. Evaluation of breast lesions with contrast-enhanced ultrasound using the microvascular imaging technique: initial observations. Breast 2008;17:532-9. [Crossref] [PubMed]
- Matsuzawa F, Omoto K, Einama T, Abe H, Suzuki T, Hamaguchi J, Kaga T, Sato M, Oomura M, Takata Y, Fujibe A, Takeda C, Tamura E, Taketomi A, Kyuno K. Accurate evaluation of axillary sentinel lymph node metastasis using contrast-enhanced ultrasonography with Sonazoid in breast cancer: a preliminary clinical trial. Springerplus 2015;4:509. [Crossref] [PubMed]
- Yang SL, Tang KQ, Tao JJ, Ao HF, Wan AH, Shen ZY. Clinical study of different parts of ultrasound contrast agent in the diagnosis of sentinel lymph node in breast cancer. Journal of Clinical and Experimental Medicine 2016;15:1773-6.
- Sun YJ, Mi CR. Compare the Value of Intravenous and Percutaneous Contrast-enhanced Ultrasound in Detection of Sentinel Lymph Nodes in Breast Cancer. Chinese Journal Ultrasound Med 2012;28:601-4.
- Qiao J, Li J, Wang L, Guo X, Bian X, Lu Z. Predictive risk factors for sentinel lymph node metastasis using preoperative contrast-enhanced ultrasound in early-stage breast cancer patients. Gland Surg 2021;10:761-9. [Crossref] [PubMed]
- Li J, Lu M, Cheng X, Hu Z, Li H, Wang H, Jiang J, Li T, Zhang Z, Zhao C, Ma Y, Tan B, Liu J, Yu Y. How Pre-operative Sentinel Lymph Node Contrast-Enhanced Ultrasound Helps Intra-operative Sentinel Lymph Node Biopsy in Breast Cancer: Initial Experience. Ultrasound Med Biol 2019;45:1865-73. [Crossref] [PubMed]
- Li JT, Zhao HM, Guo XH, Tian PQ, Lu MH, Li LF, Liu ZZ, Cui SD, Zhang HW. Preoperative evaluation of sentinel lymph node biopsy using contrast-enhanced ultrasonography in early breast cancer patients and the involved disturbing factors. National Medical Journal of China 2019;99:1086-9. [Crossref] [PubMed]
- Zheng Y, Sun J, Zhu L, Hu MS, Hou LZ, Liu JX, Dong FL. Diagnosing sentinel lymph node metastasis of T1/T2 breast cancer with conventional ultrasound combined with double contrast-enhanced ultrasound: a preliminary study. Quant Imaging Med Surg 2023;13:3451-63. [Crossref] [PubMed]
- Liu YB, Xia M, Li YJ, Li S, Li H, Li YL. Contrast-Enhanced Ultrasound in Locating Axillary Sentinel Lymph Nodes in Patients with Breast Cancer: A Prospective Study. Ultrasound Med Biol 2021;47:1475-83. [Crossref] [PubMed]
- Zhu Y, Fan X, Yang D, Dong T, Jia Y, Nie F. Contrast-Enhanced Ultrasound for Precise Sentinel Lymph Node Biopsy in Women with Early Breast Cancer: A Preliminary Study. Diagnostics (Basel) 2021;11:2104. [Crossref] [PubMed]
- Zhang Q, Agyekum EA, Zhu L, Yan L, Zhang L, Wang X, Yin L, Qian X. Clinical Value of Three Combined Ultrasonography Modalities in Predicting the Risk of Metastasis to Axillary Lymph Nodes in Breast Invasive Ductal Carcinoma. Front Oncol 2021;11:715097. [Crossref] [PubMed]
- Ma S, Xu Y, Ling F. Preoperative evaluation and influencing factors of sentinel lymph node detection for early breast cancer with contrast-enhanced ultrasonography: What matters. Medicine (Baltimore) 2021;100:e25183. [Crossref] [PubMed]
- Xie F, Zhang D, Cheng L, Yu L, Yang L, Tong F, Liu H, Wang S, Wang S. Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol 2015;13:319. [Crossref] [PubMed]
- Liu X, Wang M, Wang Q, Zhang H. Diagnostic value of contrast-enhanced ultrasound for sentinel lymph node metastasis in breast cancer: an updated meta-analysis. Breast Cancer Res Treat 2023;202:221-31. [Crossref] [PubMed]
- Xu H, Xu GL, Li XD, Su QH, Dong CZ. Correlation between the contrast-enhanced ultrasound image features and axillary lymph node metastasis of primary breast cancer and its diagnostic value. Clin Transl Oncol 2021;23:155-63. [Crossref] [PubMed]
- Wu X, Tang L, Huang W, Huang S, Peng W, Hu D. Contrast-enhanced ultrasonography and blue dye methods in detection of sentinel lymph nodes following neoadjuvant chemotherapy in initially node positive breast cancer. Arch Gynecol Obstet 2020;302:685-92. [Crossref] [PubMed]
- Tinterri C, Gentile D, Gatzemeier W, Sagona A, Barbieri E, Testori A, et al. Preservation of Axillary Lymph Nodes Compared with Complete Dissection in T1-2 Breast Cancer Patients Presenting One or Two Metastatic Sentinel Lymph Nodes: The SINODAR-ONE Multicenter Randomized Clinical Trial. Ann Surg Oncol 2022;29:5732-44. [Crossref] [PubMed]
- Tang J, Yang MT, Fan W, Wang X, Zhang X, Liang XM, Wang X, Xie ZM. Detection of sentinel lymph node in patients with early stage breast cancer. Ai Zheng 2005;24:1111-4.



