
Left pulmonary sequestration with portal venous drainage: a case description of surgical planning using IQQA-3D reconstruction
Introduction
Pulmonary sequestration (PS) is a rare congenital malformation of the pulmonary system, characterized by the presence of non-functional lung tissue that is not anatomically integrated in the normal bronchopulmonary tree. This anomaly can be categorized into three distinct types: intralobar, extralobar, and mixed. The intralobar type accounts for approximately 75% of all cases (1,2), and is usually affects the left lower lobe, which might be due to the complexity of embryological development of the pulmonary vasculature in this region (3). PS comprises between 0.2% to 6.4% of congenital pulmonary malformations, and may present in both pediatric and adult populations, with a higher incidence reported in males (4,5).
Case presentation
A 29-year-old male patient presented with a recurrent dry cough for 7 years, accompanied by occasional blood-streaked sputum. He had no complaints of fever, dizziness, chest tightness, or chest pain. Despite multiple courses of anti-infection treatment, the symptoms of cough and blood-streaked sputum persisted. The patient had no history of infectious diseases, such as hepatitis or tuberculosis, or chronic diseases, such as hypertension or diabetes. The patient had no history of drug or food allergies. The physical examination revealed symmetrical chest contours without deformities. The patient’s bilateral breath sounds were normal, and no adventitious sounds were noted. The cardiac examination revealed no evidence of palpable heaves or thrills, no cardiac enlargement, a regular rhythm, normal heart sounds across all valve areas, and an absence of pathological murmurs.
The patient initially visited a local hospital due to recurrent dry cough and blood-streaked sputum. No obvious abnormalities were found in the chest X-ray examination. However, the doctor recommended further examination to rule out potential pulmonary diseases. Subsequently, the patient underwent a chest computed tomography (CT) scan (Figure 1), which revealed a soft tissue density mass in the left lower lung, with uneven significant enhancement, suggesting the possibility of an abnormal blood supply.
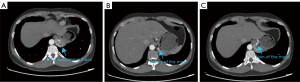
Auxiliary examination with CT angiography (CTA) of the thoracic and abdominal aorta revealed a soft tissue mass in the left lower lung (Figure 2). Post-contrast imaging showed uneven and significant enhancement of the lesion, which was supplied by an arterial branch originating from the celiac trunk and drained by a vein entering the main portal vein. These findings confirmed a diagnosis of left lower lobe PS. The CTA further revealed venous drainage of the lesion into the portal vein, indicating an infra-diaphragmatic venous drainage pattern. Arterial blood supply originated from the celiac trunk, consistent with the infra-diaphragmatic arterial supply described by Kargl et al. (6).
After admission, the patient underwent a comprehensive preoperative examination and evaluation, including blood tests, cardiopulmonary function assessment, and imaging studies, to ensure the safety and feasibility of the surgery.
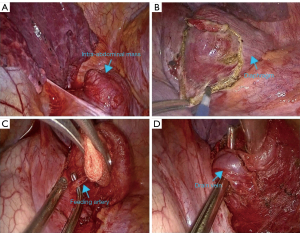
The surgery was performed in an inpatient setting. On July 25, 2022, the patient underwent a video-assisted mini-thoracotomy under general anesthesia. Intraoperatively, no significant adhesions or effusions were observed in the pleural cavity, and the interlobar fissure was well developed. The mass, located above the left diaphragm, had indistinct borders. The diaphragm tissue around the lesion was carefully separated using an electric hook (Figure 3). A single artery and vein supplying the lesion were isolated, ligated, and transected using straight cutting staplers. The base of the lesion was subsequently dissected, and the intact specimen was excised entirely. An intraoperative frozen section confirmed the benign nature of the lesion. The surgery proceeded without complications, with minimal intraoperative bleeding (20 mL). Postoperatively, the patient received prophylactic antibiotic therapy, and the incision healed uneventfully. He was discharged in a stable condition on July 31, 2022, and demonstrated no significant abnormalities during a 1-year follow-up.
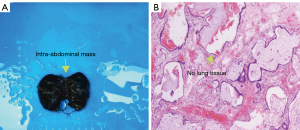
The postoperative pathological report revealed cystic dilatation of the small bronchi with fibrous tissue proliferation and chronic inflammatory cell infiltration consistent with the sequestered lung tissue. Immunohistochemical staining showed positive expression for cytokeratin-7 (CK7) in the bronchial epithelium, and positive markers for cytokeratin 5/6 (CK5/6), p63, and thyroid transcription factor-1 (TTF-1) in the basal cells, which supported the histological diagnosis (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The vascular supply to PS is often derived from one or multiple arteries, primarily originating from the thoracic and abdominal aortas. In some instances, multiple supply arteries can arise from different branches situated both above and below the diaphragm (7,8). Aberrant vascularization in PS include a wide range of atypical vessels, such as the subclavian artery, inferior phrenic artery, intercostal arteries, splenic artery, superior mesenteric artery, left gastric artery, and renal artery (8). There have also been reports of PSs receiving vascular supply from the coronary artery (9). In this case, we observed that the abnormal arterial supply originated from the celiac trunk, and the venous drainage entered the portal vein system. This vascular variation is relatively rare in PS cases, but not unprecedented. We summarized the frequencies of various arterial and venous supplies reported in recent literature for PS cases (Table 1) (3,10,11). The data indicated that the thoracic aorta and abdominal aorta are the most common sources of arterial supply, while drainage into the portal vein is extremely rare.
Table 1
| Type | Supply frequency (%) |
|---|---|
| Thoracic aorta | 60–70 |
| Abdominal aorta | 50–60 |
| Celiac trunk | 30–40 |
| Inferior vena cava | 20–30 |
| Subclavian artery | 10–15 |
| Azygos vein | 5–10 |
| Portal vein | <5 |
Less than 10% of PSs are intraperitoneal cases located below the diaphragm (12). In these cases, arterial blood supply commonly arises from the celiac trunk, while venous drainage occurs via the portal venous system; however, these cases are rare and have only been documented in a few reports (7,13). The surgical resection indications for PS mainly include recurrent infections, hemoptysis, pneumothorax, pleural adhesion, bronchiectasis, fungal infections, and heart failure. Surgical resection can significantly improve the prognosis and quality of life of patients with recurrent PS (14,15). In this case, the patient had recurrent pulmonary infections and hemoptysis that required surgical resection.
The surgical management of intraperitoneal PS adheres to standard principles applied to other forms of PS, focusing on the resection of the non-aerated lung tissue and the ligation of the aberrant arterial supply (16,17). Given the involvement of vascular and tracheal structures passing through the diaphragm, diaphragmatic defects may arise, predisposing patients to diaphragmatic weakness, and increasing the risk of herniation (6,13).
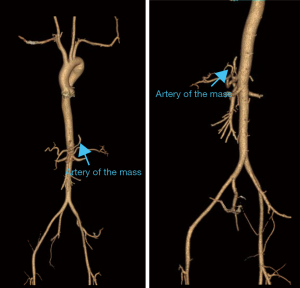
The diagnosis of PS primarily relies on identifying the aberrant arterial supply and venous drainage. Historically, digital subtraction angiography (DSA) has been considered the gold standard for diagnosing PS due to its detailed vascular imaging capabilities. However, DSA is an invasive, time-consuming, and costly procedure (16). Consequently, non-invasive imaging modalities, such as CTA and magnetic resonance angiography, have progressively supplanted DSA as the primary diagnostic tools for PS (18,19). CTA and three-dimensional (3D) reconstructions provide detailed visualizations of individual pulmonary vessels, portal venous systems, and lung tissue; however, the application of these methods to cases of PS with portal venous drainage is limited, as they cannot simultaneously display all relevant anatomical structures (10,11). Thus, we employed the intelligent/interactive qualitative and quantitative analysis-three-dimensional (IQQA-3D), EDDA Technology (Princeton, USA) reconstruction system to visualize multiple anatomical structures, including isolated lung tissue, pulmonary vessels, supplying arteries, and the portal venous system (Figure 5). This technique enabled the generation of high-definition stereoscopic 3D images that simultaneously depicted these anatomical components in cases of PS with portal venous drainage (20).
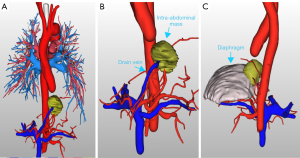
The IQQA-3D technology has significant advantages in preoperative planning. It can clearly display the 3D anatomical structure of blood vessels, including their branches, variations, and relationships with surrounding tissues. In addition, the IQQA-3D technology also enables the preoperative simulation of surgical operations and real-time intraoperative interactions. Thus, doctors can simulate surgical operations before surgery to predict potential problems that may be encountered during the surgery. During the surgery, real-time adjustments and measurements can be made on the 3D images, thereby improving the accuracy and safety of the surgery. Although the IQQA-3D technology does not change the overall surgical approach for PS, its use makes the planning of the surgical approach more efficient for surgeons. It also reduces unnecessary exploration during the surgery and significantly lowers the risk of intraoperative bleeding and complications.
To our knowledge, this study was the first to apply the IQQA-3D reconstruction system to the diagnosis and characterization of a unique subtype of PS. This technology allows for precise identification of the origins and courses of aberrant supplying arteries and draining veins, as well as their anatomical relationships with adjacent structures, thereby facilitating targeted vascular occlusion and the complete resection of isolated lung tissue during surgery (21). Such detailed preoperative mapping minimizes the need for intraoperative exploration and mitigates the risk of inadvertent injury to unidentified vessels, thereby reducing the incidence of significant intraoperative hemorrhage (22). The intraoperative blood loss in this case was only 20 milliliters, which is significantly lower than that reported in similar cases without IQQA-3D guidance. Moreover, the surgery time was 30 minutes shorter than that reported in cases without IQQA-3D guidance (20,21). The patient had an uneventful postoperative recovery and was discharged on the 6th day after surgery, with no complications occurring. These objective indicators fully demonstrate the significant advantages of IQQA-3D technology in improving surgical efficiency and safety. In the future, we anticipate that IQQA-3D technology will be widely applied in more surgical procedures, further increasing the success rate of surgeries and the quality of life of patients.
The IQQA-3D reconstruction technology has not only shown remarkable advantages in PS surgery, it has also been widely applied to a variety of other surgical procedures, significantly enhancing the precision and safety of surgeries. In hepatectomy, the IQQA-3D technology significantly reduced intraoperative blood loss, such that the patients who used IQQA-3D technology had an average blood loss of 150 milliliters, while those who did not had an average blood loss of 300 milliliters (18,23,24). The incidence of postoperative complications in patients who used the IQQA-3D technology also decreased significantly from 15% to 5% (25). Additionally, in the field of urology, this technology has been used for laparoscopic partial nephrectomy in patients with multiple renal tumors. Through preoperative 3D model reconstruction, the anatomical relationship between the tumor and the surrounding blood vessels and renal parenchyma can be clearly defined, optimizing the surgical approach and reducing the incidence of postoperative complications (26). IQQA-3D imaging transforms the surgical approach from exploratory to confirmatory, enhancing surgical precision and patient outcomes.
Conclusions
This case report revealed that venous drainage in PS may not only involve the pulmonary, azygos, and hemiazygos veins but also the portal vein system. Additionally, it showed the significance of IQQA-3D reconstruction technology in PS surgery, as well as its remarkable advantages in enhancing surgical precision and safety. Through precise preoperative planning, IQQA-3D technology significantly reduced intraoperative blood loss, shortened the duration of the surgery, and facilitated the rapid recovery of the patient. These objective findings further substantiate the application value of IQQA-3D technology in complex surgical procedures.
Acknowledgments
We would like to thank Phoebe Chi, MD, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing a draft of this manuscript.
Footnote
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2694/coif). The authors report that this study received funding from the Fujian Provincial Health Technology Project (No. 2020CXA023) and the Fujian Medical University Innovation Training Program (No. JC2022110). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cserni T, Kiss A, Józsa T, Szolosi Z, Nagy B. Extralobar pulmonary sequestration in the right upper thoracic region. Respiration 2007;74:215-9. [Crossref] [PubMed]
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg 2011;40:e39-42. [Crossref] [PubMed]
- Zhang SX, Wang HD, Yang K, Cheng W, Wu W. Retrospective review of the diagnosis and treatment of pulmonary sequestration in 28 patients: surgery or endovascular techniques? J Thorac Dis 2017;9:5153-60. [Crossref] [PubMed]
- Motono N, Iwai S, Funasaki A, Sekimura A, Usuda K, Uramoto H. Indocyanine green fluorescence-guided thoracoscopic pulmonary resection for intralobar pulmonary sequestration: a case report. J Med Case Rep 2019;13:228. [Crossref] [PubMed]
- Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med 2018;16:36. [Crossref] [PubMed]
- Kargl S, Schlader F, Scala M, Kammel J. Vascular Anatomy in Congenital Lung Lesions-Description and Classification. Front Pediatr 2022;10:900538. [Crossref] [PubMed]
- Tian Z, Zhou Y, Liu H. Extralobar pulmonary sequestration with absence of pericardium and atrial septal defect in a woman. J Cardiothorac Surg 2019;14:113. [Crossref] [PubMed]
- Zhang N, Zeng Q, Chen C, Yu J, Zhang X. Distribution, diagnosis, and treatment of pulmonary sequestration: Report of 208 cases. J Pediatr Surg 2019;54:1286-92. [Crossref] [PubMed]
- Pissarra D, Salgueiro E, Oliveira AC, Malangatana G, Pinho P, Casanova J. Pulmonary Sequestration Supplied By The Circumflex Artery - A Rare Case Report. Rev Port Cir Cardiotorac Vasc 2019;26:159-62. [PubMed]
- Chakraborty RK, Modi P, Sharma S. Pulmonary Sequestration. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Gabelloni M, Faggioni L, Accogli S, Aringhieri G, Neri E. Pulmonary sequestration: What the radiologist should know. Clin Imaging 2021;73:61-72. [Crossref] [PubMed]
- Lee CK, Lee CH, Baliski C, Zetler P. Retroperitoneal extralobar pulmonary sequestration mimicking a pheochromocytoma. Histopathology 2008;52:525-7. [Crossref] [PubMed]
- Skrabski R, Royo Y, Di Crosta I, Pueyo C, Sempere T, Maldonado J. Extralobar pulmonary sequestration with an unusual venous drainage to the portal vein: preoperative diagnosis and excision by video-assisted thoracoscopy. J Pediatr Surg 2012;47:e63-5. [Crossref] [PubMed]
- Asif A, Lilley D, Howard-Walker S, Ajab S, Qadri SS. The diagnosis and surgical management of pulmonary sequestration in adults: a case series from a single centre in the UK. Indian J Thorac Cardiovasc Surg 2024;40:91-5. [Crossref] [PubMed]
- Galanis M, Sommer E, Gioutsos K, Nguyen TL, Dorn P. Pulmonary Sequestration: A Monocentric Case Series Report. J Clin Med 2024;13:5784. [Crossref] [PubMed]
- Sudduth CL, Hill SJ, Raval MV. Presentation and management of pulmonary sequestration with total visceral inflow and outflow. Pediatr Surg Int 2016;32:709-12. [Crossref] [PubMed]
- Jariwala P, Ramesh G, Sarat Chandra K. Congenital anomalous/aberrant systemic artery to pulmonary venous fistula: closure with vascular plugs & coil embolization. Indian Heart J 2014;66:95-103. [Crossref] [PubMed]
- Wang S, Ruan Z, Liu F, Huang H, Song K. Pulmonary sequestration: angioarchitecture evaluated by three-dimensional computed tomography angiography. Thorac Cardiovasc Surg 2010;58:354-6. [Crossref] [PubMed]
- Yue SW, Guo H, Zhang YG, Gao JB, Ma XX, Ding PX. The clinical value of computer tomographic angiography for the diagnosis and therapeutic planning of patients with pulmonary sequestration. Eur J Cardiothorac Surg 2013;43:946-51. [Crossref] [PubMed]
- Xu G, Chen C, Zheng W, Zhu Y, Zheng B, Chen H. IQQA-3D imaging interpretation and analysis system-guided single-port video-assisted thoracic surgery for anatomical sub-segmentectomy (LS(1+2)a+b). J Thorac Dis 2018;10:5515-21. [Crossref] [PubMed]
- Xu G, Chen C, Zheng W, Zhu Y, Chen H, Cai B. Application of the IQQA-3D imaging interpretation and analysis system in uniportal video-assisted thoracoscopic anatomical segmentectomy: a series study. J Thorac Dis 2019;11:2058-66. [Crossref] [PubMed]
- Zheng B, Xu G, Fu X, Wu W, Liang M, Zeng T, Zhang S, Zhu Y, Zheng W, Chen C, Bédat B, Swanson SJ, Koike T, Iwata H, Bedetti B, Sato M. Management of the inter-segmental plane using the "Combined Dimensional Reduction Method" is safe and viable in uniport video-assisted thoracoscopic pulmonary segmentectomy. Transl Lung Cancer Res 2019;8:658-66. [Crossref] [PubMed]
- Luo X, Li T, Zhu JY, Huang L. Application value of three-dimensional reconstruction in preoperative evaluation of precise hepatectomy for complex primary liver cancer. Zhonghua Yi Xue Za Zhi 2021;101:2210-5. [PubMed]
- Bai L, Zhang Q, Wu L, He Y, Zhang J, Zhao J. LI T, Wen H. Clinical application of 3D reconstruction technique on precise hepatectomy for primary liver cancer. Journal of Xinjiang Medical University 2013;1234-8.
- Chen L, Luo HP, Dong SL, Chen XP. Safety assessment of hepatectomy for huge hepatocellular carcinoma by three dimensional reconstruction technique. Zhonghua Wai Ke Za Zhi 2016;54:669-74. [PubMed]
- Wei Yang, Yang Q, Pan X, Hu C, Chu J, Gan S, Ye J, Zhang X, Cui X. Application of IQQA virtual surgical planning technique in laparoscopic partial nephrectomy for multiple renal tumors. Journal of Modern Urology 2019;990-4.


