
Optical coherence tomography evaluation of vessel wall microstructure before and after endovascular stent treatment for intracranial vertebrobasilar atherosclerotic stenosis
Introduction
Stroke caused by intracranial atherosclerotic stenosis (ICAS) is the second leading cause of death globally, with China reporting one of the highest incidences of ICAS in the world (1). Among Chinese stroke patients, it has been reported that posterior circulation accounted for 26.2% of the manifestation of atherosclerotic stenosis, with intracranial artery stenosis significantly exceeding extracranial artery stenosis (2). Furthermore, among patients with symptomatic vertebrobasilar artery stenosis, the rate of recurrent stroke was higher in those with intracranial artery stenosis compared to extracranial artery stenosis (3). The PARISK (Plaque At RISK) study revealed that the recurrence rate of stroke was related not only to the degree of vascular stenosis but also to the characteristics of the stenosing plaque, such as intraplaque hemorrhage (IPH), ulceration, calcification ratio, and total plaque volume (4). ICAS can be accurately diagnosed using conventional imaging modalities, including computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA). High-resolution vessel wall magnetic resonance imaging (vwMRI) can analyze the nature and location of the plaque (5). However, vwMRI appears limited in its ability to accurately identify the structural components of the plaque. To date, standard medical treatment seems to have been the first choice for the optimal treatment for ICAS (6). Nonetheless, a recent study (7) indicated no significant difference in safety and efficacy between endovascular therapy and standard medical therapy. Endovascular treatment with stents appeared effective; however, analysis of the vessel wall structure post-stent implantation was limited by the stent material.
Optical coherence tomography (OCT) is a novel optical diagnostic technique that has rapidly developed in recent years for evaluating blood vessel walls. It employs near-infrared interferometry to receive and analyze the reflected light from biological tissue components at varying depths using an interferometer, which is then processed by a computer system to generate tomography images of biological tissues. Imaging takes only a few seconds and achieves a resolution approaching 10 µm. OCT has been approved by the Food and Drug Administration (FDA) for use in vessels affected by coronary atherosclerotic disease, as it provides a level of detail in lumen assessment that other imaging techniques cannot match (8). In ICAS, OCT can be used to analyze extracranial carotid arteries, identifying plaque characteristics and potential defects post-stent implantation, such as malapposition and plaque prolapse (9). The application of OCT in intracranial arteries is relatively rare because these arteries are often tortuous, making it difficult to completely remove blood (e.g., in the middle cerebral artery), which affects imaging results (10). Recently, some studies have explored the clinical application of OCT in the intracranial segment of the internal carotid artery and the intracranial segment of the vertebrobasilar artery (11-13). However, the application of OCT in the middle cerebral artery has only been investigated through animal experiments and human cadaveric studies (14).
There are currently limited data on the safety and feasibility of OCT in intracranial vertebrobasilar arteries. In this study, we report on the feasibility and safety of OCT in assessment of the intracranial vertebrobasilar arteries. The basilar artery perforator vessels are abundant, and the V4 segment of the vertebral artery perforator vessels are few. In order to reduce unnecessary surgical risk, we selected the V4 segment of the vertebral artery as the study target vessel.
OCT has a relatively mature role in coronary atherosclerotic disease. In this study, the criteria for morphological analysis of plaque and vessel wall structure in ICAS disease were based on OCT histopathological studies in the coronary field. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2487/rc).
Methods
Patient selection
In this retrospective case series, we analyzed the OCT images and data of 15 patients with severe intracranial vertebrobasilar artery stenosis who underwent endovascular stent treatment and were evaluated by OCT at the Department of Neurosurgery, The First Affiliated Hospital of Harbin Medical University, from 7 March 2023 to 26 March 2024. The inclusion criteria were as follows: age between 30 and 75 years, intracranial vertebrobasilar atherosclerotic stenosis refractory to antiplatelet therapy, 70–90% stenosis confirmed by DSA according to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) method (15), single-target lesions requiring surgical treatment, a modified Rankin scale (mRS) score of less than 3, and either no limitation on the onset time of the most recent transient ischemic attack (TIA) or an onset time of the most recent ischemic stroke greater than 2 weeks. The main exclusion criteria included near occlusion or complete occlusion of the target vessel, extremely tortuous vertebrobasilar arteries, severe liver or kidney dysfunction, and sensitivity to contrast media. Clinical data collected included age, gender, clinical presentation, hypertension, diabetes, dyslipidemia, coronary artery disease, smoking history, National Institutes of Health Stroke Scale (NIHSS) score, and mRS score, among others.
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Harbin Medical University (No. IRB-AF/SC-08/06.0). Written informed consent was provided by all patients or their legal representatives.
Preoperative preparation
Patients received antiplatelet therapy, including clopidogrel 75 mg/day or ticagrelor 90 mg/day with aspirin 100–325 mg/day for at least 3 days before surgery. Blood pressure and blood sugar levels were controlled. DSA was performed prior to surgery to ensure that the indication for the procedure was met.
OCT technique and endovascular stent treatment protocol
A DynaCT angiography scanner (Philips Azurion 7M20; Philips Healthcare, Amsterdam, The Netherlands) was used for the DSA examination. The OPTISTM Next Imaging Systems (Abbott Medical, Westford, MA, USA) and DragonflyTM OCT imaging catheter (Abbott Medical) were utilized for OCT evaluation. The procedure was performed under general anesthesia. A 6 F or 8 F Navien guide catheter (Medtronic, Irvine, CA, USA) was placed in the V2 segment of the vertebral artery via a transfemoral approach. Heparin was injected intravenously after positioning the guiding catheter, and heparin saline was continuously infused during the operation. Guided by a roadmap, a Traxcess 14 microguidewire (MicroVention, Aliso Viejo, CA, USA) was introduced into the target artery and advanced through the stenosed site using an Echelon-10 microcatheter (Medtronic). After withdrawing the Traxcess 14 microguidewire, the Transcend 300 microguidewire (Stryker, Kalamazoo, MI, USA) was used to reposition the microcatheter, navigating the tip to the P2 segment of the posterior cerebral artery (PCA). The Echelon microcatheter was exchanged for a 2.7 F DragonflyTM OCT imaging catheter, which was positioned distal to the area of interest. The operator manually injected 3–5 mL of iobitridol (Guerbet, Villepinte, France) to clear blood from the OCT catheter. Subsequently, a small amount of iobitridol was injected again, and the imaging catheter’s coaxial positioning and blood clearance were observed. During blood clearance, 8 mL of undiluted iobitridol was automatically injected through a 6F or 8F guide catheter at a rate of 4 mL/s at 200 psi, while the optical mirror helix of the OCT imaging catheter was retracted (20 to 25 mm/s for approximately 4 seconds) to obtain a series of cross-sectional OCT images of the vessel wall. Upon completion of image acquisition, the consecutive images were digitized and stored in AptiVueTM software (OPTISTM Next Imaging Systems, Abbott Medical) for subsequent analysis. If the image quality was suboptimal, the pullback was repeated. After obtaining clear preoperative OCT images, endovascular treatment of the target lesions was performed. While keeping the Transcend 300 microguidewire in place, the OCT imaging catheter was exchanged for the balloon-expandable stent system, the Aurora stent (Salubris, Shenzhen, China), which was delivered to the target artery stenosis. The balloon was used to gradually expand the stenosis to 90–100% of the normal vessel diameter (distal normal vessel diameter + proximal normal vessel diameter/2) to release the stent. After satisfactory stent expansion, the microguidewire was maintained in position, the balloon-expandable stent system was withdrawn, and access to the OCT imaging catheter was reestablished. The imaging catheter was again pulled back as described above to obtain additional OCT images.
Analysis of OCT images before endovascular stent treatment
Two interventional neurologists independently analyzed the DSA and OCT images. In cases of inconsistency between observers, a consensus reading was obtained from a third investigator. The definition of OCT success was based on the image quality of the target lesion in the patient.
Following established practices in the coronary literature, we scored the quality of the OCT images (16,17). A score of 0 indicated no images available; 1 indicated less than 50% of the pullback images available; 2 indicated greater than 50% of the pullback images available; and 3 indicated that all pullback images were available. The examination was deemed successful when adequate image acquisition occurred, the target lesion was fully visible, and the image quality of at least one pullback was graded as 2 or 3. Conversely, the examination was considered unsuccessful if image acquisition could not be performed due to technical or anatomical issues, if the target lesion could not be reliably assessed, or if all pullbacks received a quality grade of 0. An examination was considered partially successful if at least one pullback was graded 1 and at least a portion of the target lesion could be evaluated.
All cross-sectional OCT images within the lesion were assessed at 0.2 or 0.25 mm intervals to detect plaque composition. OCT images were analyzed using AptiVueTM software. The tissue characteristics of arterial wall plaques were defined based on prior experience in the coronary literature (18-21). Plaques were classified as follows: (I) fibrous plaque (homogeneous, high backscattering region); (II) lipid plaque (low-signal region with diffuse borders); (III) plaque rupture (fibrous plaque tissue exhibiting signal discontinuity and/or accompanied by plaque cavity formation); (IV) arterial thrombosis, defined as a mass (>250 µm in diameter) attached to the luminal surface or floating in the lumen, which included red thrombus (rich in red blood cells, high backscattering, and high attenuation); (V) white thrombus (rich in platelets, homogeneous backscattering with low attenuation); (VI) calcified plaque (area with low backscattering signal and sharp borders within a plaque); (VII) microchannels (signal-poor voids sharply delineated across multiple contiguous frames); (VIII) arterial dissection (high signal in the space between vessel walls). OCT can also identify more subtle plaque structures; (IX) macrophages (hyperintense, well-defined punctate areas with strong posterior attenuation); (X) cholesterol crystals (thin linear regions of higher signal intensity and lower attenuation); (XI) healing plaque (plaque with 1 or more layers of different light signals on the surface and a clear boundary with adjacent tissue) (22). The identification of the above plaque structures by OCT as conducted in this study is illustrated in Figure 1.
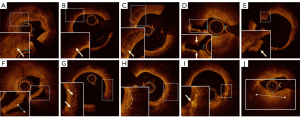
Analysis of OCT images after endovascular stent treatment
Currently, OCT is employed to analyze stent expansion rate, stent adherence, tissue prolapse, and stent thrombosis following endovascular stent treatment. Based on experience with coronary artery disease, these abnormalities are associated with poor outcomes of endovascular stent treatment (23-25). The following definitions were applied to the analysis of OCT images: (I) stent expansion rate: minimum stent area (MSA)/mean reference lumen area × 100%. The stent expansion rate can be automatically analyzed using OCT equipment. (II) Poor stent apposition: the axial distance from the surface of the stent support rod to the vessel wall stent exceeds the thickness of the stent support rod, with a length of ≥1 mm. (III) Tissue prolapse: after stent treatment, tissue on the surface of the vessel wall protrudes into the lumen through the stent mesh. Depending on the tissue composition in the protrusion, it can be classified as plaque prolapse (smooth and without significant signal decay) or thrombus prolapse (irregular and accompanied by signal attenuation). (IV) Stent thrombosis: a localized irregular mass with attenuated signal protrudes into the lumen immediately after stent treatment. The OCT image evaluation following endovascular stent treatment in this study is illustrated in Figure 2.
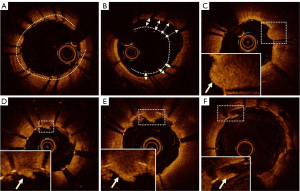
The definition of artifacts
OCT rotation artifacts were defined as oscillations during inhomogeneous axial rotation of the imaging catheter during pullback, resulting in apparent misalignment of the lumen boundary and subsequently artifacts, leading to the observed artifacts where a full rotation of the proximal side of the catheter does not line up with a full rotation at the distal tip, in a given frame. Caliber artifacts were defined as a loss of lumen integrity due to excessive vessel diameter. Blood artifacts were defined as optical occlusion resulting from inadequate blood ejection, leading to residual blood (12). The OCT image artifacts identified in this study are represented in Figure S1.
Safety evaluation
NIHSS and mRS data were obtained by two independent and experienced neurologists before, immediately after, and 24 hours after the procedure. All patients were followed up by telephone on the 7th day after endovascular stent treatment. If the postoperative NIHSS and mRS scores were higher than baseline, computed tomography (CT) or diffusion-weighted imaging (DWI) was required to confirm the occurrence of bleeding or embolic events.
Results
A total of 15 patients were included in this study. Table 1 presents the baseline characteristics of the patients; the OCT data are detailed in Table 2. The mean age was 65.7±5.9 years, and 80% of the participants were male. All lesions were located in the V4 segment of the vertebral artery (67% on the right side), and all exhibited severe stenosis (79%±6.2%). Among the participants, 12 (80%) had experienced a stroke, whereas 3 patients (20%) were hospitalized due to TIAs. There were 2 patients (13%) who scored 1 on the mRS due to previous cerebral infarctions, whereas the remaining patients had NIHSS and mRS scores of 0 at admission.
Table 1
| Characteristics | Values |
|---|---|
| Age (years) | 65.7±5.9 |
| Sex | |
| Male | 12 [80] |
| Female | 3 [20] |
| Medical history | |
| Hypertension | 11 [73] |
| Diabetes mellitus | 6 [40] |
| Hyperlipidemia | 3 [20] |
| Coronary artery disease | 3 [20] |
| Alcohol history | 4 [27] |
| Smoking history | 5 [33] |
| Clinical presentation | |
| TIA | 3 [20] |
| Stroke | 12 [80] |
| Symptomatic qualifying artery | |
| R-V4 | 10 [67] |
| L-V4 | 5 [33] |
| Stenosis of symptomatic qualifying artery (%) | 79±6.2 |
| Distribution | |
| 70–79% stenosis | 9 [60] |
| 80–90% stenosis | 6 [40] |
| NIHSS ≤1 | 15 [100] |
| mRS ≤2 | 15 [100] |
Continuous variables are presented as mean ± SD, whereas categorical variables are presented as frequency [%]. mRS, modified Rankin scale; L, left vertebral artery; NIHSS, National Institutes of Health Stroke Scale; R, right vertebral artery; SD, standard deviation; TIA, transient ischemic attack.
Table 2
| Variables | Cases | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Before endovascular stent treatment | |||||||||||||||
| Pullbacks† | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 |
| Image quality‡ | 3 | 3 | 1/2 | – | 3 | 3 | – | 3 | 3 | 0/1/1 | 3 | – | – | 2 | 3 |
| Success index | S | S | S | F | S | S | F | S | S | PS | S | F | F | S | S |
| Rotational artifacts† | 0 | 0 | 1 | – | 0 | 0 | – | 0 | 0 | 0 | 0 | – | – | 1 | 0 |
| Caliber artifacts† | 0 | 0 | 2 | – | 0 | 0 | – | 0 | 0 | 3 | 1 | – | – | 0 | 0 |
| Blood artifacts† | 0 | 0 | 1 | – | 0 | 1 | – | 0 | 0 | 3 | 1 | – | – | 1 | 1 |
| Area of stenosis (%) | 79 | 90 | 92 | – | 88 | 79 | – | 77 | 86 | 75 | 80 | – | – | 90 | 89 |
| Fibrous plaques | Y | Y | Y | – | Y | Y | – | Y | Y | Y | Y | – | – | Y | Y |
| Lipid plaques | Y | Y | Y | – | Y | Y | – | Y | Y | Y | Y | – | – | Y | Y |
| Calcified plaques | Y | Y | Y | – | Y | Y | – | Y | Y | Y | Y | – | – | Y | Y |
| Ruptured plaques | N | N | Y | – | N | N | – | N | Y | N | N | – | – | Y | N |
| White thrombus | N | N | Y | – | Y | N | – | N | N | N | Y | – | – | Y | N |
| Red thrombus | N | N | N | – | N | N | – | N | N | N | N | – | – | N | N |
| Vascular dissection | Y | N | Y | – | Y | N | – | N | Y | N | Y | – | – | N | N |
| Microchannel | Y | Y | N | – | Y | Y | – | Y | Y | Y | N | – | – | N | Y |
| Cholesterol crystal | Y | N | Y | – | N | N | – | N | N | N | Y | – | – | N | Y |
| Macrophages | Y | N | N | – | N | N | – | N | N | N | N | – | – | Y | N |
| Healing plaque | Y | N | N | – | N | N | – | N | N | N | N | – | – | Y | Y |
| After endovascular stent treatment | |||||||||||||||
| Pullbacks† | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Image quality‡ | 3 | 3 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | – | 2 | 3 |
| Success index | S | S | PS | S | S | S | S | S | S | F | S | S | F | S | S |
| Rotational artifacts† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 |
| Caliber artifacts† | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | – | 0 | 0 |
| Blood artifacts† | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | – | 1 | 0 |
| Expansion rate (%) | 77 | 69 | 67 | 63 | 84 | 79 | 47 | 67 | 88 | – | 60 | 33 | – | 76 | 63 |
| Stent attach | P | P | W | W | W | W | W | W | W | – | P | P | – | W | W |
| Plaque prolapse | Y | N | Y | N | N | Y | N | N | Y | – | N | N | – | Y | Y |
| Thrombus prolapse | N | N | N | N | N | N | Y | Y | N | – | Y | Y | – | Y | Y |
| Stent thrombosis | Y | N | N | N | N | Y | N | N | N | – | N | N | – | N | N |
| Vascular dissection | N | N | N | N | N | N | N | N | N | – | N | N | – | N | Y |
Area of stenosis: the results were automatically obtained by OCT imaging equipment—The OPTISTM Next Imaging Systems (Abbott Medical, Westford, MA, USA) analysis. †, “0”, “1”, “2”, “3” represent the frequency of occurrence; ‡, “0”, “1”, “2”, “3” represent the quality of the image. F, failure; N, not have; OCT, optical coherence tomography; P, stent attached poorly; PS, partial success; S, success; W, stent attached well; Y, yes.
During the procedure, the OCT catheter could not pass through the diseased vessel in 3 patients due to the severity of stenosis, resulting in the failure of the preoperative OCT examination. Additionally, preoperative and postoperative OCT examinations failed in 1 patient due to excessive curvature of the V3 segment of the vertebral artery. OCT pullbacks were performed 14 times (average 0.9±0.8 per person) before endovascular stent treatment, during which the imaging quality was graded as follows: grade 0 once (8%), grade 1 three times (21%), grade 2 two times (14%), and grade 3 eight times (57%). After endovascular stent treatment, OCT pullbacks were performed 14 times (average 0.9±0.2 per person), with imaging quality graded as grade 1 twice (14%), grade 2 twice (14%), and grade 3 10 times (72%). The rotational artifacts, caliber artifacts, and blood artifacts in OCT images before and after endovascular stent implantation were 14%, 0%; 43%, 21%; and 57%, 43%, respectively. Among the 15 patients, the success, partial success, and failure rates of OCT pullbacks before stenting were 10/15 (67%), 1/15 (7%), and 4/15 (26%), respectively. After stenting, the success, partial success, and failure rates of OCT pullbacks were 12/15 (80%), 1/15 (7%), and 2/15 (13%), respectively.
Analysis was performed on patients with successful and partially successful OCT pullbacks before endovascular stenting (11 patients). The area of stenosis in the target lesion vessel was 84%±6% on OCT examination prior to endovascular stenting. Among the atherosclerotic plaques, fibrous plaques were identified in all 11 patients (100%), lipid plaques in 11 patients (100%), calcified plaques in 11 patients (100%), ruptured plaques in 3 patients (27%), white thrombus in 4 patients (36%), vascular dissection in 5 patients (45%), microchannels in 8 patients (73%), and red thrombus in none (0%). In terms of more subtle structures, macrophages were found in 2 patients (18%), cholesterol crystals in 4 patients (36%), and healing plaques in 3 patients (27%). Analysis was also conducted of patients with successful and partially successful OCT pullbacks after endovascular stenting (13 patients). The OCT scan analysis revealed that the stent expansion rate was 67%±14%, with the stent well attached in 9 patients (69%). A total of 6 patients (46%) exhibited plaque prolapse, and 6 patients (46%) had thrombus prolapse (all consisting of white thrombus). Additionally, 2 patients (15%) experienced stent thrombosis (all white thrombus). In 1 patient, a dissection of the vessel wall was observed after stent therapy, where the peeled vessel wall had been tightly adhered to by the stent trabeculator. We administered 10 mL of tirofiban intra-arterially, followed by a continuous intravenous pump of tirofiban for 24 hours post-surgery, with no adverse events reported (Figure 3).
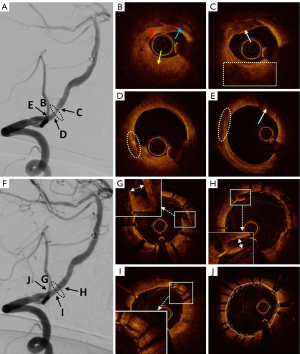
After endovascular treatment, all patients were evaluated using mRS and NIHSS scores. One patient developed bilateral left gaze and dizziness after the procedure. DWI examination revealed spotted abnormal signals in the pons, right cerebellar hemisphere, left occipital lobe, and left thalamus. The symptoms improved on the third day after the administration of tirofiban to enhance blood circulation. The postoperative mRS and NIHSS scores of the remaining 14 patients did not differ from their preoperative values. All patients were followed up by telephone 7 days after endovascular stent treatment, with no deaths or other serious surgical complications reported.
Discussion
In this study, we performed OCT analysis before and after endovascular stenting in ICAS patients with severe vertebrobasilar artery stenosis (Figure 4). In 2020, Xu et al. reported a series of case studies on patients with vertebrobasilar artery stenosis using OCT (11); thereafter, Yang et al. conducted a preliminary safety analysis of the application of OCT in intracranial ICAS lesions (12). Building on the experiences of these previous studies, we further analyzed the morphological characteristics of plaques in vertebrobasilar artery stenosis, including fibrous plaques, lipid plaques, calcified plaques, intraluminal thrombosis, vascular dissection, and plaque rupture, as well as stent adherence, stent expansion rate, tissue and thrombus prolapse, and stent thrombosis after endovascular stent implantation. These structural features may be related to the incidence of posterior circulation strokes and the recurrence rate of strokes. The identification of these features through OCT can provide guidance for medical and endovascular treatment strategies for intracranial vertebrobasilar artery stenosis.
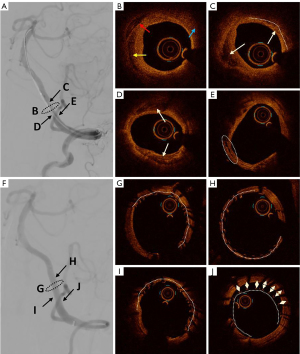
In 2007, Zimarino et al. were the first to accurately detect carotid atherosclerotic lesions using OCT in rabbits (26). Since then, OCT has demonstrated high sensitivity and specificity for identifying different types of atherosclerotic plaques. Previous studies have shown that the risk of stroke is related not only to the degree of stenosis but also to the histopathology of arterial plaque. In 2019, Saba et al. analyzed imaging biomarkers of carotid plaque and suggested that IPH, plaque volume, neovascularization, and inflammation are potential biomarkers for carotid plaque vulnerability, which may predict the occurrence of stroke (27). Additionally, several studies have indicated that the area, volume, thickness, shape, and location of calcified plaques might play a key role in predicting stroke occurrence in vulnerable plaques (28,29). Furthermore, research on more subtle lesion characteristics has found that the presence of macrophages and microcalcifications is associated with plaque vulnerability (30). Ruptured thin-cap fibroatheromas (TCFA), intraluminal white thrombus, and cholesterol crystals within plaques have been identified as predictors of stroke (31-33). These biomarkers could potentially alter current treatment strategies that rely solely on the degree of stenosis. In this study, OCT was utilized to identify the microstructure of atherosclerotic plaques in intracranial vertebrobasilar artery stenosis, providing preliminary experience for further investigation into vulnerable plaques.
Endovascular stent treatment can effectively improve intracranial blood perfusion and alleviate ischemic symptoms. However, this therapy may also lead to intraoperative complications and postoperative adverse events. The application of OCT to accurately evaluate the histopathological characteristics of stenotic plaques can aid in selecting the most appropriate treatment plan for patients. Yoshimura et al. utilized OCT for plaque analysis in patients with carotid artery stenosis, allowing for the selection of more suitable treatment plans to reduce complications (34,35). In this case series, balloon-expanded stent placement was shown to be feasible in patients who were successfully visualized by OCT prior to treatment. One patient developed bilateral left gaze and dizziness after endovascular stent treatment. DWI confirmed multiple punctate infarcts, which were believed to be caused by plaque detachment and embolization. Following treatment to improve blood circulation, the symptoms were alleviated at discharge. When using OCT imaging, it is necessary to pass the OCT imaging catheter 5 mm distal to the lesion site and then inject iobitridol for pullback imaging, which may lead to fragmentation and detachment of the plaque. However, the procedure of balloon dilation and stent implantation also carries the risk of plaque detachment. Therefore, the specific causes of postoperative complications are currently unknown. After endovascular stent treatment, we successfully used OCT to assess the vessel wall structure. In coronary practice, a stent inflation rate of ≥80% is generally considered the goal for optimal stent expansion. A larger stent inflation rate is typically associated with better long-term stent patency, improved clinical outcomes, and a reduced risk of stent failure (22). In our case series, the mean stent inflation rate was 67%±14%, which was below the target value of 80%. However, further follow-up studies are needed to determine whether experiences from coronary practice are applicable to ICAS lesions. In our study, one patient was found to have a vascular wall dissection after stent treatment, which was tightly attached by the trabeculae of the stent. The patient received treatment to enhance blood circulation without postoperative adverse reactions. Based on experiences with coronary arteries, such minor structural abnormalities in the vessel wall following stent placement typically do not result in serious complications and do not necessitate additional treatment (36). Nonetheless, there is a lack of effective evidence to confirm whether this experience is applicable to ICAS lesions. In-stent restenosis (ISR) remains a significant challenge in endovascular stent therapy, and a previous study demonstrated that ISR is highly correlated with the rate of recurrent stroke (37). Drug-eluting stents (DES) have been shown to be effective in reducing the incidence of ISR. In this study, all patients were treated with the Maurora-DES. Intimal hyperplasia is the primary pathological mechanism leading to ISR, and macrocalcification within atherosclerotic plaques may contribute to its occurrence (38).
In this study, three patients were unable to pass the OCT catheter through the diseased vessel due to the severity of stenosis. Our strategy involved performing a 2 mm balloon dilation within the severely stenosed lesion to facilitate the smooth passage of the OCT catheter. One patient could not accommodate the OCT catheter due to excessive curvature in the V3 segment of the vertebral artery. The Dragonfly catheter was originally designed for detecting coronary artery disease without specific consideration for the anatomical characteristics of intracranial arteries, which resulted in challenges for some patients with high vessel tortuosity. Therefore, efforts are being made in the field of OCT imaging to achieve smaller shapes, reduced stiffness, and increased flexibility for the needs of neurointerventional surgery (39-41). In 2024, Pereira et al. performed an imaging study using neuro-OCT (nOCT) in 32 patients with intracranial arterial disease and showed high-resolution imaging of various intracranial arterial diseases with tortuous paths using nOCT. In this study, nOCT was successfully applied to cerebrovascular examination, demonstrating the artifact-free and high-resolution long-segment imaging of intracranial arteries, which provides valuable experience for the future research of OCT catheters dedicated to intracranial arteries (42).
This study has several limitations. First, it is a single-center study with a limited number of patients enrolled, resulting in insufficient data for correlation analysis; thus, no associations between plaque structure and the occurrence of clinical events were identified. Second, we did not conduct long-term follow-up to determine the long-term risks.
Conclusions
This study offers valuable experience regarding the application of OCT technology in intracranial vertebrobasilar arteries. Preliminary results indicate that OCT is feasible for identifying atherosclerotic plaques and assessing vessel wall status following endovascular stent treatment. Compared with other imaging methods, OCT possesses unique characteristics and can provide new research insights for the treatment of intracranial vertebrobasilar artery conditions.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2487/rc
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2487/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Harbin Medical University (No. IRB-AF/SC-08/06.0) and informed consent was provided by all patients or their legal representatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158-9. [Crossref] [PubMed]
- Hua Y, Jia L, Xing Y, Hui P, Meng X, Yu D, et al. Distribution Pattern of Atherosclerotic Stenosis in Chinese Patients with Stroke: A Multicenter Registry Study. Aging Dis 2019;10:62-70. [Crossref] [PubMed]
- Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke 2013;44:598-604. [Crossref] [PubMed]
- van Dam-Nolen DHK, Truijman MTB, van der Kolk AG, Liem MI, Schreuder FHBM, Boersma E, Daemen MJAP, Mess WH, van Oostenbrugge RJ, van der Steen AFW, Bos D, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, van der Lugt A, Kooi MEPARISK Study Group. Carotid Plaque Characteristics Predict Recurrent Ischemic Stroke and TIA: The PARISK (Plaque At RISK) Study. JACC Cardiovasc Imaging 2022;15:1715-26. [Crossref] [PubMed]
- de Havenon A, Mossa-Basha M, Shah L, Kim SE, Park M, Parker D, McNally JS. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology 2017;59:1193-202. [Crossref] [PubMed]
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021;52:e364-467. [Crossref] [PubMed]
- Gao P, Wang T, Wang D, Liebeskind DS, Shi H, Li T, Zhao Z, Cai Y, Wu W, He W, Yu J, Zheng B, Wang H, Wu Y, Dmytriw AA, Krings T, Derdeyn CP, Jiao LCASSISS Trial Investigators. Effect of Stenting Plus Medical Therapy vs Medical Therapy Alone on Risk of Stroke and Death in Patients With Symptomatic Intracranial Stenosis: The CASSISS Randomized Clinical Trial. JAMA 2022;328:534-42. [Crossref] [PubMed]
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- de Donato G, Pasqui E, Alba G, Giannace G, Panzano C, Cappelli A, Setacci C, Palasciano G. Clinical considerations and recommendations for OCT-guided carotid artery stenting. Expert Rev Cardiovasc Ther 2020;18:219-29. [Crossref] [PubMed]
- Chen CJ, Kumar JS, Chen SH, Ding D, Buell TJ, Sur S, Ironside N, Luther E, Ragosta M 3rd, Park MS, Kalani MY, Liu KC, Starke RM. Optical Coherence Tomography: Future Applications in Cerebrovascular Imaging. Stroke 2018;49:1044-50. [Crossref] [PubMed]
- Xu X, Li M, Liu R, Yin Q, Shi X, Wang F, Gao J, Xu G, Ye R, Liu X. Optical coherence tomography evaluation of vertebrobasilar artery stenosis: case series and literature review. J Neurointerv Surg 2020;12:809-13. [Crossref] [PubMed]
- Yang B, Feng Y, Ma Y, Wang Y, Chen J, Li L, Dong J, Zhang B, Gao P, Chen Y, Dmytriw AA, Jiao L. Frequency-Domain Optical Coherence Tomography for Intracranial Atherosclerotic Stenosis: Feasibility, Safety, and Preliminary Experience. Front Neurol 2021;12:678443. [Crossref] [PubMed]
- Given CA 2nd, Ramsey CN 3rd, Attizzani GF, Jones MR, Brooks WH, Bezerra HG, Costa MA. Optical coherence tomography of the intracranial vasculature and Wingspan stent in a patient. BMJ Case Rep 2014;2014: [Crossref] [PubMed]
- Mathews MS, Su J, Heidari E, Levy EI, Linskey ME, Chen Z. Neuroendovascular optical coherence tomography imaging and histological analysis. Neurosurgery 2011;69:430-9. [Crossref] [PubMed]
- Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643-6.
- Motreff P, Levesque S, Souteyrand G, Sarry L, Ouchchane L, Citron B, Cassagnes J, Lusson JR. High-resolution coronary imaging by optical coherence tomography: Feasibility, pitfalls and artefact analysis. Arch Cardiovasc Dis 2010;103:215-26. [Crossref] [PubMed]
- Lehtinen T, Nammas W, Airaksinen JK, Karjalainen PP. Feasibility and safety of frequency-domain optical coherence tomography for coronary artery evaluation: a single-center study. Int J Cardiovasc Imaging 2013;29:997-1005. [Crossref] [PubMed]
- Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39:604-9. [Crossref] [PubMed]
- Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002;106:1640-5. [Crossref] [PubMed]
- Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058-72. Erratum in: J Am Coll Cardiol 2012;59:1662. [Crossref] [PubMed]
- Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748-58. [Crossref] [PubMed]
- Sonoda S, Morino Y, Ako J, Terashima M, Hassan AH, Bonneau HN, Leon MB, Moses JW, Yock PG, Honda Y, Kuntz RE, Fitzgerald PJ. SIRIUS Investigators. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the sirius trial. J Am Coll Cardiol 2004;43:1959-63. [Crossref] [PubMed]
- Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, Kim YH, Cheong SS, Song JK, Kim JJ, Park SW, Park SJ. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J 2006;27:1305-10. [Crossref] [PubMed]
- Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39:3281-300. [Crossref] [PubMed]
- Hong YJ, Jeong MH, Choi YH, Song JA, Kim DH, Lee KH, Yamanaka F, Lee MG, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Impact of tissue prolapse after stent implantation on short- and long-term clinical outcomes in patients with acute myocardial infarction: an intravascular ultrasound analysis. Int J Cardiol 2013;166:646-51. [Crossref] [PubMed]
- Zimarino M, Prati F, Stabile E, Pizzicannella J, Fouad T, Filippini A, Rabozzi R, Trubiani O, Pizzicannella G, De Caterina R. Optical coherence tomography accurately identifies intermediate atherosclerotic lesions--an in vivo evaluation in the rabbit carotid artery. Atherosclerosis 2007;193:94-101. [Crossref] [PubMed]
- Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D, Wasserman BA, Bonati LH, Wintermark M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Mushenkova NV, Summerhill VI, Zhang D, Romanenko EB, Grechko AV, Orekhov AN. Current Advances in the Diagnostic Imaging of Atherosclerosis: Insights into the Pathophysiology of Vulnerable Plaque. Int J Mol Sci 2020;21:2992. [Crossref] [PubMed]
- Shi X, Han Y, Li M, Yin Q, Liu R, Wang F, Xu X, Xiong Y, Ye R, Liu X. Superficial Calcification With Rotund Shape Is Associated With Carotid Plaque Rupture: An Optical Coherence Tomography Study. Front Neurol 2020;11:563334. [Crossref] [PubMed]
- Burgmaier M, Milzi A, Dettori R, Burgmaier K, Marx N, Reith S. Co-localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS One 2018;13:e0205984. [Crossref] [PubMed]
- Jones MR, Attizzani GF, Given CA 2nd, Brooks WH, Ganocy SJ, Ramsey CN, Fujino Y, Bezerra HG, Costa MA. Intravascular frequency-domain optical coherence tomography assessment of carotid artery disease in symptomatic and asymptomatic patients. JACC Cardiovasc Interv 2014;7:674-84. [Crossref] [PubMed]
- Yang Q, Guo H, Shi X, Xu X, Zha M, Cai H, Yang D, Huang F, Zhang X, Lv Q, Liu R, Liu X. Identification of Symptomatic Carotid Artery Plaque: A Three-Item Scale Combined Angiography With Optical Coherence Tomography. Front Neurosci 2021;15:792437. [Crossref] [PubMed]
- Shi X, Cai H, Wang F, Liu R, Xu X, Li M, Han Y, Yin Q, Ye R, Liu X. Cholesterol Crystals are Associated with Carotid Plaque Vulnerability: An Optical Coherence Tomography Study. J Stroke Cerebrovasc Dis 2020;29:104579. [Crossref] [PubMed]
- Yoshimura S, Kawasaki M, Hattori A, Nishigaki K, Minatoguchi S, Iwama T. Demonstration of intraluminal thrombus in the carotid artery by optical coherence tomography: technical case report. Neurosurgery 2010;67:onsE305-discussion onsE305. [Crossref] [PubMed]
- Kawasaki M, Yoshimura S, Yamada K, Hattori A, Ishihara Y, Nishigaki K, Takemura G, Iwama T, Minatoguchi S. Carotid artery OCT in cerebral infarction. JACC Cardiovasc Imaging 2013;6:1215-6. [Crossref] [PubMed]
- Kawamori H, Shite J, Shinke T, Otake H, Matsumoto D, Nakagawa M, Nagoshi R, Kozuki A, Hariki H, Inoue T, Osue T, Taniguchi Y, Nishio R, Hiranuma N, Hirata K. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur Heart J Cardiovasc Imaging 2013;14:865-75. [Crossref] [PubMed]
- Derdeyn CP, Fiorella D, Lynn MJ, Turan TN, Cotsonis GA, Lane BF, Montgomery J, Janis LS, Chimowitz MI. SAMMPRIS Investigators. Nonprocedural Symptomatic Infarction and In-Stent Restenosis After Intracranial Angioplasty and Stenting in the SAMMPRIS Trial (Stenting and Aggressive Medical Management for the Prevention of Recurrent Stroke in Intracranial Stenosis). Stroke 2017;48:1501-6. [Crossref] [PubMed]
- Xu R, Yang B, Li L, Wang T, Lu X, Luo J, Zhang X, Dong J, Wang Y, Hua Y, Ma Y, Jiao L. Macrocalcification of intracranial vertebral artery may be related to in-stent restenosis: lessons learned from optical coherence tomography. J Neurointerv Surg 2022;14: [Crossref] [PubMed]
- Gounis MJ, Ughi GJ, Marosfoi M, Lopes DK, Fiorella D, Bezerra HG, Liang CW, Puri AS. Intravascular Optical Coherence Tomography for Neurointerventional Surgery. Stroke 2019;50:218-23. [Crossref] [PubMed]
- Ughi GJ, Marosfoi MG, King RM, Caroff J, Peterson LM, Duncan BH, Langan ET, Collins A, Leporati A, Rousselle S, Lopes DK, Gounis MJ, Puri AS. A neurovascular high-frequency optical coherence tomography system enables in situ cerebrovascular volumetric microscopy. Nat Commun 2020;11:3851. [Crossref] [PubMed]
- Anagnostakou V, Ughi GJ, Puri AS, Gounis MJ. Optical Coherence Tomography for Neurovascular Disorders. Neuroscience 2021;474:134-44. [Crossref] [PubMed]
- Pereira VM, Lylyk P, Cancelliere N, Lylyk PN, Lylyk I, Anagnostakou V, Bleise C, Nishi H, Epshtein M, King RM, Shazeeb MS, Puri AS, Liang CW, Hanel RA, Spears J, Marotta TR, Lopes DK, Gounis MJ, Ughi GJ. Volumetric microscopy of cerebral arteries with a miniaturized optical coherence tomography imaging probe. Sci Transl Med 2024;16:eadl4497. [Crossref] [PubMed]


