
The diagnosis and pathology of cystic echinococcosis of the bone: a description of two cases
Introduction
Cystic echinococcosis (CE) is a parasitic disease caused by the Echinococcus granulosus tapeworm and is one of the most common types of echinococcosis in the world (1). Approximately 70% of lesions are found in the liver, while the lungs are the second most common site, with 15–30%, primarily affecting the lower lobes. The incidence of bone involvement in all cases of CE ranges from 0.5% to 4% (2). In the skeletal system, it most frequently occurs in the spine, followed by the pelvis, femur, and tibia, while involvement of the humerus is relatively rare (3). This report presents two cases of CE affecting the bone. In a patient with CE of the humerus, surgical treatment was applied, and the patient was discharged successfully.
Case presentation
Case 1
A 53-year-old male patient discovered a mass in his right upper arm 15 years prior to attending hospital. Over the years, the mass gradually increased in size; however, the patient did not seek medical treatment. In 2024, the patient fell and experienced pain in his right shoulder joint, along with limited movement, prompting him to visit Gansu Provincial Hospital. The patient’s laboratory tests showed no significant abnormalities. Imaging examinations upon admission were as follows: X-ray showed bone destruction in the upper segment of the right humerus, a surgical neck fracture of the humerus, and soft tissue swelling with high-density shadows (Figure 1A). Computed tomography (CT) examination indicated a pathological fracture of the upper segment of the humerus, with surrounding soft tissue swelling and multiple cystic foci, along with calcification of the cyst walls (Figure 1B,1C). Magnetic resonance imaging (MRI) showed irregular bone destruction in the upper segment of the right humerus, with uneven cortical thickness and discontinuity. Displacement changes were noted at the ends of the surgical neck, with localized narrowing of the medullary cavity, but no significant bone marrow edema observed. There were widely irregular long T1 and long T2 signals for the cystic lesions in the surrounding soft tissues of the humerus and between the pectoralis major and subscapularis muscles, with mixed high signals in diffusion-weighted imaging (DWI) and well-defined boundaries. The largest lesion in the soft tissue posterior to the humerus measured approximately 13 cm in the long axis (Figure 1D-1G).
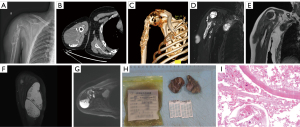
The details of pathological examination were as follows: gross examination revealed a right subclavian mass, with an irregular gray-yellow tissue fragment (5 cm × 4 cm × 0.8 cm) that was partially cystic, along with a separate gray-white cystic structure (4 cm × 3 cm × 0.8 cm) with a gelatinous cut surface. A right shoulder mass was also detected, with two irregular gray-yellow and gray-brown tissue fragments (aggregate dimensions 8 cm × 4 cm × 2 cm) with cystic architecture. The cyst walls measured 0.2–0.3 cm in thickness, with the small portion (4 cm × 4 cm × 1 cm) containing minimal gray-white membranous material and a soft cut surface. On microscopic examination, the cyst walls demonstrated characteristic features of CE, including a laminated germinal layer with hooklets and protoscolices (Figure 1H,1I).
Case 2
A 45-year-old male patient attended hospital with a complaint of numbness in both lower limbs beginning 2 months prior without any obvious cause. At the time, the patient was not concerned and did not seek treatment. Later, he noticed that the symptoms had worsened, and he experienced weakness in both lower limbs and instability while walking, which prompted him to visit our hospital. The patient’s laboratory tests showed no significant abnormalities. Meanwhile, the imaging examinations were as follows: X-ray showed a slightly high-density mass at the right edge of the T3–5 vertebrae (Figure 2A). A CT scan demonstrated a space-occupying lesion in the right accessory area of the T5 vertebra, with significant enhancement on contrast-enhanced imaging, as well as bone destruction in the costovertebral joints and pedicles at T4–5 (Figure 2B,2C). MRI revealed a soft tissue lesion in the right accessory region corresponding to the T4–5 vertebrae that exhibited slightly long T1 and long T2 signals, with high signal on fat suppression images. The lesion was irregularly shaped, had uniform signal characteristics, and measured approximately 3.55 cm × 2.12 cm × 2.08 cm. Axial images showed irregular bone structures in the right pedicles of the T4 and T5 vertebrae, and the signal of the right rib neck at the corresponding level was uneven. There were curvilinear long T1 and long T2 fluid signal shadows observed in the surrounding spaces (Figure 2D-2G).
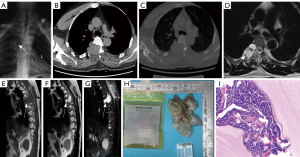
The details of pathological examination were as follows: Vertebral body and paravertebral tissue specimens showed aggregated gray-white fragmented membranous tissue (7 cm × 5 cm × 1 cm) with localized gelatinous appearance, and the cyst walls measured 0.1–0.2 cm in thickness. Histological analysis under microscopy revealed a laminated germinal layer and detached endogenous daughter cysts, confirming CE (Figure 2H,2I).
All images in this study were obtained from the Department of Radiology and Department of Pathology of Gansu Provincial People’s Hospital. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patients and their families for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CE of the bone is a rare parasitic disease caused by the larval stage of the Echinococcus granulosus tapeworm. It is primarily transmitted through food or water contaminated by feces from definitive hosts such as canines. When humans inadvertently ingest infected food or water, the larvae enter the bloodstream and eventually colonize the bones, forming cysts (4). In bone, these cysts cannot expand freely as they do in soft tissues; instead, they grow invasively, destroying trabecular bone and leading to bone resorption and destruction. Clinically and radiologically, bone CE can be classified into an active stage or an inactive stage based on the mobility of the cysts. During the active stage, it is common to observe continuous cystic growth, a rapid aggravation of bone destruction, and an increased risk of pathological fracture. In the inactive stage, there is often cyst wall calcification or fibrosis, and the degree of bone destruction slows down or even ceases. Clinically, symptoms of bone echinococcosis are diverse and characterized by a slow progression. The most commonly affected sites include the spine, pelvis, and femur. Patients typically experience local persistent pain first, which is mostly related to bone destruction and local inflammatory reactions during the active stage. When the lesion expands, especially during the active stage, it is more likely to cause pathological fracture. If the spine is affected, neural symptoms such as hypoesthesia, motor dysfunction, and even paralysis may occur due to spinal cord compression (5). After the inactive stage, due to the slowdown of cyst proliferation, the destruction to bone also slows.
CE has a wide global distribution. Its endemic areas principally encompass regions with developed animal husbandry, such as parts of Asia, Africa, South America, and Europe (6). In these areas, due to the close contact between humans and livestock, the eggs contaminate the environment, increasing the risk of infection (7). For example, in Xinjiang, Qinghai, and other areas in China, herders live with livestock, such as cattle and sheep, for extended periods, and are thus at high risk for CE of the bone (8). However, with the acceleration of globalization and the intensification of human migration, CE of the bone has spread from traditional endemic areas to nonendemic areas. For instance, migrant workers from endemic areas moving into cities may carry the pathogen to these relatively less exposed regions (6,8). Moreover, the trade and transportation of animals have also become important pathways for the long distance spread of eggs. The occurrence of sporadic cases in some originally nonendemic cities is strong evidence for the effect of human and animal mobility on disease spread (9) and suggests that the prevention and control of CE of the bone should no longer be limited to the traditional endemic areas. Indeed, surveillance and prevention and control measures should be strengthened in these nonendemic areas.
Laboratory evaluation for CE of the bone primarily includes peripheral blood eosinophil counts and serological tests. Peripheral eosinophilia is frequently observed in patients with CE, but only about 25% exhibit a pronounced increase (usually below 10%, with occasional increase up to 70%), thus limiting both its sensitivity and specificity (10). Serological tests involve the detection of echinococcal antibodies and antigens. Antibody-based assays are generally more sensitive, and a positive result strongly indicates CE infection. However, 10–15% of cases with calcified, degenerated, or necrotic cysts may yield false-negative results, making it impossible to rule out infection solely on serologic grounds (11). Moreover, normal eosinophil levels do not exclude infection in bone or other atypical sites, while markers such as C-reactive protein and erythrocyte sedimentation rate may be elevated in secondary infections or postoperatively but lack disease specificity (12). In the cases of CE of the bone described here, no laboratory abnormalities were observed, underscoring the necessity of integrating imaging and histopathological examinations to achieve a definitive diagnosis.
In terms of gross morphology, the bone tissues affected by CE in our two cases appeared as fragmented, grayish-red bone-like structures, with occasional patches of grayish-white membranous material and a relatively soft cut surface. These findings are consistent with previous reports, such as the case samples described by Zhang and Ma, where the submitted specimens consisted of fragmented grayish-red bone-like tissue. These gross features suggest significant destruction and fragility of the bone structure due to parasitic invasion (13).
Microscopic examination of CE of the bone involves the pathological hallmarks of CE, namely the degenerative Echinococcus granulosus larvae and their cystic structures. The cyst wall is composed of two distinct layers: (I) the outer laminated layer, which is acellular, laminar in structure, and stains red or blue; and (II) the inner germinal layer, consisting of a single row of nucleated cells that stain deeply red. The cyst cavity is filled with hydatid fluid and contains numerous protoscolices and brood capsules. Protoscolices are the inverted larval forms of the parasite, typically spherical or oval in shape, with visible retracted hooklets. Brood capsules, derived from the germinal layer, are cystic structures with walls composed solely of the germinal layer, lacking the laminated layer, and can give rise to new protoscolices. These histological features are diagnostic of echinococcosis and reflect the parasite’s growth and development within the host (14,15).
In addition to the parasitic structures, CE of bone is often accompanied by secondary pathological changes, including localized necrosis, calcification, and multinucleated giant cell reactions. These changes represent the host’s immune response to chronic parasitic infection and tissue repair mechanisms. The mechanism of bone destruction is primarily associated with the growth and expansion of hydatid cysts within the bone. Unlike the cysts in other organs, those for CE of the bone are not encapsulated by the host’s fibrous tissue but instead infiltrate and expand along the trabecular bone structure, resulting in the formation of multiple cystic lesions. The growth of the cysts exerts mechanical pressure on surrounding bone tissue, leading to atrophy, necrosis, and eventual resorption of bone. As the disease progresses, this process culminates in cystic transformation and bone lysis. As described by Cattaneo et al., this unique pathophysiological mechanism underscores the more invasive and destructive nature of CE of the bone as compared to that of soft tissue (14).
In recent years, polymerase chain reaction (PCR) technology has demonstrated remarkable advantages in the diagnosis of CE of the bone. Through the PCR technology, it is possible to specifically amplify the DNA markers of Echinococcus granulosus and related species. Through the analysis and identification of these amplified products, different forms of echinococcosis can be precisely distinguished at the genetic level. Given that the clinical manifestations and treatment requirements of different types of echinococcosis in bone tissue vary significantly, this precise diagnostic ability provides critical evidence for clinicians to formulate personalized and efficient treatment strategies. Moreover, this significantly enhances the accuracy of disease diagnosis, the pertinence of treatment, and the diagnosis and treatment outcomes of patients (16).
Addition to PCR, reverse transcription-PCR (RT-PCR) has become a prominent means to evaluating the efficacy of drug interventions for CE of the bone. By quantifying the expression changes of Th1/Th2 cytokine messenger RNA in the peripheral blood mononuclear cells of treated patients, RT-PCR can enable clinicians to characterize the host’s immune response to treatment at the molecular level. This information helps in evaluating the efficacy of the treatment regimen in real time and allows for the prediction of treatment outcomes, further aiding clinicians in optimizing treatment plans in a timely manner and effectively improving the treatment success rate and the prognosis of patients (17).
Moreover, the organic combination of PCR and next-generation sequencing technology constitutes a powerful tool for the epidemiological study of CE of the bone. Through the targeting of the conserved 18S ribosomal RNA gene region of Taeniidae, it is possible to detect the DNA of Echinococcus in dog feces with high sensitivity, thus confirming the presence of the parasite in the definitive host. These data facilitate a more comprehensive understanding of the transmission dynamics, transmission routes, and epidemic patterns of CE of the bone and serve as the basis for formulating and implementing effective prevention and control measures (18).
Imaging examinations play a crucial role in the diagnosis of CE of the bone, with the early lesions of this disease often being difficult to detect via X-ray alone. As the condition progresses, late-stage X-rays may reveal bone density loss, thinning of the cortical bone, and irregularly bordered cystic lucencies, along with the calcification of localized cyst walls, reflecting the expansion of the cysts and erosion of bone. Additionally, pathological fractures or significant deformation of bone structures may occur in the affected areas.
CT scans provide more detailed images of the lesions and are especially useful for characterizing the internal structures of the cysts and the extent of bone destruction in the lesion area. CT can accurately display the size, shape, and effects of the cysts on surrounding soft tissues, such as the degree of cortical bone destruction, expansion of the medullary cavity, and possible inflammatory responses in surrounding soft tissues. In some cases, CT can also identify septations within the cysts, suggesting the presence of multiloculated lesions. In MRI, the cystic lesions typically appear as cysts within soft tissues, often containing multiple daughter cysts, also known as “cysts within cysts”. Compared to the mother cyst, these internal cysts appear with a low signal on T1-weighted images and may either have a low or high signal on T2-weighted images (19). MRI is particularly suited to assessing the effect of lesions on bone marrow, helping distinguish intrabone lesions from external soft tissue lesions, while clearly visualizing the boundaries of the lesions with surrounding tissues, aiding in the identification of nerve compression or soft tissue infection spread.
In recent years, the potential application value of positron emission tomography-CT (PET/CT) or PET-MRI (PET/MRI) in the diagnosis or differential diagnosis of CE has garnered increased research focus. In PET, detection of 18F fludeoxyglucose (FDG) uptake can reveal the metabolic activity of lesions, which can help to distinguish active lesions from degenerated, calcified, or necrotic ones, thereby improving the accuracy lesion diagnosis (20-22). Some studies have reported that bone or extrahepatic CE lesions show a moderate-to-high degree of FDG uptake in PET imaging, demonstrating certain metabolic similarities with malignant tumors and thus may be prone to being misdiagnosed clinically (21). However, a multidimensional analysis consisting of lesion morphology, metabolic distribution, and delayed imaging, combined with the high-resolution imaging of the cyst wall structure and adjacent tissues via MRI, can provide an accurate differential diagnosis (23,24). In addition, PET/CT or PET/MRI can also dynamically assess the activity of parasites during the treatment process, assist in determining the efficacy of antiparasitic treatment, and monitor for early recurrence. The major of the literature on echinococcosis relates to the alveolar type, and reports related to CE are relatively limited. However, the available data suggest that PET bears important clinical significance in identifying multi-organ systemic infections or the coexistence of other tumors (22,25). With the implementation of large-sample, multicenter studies, the application prospects of PET/CT and PET/MRI in the diagnosis and differential diagnosis of CE will become clearer.
Differential diagnosis is an important function of imaging examinations, and CE of the bone must be distinguished from various bone diseases, including osteosarcoma, metastatic bone cancer, osteitis tuberculosis, chronic osteomyelitis, and other parasitic infections. Accurate differential diagnosis is crucial for avoiding misdiagnosis and selecting appropriate treatment options.
First, bone tumors and metastatic bone cancer typically present with irregular bone destruction and soft tissue masses, whereas CE of the bone is characterized by cystic lesions with relatively uniform borders. Additionally, tumor lesions usually exhibit marrow infiltration and significant periosteal reactions, which are relatively rare in echinococcosis. MRI can assist in distinguishing these lesions by identifying the characteristic signals of the tumor tissue. Second, the imaging manifestations of osteitis tuberculosis and CE of the bone may be highly similar, particularly in advanced stages of bone tuberculosis, in which lesions can appear cystic or lytic. However, bone tuberculosis is often accompanied by significant soft tissue swelling and sinus formation, whereas echinococcosis is characterized by isolated cysts without significant surrounding soft tissue reactions. Moreover, tuberculous lesions often present as multiple foci, while echinococcosis commonly presents as singular lesions. Furthermore, chronic osteomyelitis may be confused with CE of the bone since both can exhibit bone destruction and chronic inflammation. However, chronic osteomyelitis typically features bone proliferation and periosteal reactions, with imaging often revealing unevenly dense sclerotic bands and dead bone, which are not commonly seen in echinococcosis. Serological tests combined with imaging examinations can help differentiate these two conditions. CE poses a significant threat to human and animal health. Therefore, research on its diagnostic techniques is of the utmost importance. Three recent studies examined novel diagnostic methods from different perspectives. Dawuti et al. combined serum Fourier-transform infrared (FT-IR) spectroscopy with machine learning algorithms. By testing the sera of 77 infected sheep and 121 healthy control sheep, they found that the 1500–1700 band had the best classification performance. The diagnostic sensitivity, specificity, and accuracy of the relevant model reached 100%, 95.74%, and 96.66% (26), respectively. Meanwhile, Brunner et al. used Fourier-transform infrared microscopy to examine the human tissue sections of 11 confirmed patients. The results showed that this method could distinguish echinococcus components from human tissues, regardless of the organ type (27). Finally, Yang et al. conducted a retrospective, large-scale, multicenter study, in which they applied a deep convolutional neural network model for the ultrasound identification of hepatic echinococcosis, opening up a new path for diagnosis based on artificial intelligence (28). These achievements not only improve the diagnostic tools for CE but also prompt further research in this field. In the future, efforts should be focused on integrating and innovating multiple technologies, expanding the scope of clinical validation, and developing convenient diagnostic devices. This will facilitate the translation of diagnostic technologies into clinical practice, enhance the global prevention and control of CE, and safeguard the health of humans and animals.
Surgical treatment is the primary approach for treating CE of the bone. The aims are to completely remove the lesions, prevent recurrence, and preserve limb function and spinal stability as much as possible. During the surgical procedure, it is crucial that the spillage of cyst fluid is minimized to prevent anaphylactic shock and implantation metastasis. For lesions in the long bones of the extremities such as the humerus, methods including lesion curettage and bone grafting are commonly employed (5). For spinal lesions, the surgical operation is more complex, requiring not only the removal of the lesions but also spinal decompression and stability reconstruction to prevent further aggravation of spinal cord injury (29).
Albendazole may also provide considerable treatment effect for patients with CE of the bone. Albendazole can inhibit the growth and reproduction of Echinococcus granulosus. Pretreatment with albendazole before surgery can reduce the size of the cysts, thereby decreasing the surgical difficulty and the risk of recurrence. Adjuvant treatment with albendazole after surgery can kill residual parasites and reduce the likelihood of recurrence (8). However, the efficacy of albendazole is influenced by various factors, such as drug dosage, treatment course, and individual differences among patients. In addition, long-term use of albendazole may cause adverse reactions, such as liver function impairment and leukopenia. Therefore, during the treatment process, it is necessary to closely monitor the patient’s liver function and blood routine (30).
This report describes two cases of CE of the bone. Through the comprehensive application of CT and MRI imaging techniques, precise lesion localization and disease assessment were achieved. Pathological examination via hematoxylin and eosin staining was performed, and the PCR detection method was employed, providing strong support for the final diagnosis. Serological tests may provide auxiliary value in diagnosis. Although neither of the two cases received albendazole pretreatment, the literature indicates that its application can help in reducing surgical difficulty and recurrence risk. Based on the comprehensive case analysis and a review of the recent literature, this study emphasizes the crucial role of early diagnosis, pathological biopsy, and multimodal assessment in the diagnosis and treatment of CE of the bone and may serve as a key reference for improving disease management strategies.
Acknowledgments
The authors would like to sincerely thank all the mentors involved in this research as well as the patients and their families for their cooperation and trust, which made it possible to complete this work smoothly. The authors also express their special thanks to the Radiology and Pathology Departments of Gansu Provincial People’s Hospital for their hard work, which has been the foundation for the success of this study.
Footnote
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2292/coif). S.Z. reports funding from the Gansu Provincial Natural Science Foundation (No. 22JR5RA659) and the Gansu Provincial Joint Research Fund General Project (No. 23JRRA1542). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patients and their families for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003;362:1295-304. [Crossref] [PubMed]
- Loudiye H, Aktaou S, Hassikou H, El-Bardouni A, El-Manouar M, Fizazi M, Tazi A, Hajjaj-Hassouni N. Hydatid disease of bone. Review of 11 cases. Joint Bone Spine 2003;70:352-5. [Crossref] [PubMed]
- Monge-Maillo B, Lopez-Velez R. Cystic echinococcosis of the bone. Curr Opin Infect Dis 2023;36:341-7. [Crossref] [PubMed]
- Sun H, Wang S, Tan W, Li Y, Ren Q, Liu Y, Huang Y, Shi C, Li J. Echinococcus granulosus promotes bone resorption by increasing osteoclasts differentiation. Acta Trop 2023;248:107027. [Crossref] [PubMed]
- Steinmetz S, Racloz G, Stern R, Dominguez D, Al-Mayahi M, Schibler M, Lew D, Hoffmeyer P, Uçkay I. Treatment challenges associated with bone echinococcosis. J Antimicrob Chemother 2014;69:821-6. [Crossref] [PubMed]
- Moro PL, Schantz PM. Echinococcosis: historical landmarks and progress in research and control. Ann Trop Med Parasitol 2006;100:703-14. [Crossref] [PubMed]
- Torgerson PR, Robertson LJ, Enemark HL, Foehr J, van der Giessen JWB, Kapel CMO, Klun I, Trevisan C. Source attribution of human echinococcosis: A systematic review and meta-analysis. PLoS Negl Trop Dis 2020;14:e0008382. [Crossref] [PubMed]
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004;17:107-35. [Crossref] [PubMed]
- Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010;114:1-16. [Crossref] [PubMed]
- Gdoura F, Trigui M, Zribi W, Ellouze Z, Bouzidi R, Ayedi K, Keskes H. Pelvic bone hydatidosis. Orthop Traumatol Surg Res 2010;96:85-9. [Crossref] [PubMed]
- Myjak P, Nahorski W, Pietkiewicz H, von Nickisch-Rosenegk M, Stolarczyk J, Kacprzak E, Felczak-Korzybska I, Szostakowska B, Lucius R. Molecular confirmation of human alveolar echinococcosis in Poland. Clin Infect Dis 2003;37:e121-5. [Crossref] [PubMed]
- Sioutis S, Reppas L, Bekos A, Soulioti E, Saranteas T, Koulalis D, Sapkas G, Mavrogenis AF. Echinococcosis of the spine. EFORT Open Rev 2021;6:288-96. [Crossref] [PubMed]
- Zhuanmin Z, Shirong M. Bone echinococcosis: report of a case. Chinese Journal of Pathology 2025;54:81-3. [PubMed]
- Cattaneo L, Manciulli T, Cretu CM, Giordani MT, Angheben A, Bartoloni A, Zammarchi L, Bartalesi F, Richter J, Chiodini P, Godbole G, Junghanss T, Stojkovic M, Sammarchi L, Dore R, Vercelli A, Benazzo F, Cuzzocrea F, Tamarozzi F, Brunetti E. Cystic Echinococcosis of the Bone: A European Multicenter Study. Am J Trop Med Hyg 2019;100:617-21. [Crossref] [PubMed]
- Jain S, Chopra P. Cystic echinococcosis of the pelvic bone with recurrences: a case report. Korean J Parasitol 2011;49:277-9. [Crossref] [PubMed]
- Gottstein B. Molecular and immunological diagnosis of echinococcosis. Clin Microbiol Rev 1992;5:248-61. [Crossref] [PubMed]
- Riganò R, Profumo E, Buttari B, Teggi A, Siracusano A. Cytokine gene expression in peripheral blood mononuclear cells (PBMC) from patients with pharmacologically treated cystic echinococcosis. Clin Exp Immunol 1999;118:95-101. [Crossref] [PubMed]
- Abu-Helu R, Kokaly G, Nojoum S, Matouk I, Ibrahim M, Abbasi I. Molecular identification of echinococcus spp. and other taeniid tapeworms using next-generation sequence analysis of PCR amplified 18s rRNA gene. Am J Mol Biol 2024;15:75-87. [Crossref]
- Arkun R, Mete BD. Musculoskeletal hydatid disease. Semin Musculoskelet Radiol 2011;15:527-40. [Crossref] [PubMed]
- Maurer A, Kotasidis F, Deibel A, Burger IA, Huellner MW. Whole-Body 18 F-FDG PET/CT Patlak Parametric Imaging of Hepatic Alveolar Echinococcosis. Clin Nucl Med 2023;48:1089-90. [Crossref] [PubMed]
- Takenaka J, Hirata K, Watanabe S, Takahata M, Kudo K. Bone Echinococcosis Mimicking Malignancy on FDG PET. Clin Nucl Med 2023;48:e523-5. [Crossref] [PubMed]
- Niccoli Asabella A, Altini C, Pisani AR, Ingravallo G, Rubini G. 18F-FDG PET/CT metabolic activity assessment in infective and neoplastic diseases: a patient with systemic hydatidosis and concomitant Burkitt lymphoma. Clin Nucl Med 2013;38:546-9. [Crossref] [PubMed]
- Caoduro C, Porot C, Vuitton DA, Bresson-Hadni S, Grenouillet F, Richou C, Boulahdour H, Blagosklonov O. The role of delayed 18F-FDG PET imaging in the follow-up of patients with alveolar echinococcosis. J Nucl Med 2013;54:358-63. [Crossref] [PubMed]
- Reuter S, Schirrmeister H, Kratzer W, Dreweck C, Reske SN, Kern P. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin Infect Dis 1999;29:1157-63. [Crossref] [PubMed]
- Spolverato G, Pawlik TM. Images of the month. Hydatid disease. Am J Gastroenterol 2014;109:1526. [Crossref] [PubMed]
- Dawuti W, Dou J, Zheng X, Lü X, Zhao H, Yang L, Lin R, Lü G. Rapid and accurate screening of cystic echinococcosis in sheep based on serum Fourier-transform infrared spectroscopy combined with machine learning algorithms. J Biophotonics 2023;16:e202200320. [Crossref] [PubMed]
- Brunner A, Unterberger SH, Auer H, Hautz T, Schneeberger S, Stalder R, Badzoka J, Kappacher C, Huck CW, Zelger B, Pallua JD. Suitability of Fourier transform infrared microscopy for the diagnosis of cystic echinococcosis in human tissue sections. J Biophotonics 2024;17:e202300513. [Crossref] [PubMed]
- Yang Y, Cairang Y, Jiang T, Zhou J, Zhang L, Qi B, et al. Ultrasound identification of hepatic echinococcosis using a deep convolutional neural network model in China: a retrospective, large-scale, multicentre, diagnostic accuracy study. Lancet Digit Health 2023;5:e503-14. [Crossref] [PubMed]
- Mrabet D, Rekik S, Khiari H, Mizouni H, Meddeb N, Cheour I, Elleuch M, Mnif E, Mrabet A, Sahli H, Sellami S. Back pain caused by a pseudo-tumorous vertebral collapse: atypical presentation of primary vertebral hydatidosis. BMJ Case Rep 2011;2011:bcr0220113853. [Crossref] [PubMed]
- Badeliya SN, Chauhan NF, Patel NR, Trivedi LV, Patel MP, Patel SD, Dave SP. Advanced synthetic entities penetrating in the helminthiasis remedy: an extensive review. Adv Synth Entities Penetrating Helminthiasis Rem: Extensive Rev 2023. Available online: https://ijbpas.com/archive/archive-single-pdf/5591


