
Characterization of brain metastases from lung cancer using percentagewise quantification of intratumoral susceptibility signals
Introduction
Lung cancer is the most common cause of cancer-related mortality in humans (1). It easily metastasizes to the central nervous system (CNS), and accounts for about 50% of brain metastases (BMs) cases (2). BMs are devastating complications and contribute significantly to mortality (3,4). Due to high risk of developing BMs, the patients of advanced lung cancers are recommended for brain magnetic resonance imaging (MRI) even without neurological symptoms (5).
Nowadays, detection and assessment of BMs could be accomplished by conventional contrast-enhanced MRI sequences, including T1-weighted imaging (T1WI) before and after gadolinium (Gd) contrast administration, T2-weighted imaging (T2WI), fluid attenuated inversion recovery (FLAIR) and diffusion weighed imaging (DWI). The common imaging features of BMs are single or multiple spherical and well-enhanced solid lesions or ring enhancement of larger lesions in the grey-white matter junction with strikingly disproportionate edema (6), which lack specificity for the pathological types of original tumors. Lung cancer is an entity of heterogeneous items, which can be histologically divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for approximately 85% of lung cancers, which mainly includes adenocarcinoma (AD) and squamous cell carcinoma (SCC), and SCLC accounts for approximately 15% (7,8). These subtypes require distinctive treatment protocols and suggest different prognoses. Up to 30% of patients with BMs have synchronous presentation (BMs and primary tumor diagnosed at the same time) (9). For patients with lung cancer and synchronous BMs, fast and efficient identification of pathological subtypes is critical to optimize clinical decision-making and accelerate clinical practice. Furthermore, when the primary tumor is inaccessible for biopsy or poses significant procedural risks, a non-invasive method to determine the subtypes based on the magnetic resonance (MR) images of BMs would be valuable. However, non-invasive characterization and differential diagnosis of BMs in this systemic malignancy is challenging on conventional MRI.
Susceptibility weighted imaging (SWI) is a high-resolution, three dimensional (3D), fully velocity-compensated gradient-echo sequence, which is optimized for magnetic susceptibility effects and more sensitive than conventional MRI sequences for detecting neovascularization or blood products (10). The intratumoral susceptibility signals (ITSS), composed of “low-signal tubular structures or dot-like structures with or without conglomeration within a tumor” on SWI images, demonstrated neovasculature or microbleedings in the brain masses (11). Using semiquantitative grading of ITSS, Kim et al. (12) showed that the solitary BM (9 from NSCLC, 3 from SCLC in a total of 15 metastatic tumors) could be differentiated from glioblastoma multiforme (GBM) due to higher ITSS numbers in GBM. Although ITSS could provide more information of the intra-tumoral microenvironment and indicate highly malignant lesions, such as glioblastoma (13), the previous method of categorizing ITSS was semiquantitative and relatively reader-subjective (11-14). Percentagewise quantification (PQ) of ITSS has been introduced as a more objective and less reader-dependent measure to assess ITSS. It was reported that higher PQ of ITSS helped in differentiating malignant melanoma (MM) from namely bronchial carcinoma (BC) or mamma carcinoma (MC), but had poor diagnostic performance for the discrimination of BC and MC (15). These findings suggest that ITSS may serve as imaging biomarkers to differentiate brain metastatic entities with distinct biological profiles characterized by heterogeneous neoangiogenesis and tumor aggressiveness. Nevertheless, critical limitations persist in existing studies, including limited sample sizes of BMs from lung cancer, selection bias towards larger solitary metastatic lesions or unclear pathological subtypes of lung cancer, which may compromise the generalizability of ITSS interpretations and introduce systematic biases in understanding vascular heterogeneity across lung cancer subtypes. In addition, previous studies have shown that the image quality of SWI can be improved by application of contrast agent, therefore tumor boundaries of enhancement and intralesional susceptibility effects can be visualized on contrast-enhanced SWI (CE-SWI) without information loss (16,17). Also, no statistical differences in calculating the major diameters of the brain tumors were observed between CE-SWI and 3D T1WI (18). Thus, the brain tumors could be well delineated for volume calculation and ITSS be percentage-wisely quantified on CE-SWI.
ITSS occurs in a high percentage of BMs from lung cancer (15,19), but little is known about the characteristics of ITSS in BMs of its pathological subtypes. In this study, we aimed to investigate the differences in PQ of ITSS from different sizes of synchronous BMs on CE-SWI by retrospectively analyzing a cohort of 213 patients with lung cancer (AD, SCC, and SCLC). We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1505/rc).
Methods
Patients
This retrospective study was approved by the institutional review board of Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University (No. KS23008), and informed consent was waived for the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Two hundred and fifty-eight patients who underwent brain conventional contrast-enhanced MRI and CE-SWI from March 2020 to December 2023 in Shanghai Chest Hospital were initially included, and 213 patients were finally enrolled into this study. The inclusion criteria were as follows: (I) patients with synchronous BMs of primary AD, SCC, and SCLC (confirmed by cytology, biopsy or surgery); (II) patients underwent follow-up MRI within the next 6 months and BMs were finally confirmed by overall consideration; (III) the metastatic lesions must be located in the brain parenchyma and identified both on CE-T1WI and CE-SWI. The exclusion criteria were as follows: (I) patients with other subtypes of lung cancer, such as large cell lung carcinoma; (II) multiple malignant tumors, history of brain radiation therapy or prior neurosurgery, multiple microbleeds due to cerebral small vessel disease (CSVD); (III) poor imaging quality of CE-SWI.
MRI acquisitions
All MR imagings were performed on a 3.0-T clinical MR imager (Prisma or Vida, SIEMENS Medical Systems; Erlangen, Germany) with a 20-channel head-matrix coil. The MRI acquisition workflow included: T1WI (3 minutes and 30 second) → T2WI (29 seconds) → DWI (49 seconds) → FLAIR (48 seconds) → administration of contrast agents → T1WI → SWI (3 minutes and 40 second). CE-T1WI and CE-SWI was performed after intravenous bolus injection [0.1 mmol/kg gadobenate dimeglumine (Gd-BOPTA)]. The parameters of T1WI and SWI were as follows: T1WI: repetition time (TR) =3.8 ms, echo time (TE) =1.64 ms, number of excitations (NEX) =1, matrix =256×256, field of view (FOV) =240 mm, slice thickness =1 mm; SWI: TR =27 ms; TE =20 ms, flip angle =15°, bandwidth =140 Hz, matrix =332×332; FOV =220 mm, slice thickness =1.5 mm.
Image processing and analysis
All BMs on CE-SWI for each patient were identified with reference to CE-T1WI by mutual consent of two radiologists at a workstation, who were blinded to the pathological subtypes of lung cancer. The image processing consisted of three steps: (I) image preprocessing: all BMs were manually delineated slice-by-slice on CE-SWI using the ITK-SNAP software (Version 3.2), and then all images of CE-SWI were resampled to voxel size of 1×1×1 mm3 by the Simple Insight Segmentation and Registration Toolkit (Simple ITK; https://github.com/SimpleITK/SimpleITK) package to ensure the physical space consistency. The bias field correction or receiver-field inhomogeneity correction was conducted to reduce the nonuniformity of the low-frequency signal intensity. After image segmentation and filtering, the segmented tumor areas were classified into two parts: the part of ITSS (whose intensity was below the value of the ventricular system) and the part of non-ITSS (including the areas of tumor enhancement, necrosis and cystic degeneration, the intensity ≥ the value of the ventricular system). (II) Network training: network programming was performed using PyCharm (Python version 3.7.0) and Compute Unified Device Architecture (CUDA) as the training platform. The Adaptive Moment Estimation optimizer (Adam optimizer) and the weighted binary cross entropy (BCE) were applied for updating the parameters of the network and minimizing the loss function. A certain amount of imaging data was put into the U-Net network for training to get the best network model. (III) Network prediction: the test set of image data was put into the best trained U-Net network model to generate predicted images with two colors. In delineated BMs, the white represented ITSS and the black non-ITSS. And then PQ of ITSS in each BM was calculated. The BMs are classified into three categories based on sizes: Group 1 (micrometastases ≤0.125 cm3), Group 2 (middle metastases, 0.125 cm3< size <27 cm3) and Group 3 (macrometastases ≥27 cm3). The smaller lesions (<0.027 cm3/27 voxels) are not recognized due to the technical limitations of the model. In fact, a measurable micrometastatic lesion corresponds to 27–125 voxels in this study. Within the delineated BMs, all voxels below the value of the ventricular system (presenting ITSS) were visualized and percentagewise calculated as shown in Figure 1.
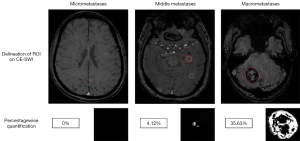
Statistical analysis
Statistical analysis was performed using SPSS package (Version 23.) and GraphPad Prism package (Version 10.2). The measured data were presented as mean ± standard deviation (SD). Fisher exact test was performed to examine differences in the contingency tables. PQ values of ITSS from all BMs in three subtypes were compared among Groups 1, 2 and 3 using analysis of variance (ANOVA) or nonparametric tests, and post hoc pairwise comparisons were conducted using Dunn’s test with Bonferroni correction following a significant result. Receiver operating characteristic (ROC) curve-analysis was performed for the differentiation of AD vs. non-AD in macrometastases. P<0.05 was statistically significant.
Results
There were 213 patients (137 men, 76 women; age range, 23–81 years; mean age 60.96 years) enrolled in this study. A total of 1,060 BMs (702 in AD, 114 in SCC, and 244 in SCLC) were identified on CE-SWI, in which there were 390 in Group 1, 617 in Group 2, and 53 in Group 3. The characteristics of patients with BMs from AD, SCC, and SCLC were shown in Table 1.
Table 1
| Characteristics | AD | SCC | SCLC |
|---|---|---|---|
| Patients, n | 141 | 28 | 44 |
| Male | 75 | 22 | 40 |
| Female | 66 | 6 | 4 |
| Age (years) | |||
| Range | 26–81 | 23–75 | 48–78 |
| Median | 61 | 66 | 64 |
| Mean ± SD | 59.94±9.92 | 63.13±12.21 | 62.83±6.64 |
| No. of BMs (%) | |||
| Total | 702 (42.74) | 114 (33.33) | 244 (49.18) |
| Group 1 | 261 (77.01) | 28 (64.29) | 101 (83.17) |
| Group 2 | 405 (24.44) | 78 (25.64) | 134 (26.87) |
| Group 3 | 36 (0.00) | 8 (0.00) | 9 (0.00) |
In the No. of BMs, the incidence of BMs with PQ <1% (no ITSS) in each group was shown in parentheses. Group 1 represents micrometastases (≤0.125 cm3); Group 2 represents middle metastases (0.125 cm3< size <27 cm3); Group 3 represents macrometastases (≥27 cm3). AD, adenocarcinoma; BMs, brain metastases; ITSS, intratumoral susceptibility signals; PQ, percentagewise quantification; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; SD, standard deviation.
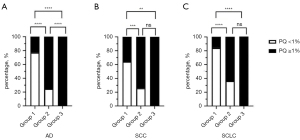
PQ values below 1% were considered as nearly no ITSS or ITSS negative. Four hundred and fifty-eight of 1,060 BMs had no ITSS, and the overall incidence of no-ITSS BMs was 43.21%. The incidence of BMs with no ITSS in each group was shown in parentheses in the fourth row of Table 1. The incidence of no ITSS was 77.01% in Group 1, 24.44% in Group 2, and 0% in Group 3 from AD; the incidence of no ITSS was 64.29% in Group 1, 25.64% in Group 2, and 0% in Group 3 from SCC; the incidence of no ITSS was 83.17% in Group 1, 26.87% in Group 2, and 0% in Group 3 from SCLC (Figure 2). It indicated larger lesions (middle metastases and macrometastases) of BMs were more likely to have positive ITSS than small ones (micrometastases) in the three subtypes of lung cancer.
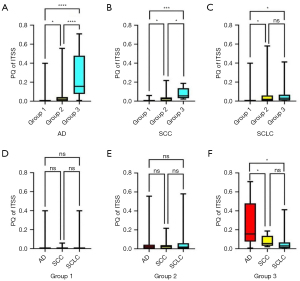
PQ values of ITSS in different sizes of BMs from AD, SCC, and SCLC were shown (Table 2). As shown in Figure 3A-3C, BMs in Group 2 and Group 3 had higher PQ of ITSS than those in Group 1. Notably, there was no difference in PQ of ITSS for middle metastases and macrometastases in SCLC. As shown in Figure 3D,3E, there were no differences in Group 1 and Group 2 among AD, SCC, and SCLC. In Group 3 (Figure 3F), PQ of ITSS in AD was higher than that in SCC (P=0.016) and in SCLC (P=0.010), while there was no difference between SCC and SCLC (P=0.921).
Table 2
| Characteristics | AD | SCC | SCLC | P value | |||
|---|---|---|---|---|---|---|---|
| All | AD vs. SCC | AD vs. SCLC | SCC vs. SCLC | ||||
| PQ in G1 | 0.320 | 0.487 | 0.530 | 0.702 | |||
| Range | 0–39.87 | 0–5.89 | 0–39.87 | ||||
| Median | 0 | 0 | 0 | ||||
| Mean ± SD | 1.51±5.38 | 0.55±1.64 | 1.09±4.53 | ||||
| PQ in G2 | 0.720 | 0.484 | 0.887 | 0.423 | |||
| Range | 0–55.57 | 0–21.71 | 0–58.01 | ||||
| Median | 2.12 | 1.39 | 1.81 | ||||
| Mean ± SD | 4.57±8.43 | 3.46±4.33 | 4.70±8.63 | ||||
| PQ in G3 | 0.014 | 0.016 | 0.010 | 0.921 | |||
| Range | 6.73–70.82 | 2.19–18.68 | 0–41.17 | ||||
| Median | 15.57 | 5.48 | 3.06 | ||||
| Mean ± SD | 26.13±23.98 | 7.94±6.02 | 7.11±13.08 | ||||
| P value | <0.001 | 0.001 | <0.001 | – | – | – | – |
| G1 vs. G2 | 0.013 | 0.037 | <0.001 | ||||
| G1 vs. G3 | <0.001 | <0.001 | 0.021 | ||||
| G2 vs. G3 | <0.001 | 0.008 | 0.348 | ||||
G1, G2, G3: Group 1, Group 2, Group 3. Group 1 represents micrometastases (≤0.125 cm3); Group 2 represents middle metastases (0.125 cm3< size <27 cm3); Group 3 represents macrometastases (≥27 cm3). AD, adenocarcinoma; BMs, brain metastases; ITSS, intratumoral susceptibility signals; PQ, percentagewise quantification; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; SD, standard deviation.
Diagnostic performance for the discrimination of AD and non-AD (SCC and SCLC) in macro-metastases was good [area under the receiver operating characteristic curve (AUC) =0.81; 95% confidence interval (CI): 0.64, 0.98] (Figure 4). At a cut off value of 6.68%, the sensitivity and specificity for differentiation of AD and non-AD were 0.92 and 0.71.

Discussion
In this study, we found that PQ of ITSS can contribute to the non-invasive characterization of lung cancer BMs and provided for the first time the differential diagnosis of AD and non-AD on CE-SWI. The incidence of no ITSS decreased and PQ of ITSS increased as the tumor sizes of BMs got larger. Besides, PQ of ITSS in macrometastases (≥27 cm3) from AD was higher than that from non-AD (SCC and SCLC), while there were no differences in micrometastases (≤0.125 cm3) and middle metastases (0.125 cm3< size <27 cm3) among the three subtypes. By choosing a cut off value of 6.68%, a good differentiation performance between patients suffering from macrometastases of AD and non-AD was demonstrated.
To date, several studies have dealt with correlations between ITSS in brain tumors and the underlying tumor entities. A correlation between ITSS and sizes of BMs from breast cancer or melanoma was reported (20), which indicated that the presence of intratumoral hemorrhage was uncommon in micrometastases but common in metastases greater than 0.1 cm3. Our study showed similar tendency in BMs from the three subtypes of lung cancer. ITSS occurred less frequently in micrometastases, while increased dramatically in middle metastases and macrometastases. It was reported that small lesions of BMs usually responded much better to treatment and could be controlled at a substantially higher rate, compared to larger lesions (21,22). From a pathophysiological point of view, the absence of angiogenesis and microbleedings in micrometastases presumably indicated less expansion of the tumor population regardless of the proliferative capacity of tumor cells and better response to therapies. Moreover, Radbruch et al. (15) revealed that PQ of ITSS in 75 BMs from 45 patients with BC metastases ranged from 0% to 90.9%, and mean was 14.3%±27.7%. Also, diagnostic performance of PQ of ITSS was good for the discrimination of MM and BC (AUC =0.81, a cut off value =20%), but poor for the discrimination of MC and BC (AUC =0.60). In our study, we included a large number of cases and conducted a more in-depth investigation on PQ of ITSS in different sizes of BMs from the three subtypes of lung cancer. According to our results, diagnostic performance of PQ of ITSS in macrometastases was good (AUC =0.81, a cut off value =6.68%) to discriminate AD from non-AD. If the patient of lung cancer had synchronous brain macrometastases with PQ of ITSS >6.68%, it indicated the primary tumor was more likely to be AD, rather than SCC or SCLC. This might expedite the treatment based on histological classification of lung cancer. Also, the cognition of the subtype and its bleeding-related characteristics in advance can help in optimizing the treatment plan.
Angiogenesis is one of the hallmarks of cancers and promotes the proliferation and infiltration of metastatic cells (23,24). These tortuous and enlarged neocapillaries of tumors are different from the normal capillaries by their immature walls and increased endothelial gaps, which tend to be leaky and cause intratumoral hemorrhage (25). Observation and identification of tumor vasculature and hemorrhage not only provides supporting evidence for tumor diagnosis, but also is an important indicator for monitoring the efficacy of tumor radiotherapy and chemotherapy (13). In our research, we investigated angiogenesis and the intra-tumor blood products in BMs of lung cancer subtypes by the quantitative method. Our results showed that macrometastases from AD exhibited significantly higher PQ of ITSS (indicative of increased microbleeding and angiogenesis) comparing with those from SCC and SCLC, while no differences in micrometastases and middle metastases between AD and non-AD were observed. This discrepancy may be attributed to biological heterogeneity of tumor subtypes. Separate metastatic cell clusters develop into micrometastases without requiring substantial vascular remodeling or extensive blood supply. In contrast, macrometastases necessitate robust neoangiogenesis to sustain their growth, thereby truly reflecting their biological heterogeneity of neo-angiogenesis among different subtypes (26). Microvessel density (MVD), defined as the number of microvessels per square millimeter (vessels/mm2) or per high-power field (vessels/HPF) following immunohistochemical labeling of vascular endothelial markers [e.g., cluster of differentiation 31 (CD31), cluster of differentiation 34 (CD34), or vascular endothelial growth factor receptor 2 (VEGFR2)], serves as a key quantitative measure of tumor angiogenesis (27). Notably, MVD varies significantly across the pathological subtypes of lung cancer. Studies reported higher MVD in AD (30–40 vessels/mm2) compared to SCC (15–25 vessels/mm2) (28,29), and the disparity in angiogenesis aligned with our findings of distinct ITSS profiles between AD and SCC in macrometastases. Unfortunately, there is a paucity of studies investigating MVD in SCLC, highlighting a critical gap in our microscopic understanding of its angiogenic profile. Radiologically, our study revealed that SCLC-derived BMs exhibited limited angiogenic alterations after progressing to middle metastases, in contrast to the pronounced angiogenesis observed in AD, which will be explored in future. To the best of our knowledge, our work is the first study to demonstrate the heterogeneity of angiogenesis and microbleeding in different sizes of BMs from lung cancer subtypes using CE-SWI.
There are several limitations in our study. (I) The relatively small sample sizes of macrometastases, such as 8 for SCC and 9 for SCLC, may not provide sufficient statistical power to detect significant differences in Group 3, with a higher chance of Type II errors. We will be actively engaged in expanding our sample collection for a further study. (II) The phase mask in SWI is geometry dependent, so it can lose susceptibility contrast and does not provide exact quantitative measures of magnetic susceptibility (30). Although this limitation is currently being addressed with the development of quantitative susceptibility mapping (QSM) (31), its additional scanning time (approximately 5 minutes for the whole brain) is challenging for weak and elderly cancer patients. (III) BMs from other pathological subtypes of lung cancer, such as large cell lung carcinoma and carcinoid, were not included due to lower incidence. Yet, the substantial expansion of the sample size would be helpful in extending the perception and characterization of ITSS in BMs from lung cancer. (IV) Both microbleeds and vascular structures are categorized as ITSS, but they cannot be completely distinguished on CE-SWI, especially in BMs with lots of hemorrhage. (V) PQ of ITSS on CE-SWI provides an array of information regarding the microstructural and pathophysiological information of BMs, but fails to offer metabolic and functional properties. In future investigations, we plan to explore the utility of PQ in combination with other functional MRI sequences [e.g., dynamic contrast enhanced (DCE) MRI] for predicting response to antiangiogenic therapies.
Conclusions
PQ of ITSS on CE-SWI is a promising tool for noninvasively characterizing BMs from the subtypes of lung cancer, which is able to show the intratumoral bleeding and neovascularization increase when the BMs are getting larger and is helpful in differentiating macrometastses from AD and non-AD.
Acknowledgments
We would like to thank Jercy Chen for her help in polishing our paper.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1505/rc
Funding: This study received funding by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1505/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the institutional review board of Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University (No. KS23008), and informed consent was waived for the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W, Heimans J, Hildebrand J, Hoskin P, Kalljo M, Krauseneck P, Marosi C, Siegal T, Vecht C. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol 2006;13:674-81. [Crossref] [PubMed]
- Tagore S, Caprio L, Amin AD, Bestak K, Luthria K, D’Souza E, et al. Single-cell and spatial genomic landscape of non-small cell lung cancer brain metastases. Nat Med 2025;31:1351-63. [Crossref] [PubMed]
- Mujoomdar A, Austin JH, Malhotra R, Powell CA, Pearson GD, Shiau MC, Raftopoulos H. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882-8. [Crossref] [PubMed]
- Levy A, Faivre-Finn C, Hasan B, De Maio E, Berghoff AS, Girard N, Greillier L, Lantuéjoul S, O'Brien M, Reck M, Dingemans AC, Novello S, Berghmans T, Besse B, Hendriks LYoung Investigators EORTC Lung Cancer Group. (YI EORTC LCG). Diversity of brain metastases screening and management in non-small cell lung cancer in Europe: Results of the European Organisation for Research and Treatment of Cancer Lung Cancer Group survey. Eur J Cancer 2018;93:37-46. [Crossref] [PubMed]
- Pope WB. Brain metastases: neuroimaging. Handb Clin Neurol 2018;149:89-112. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Wen P, Wang TF, Li M, Yu Y, Zhou YL, Wu CL. Meta-analysis of prophylactic cranial irradiation or not in treatment of extensive-stage small-cell lung cancer: The dilemma remains. Cancer Radiother 2020;24:44-52. [Crossref] [PubMed]
- Kniep HC, Madesta F, Schneider T, Hanning U, Schönfeld MH, Schön G, Fiehler J, Gauer T, Werner R, Gellissen S. Radiomics of Brain MRI: Utility in Prediction of Metastatic Tumor Type. Radiology 2019;290:479-87. [Crossref] [PubMed]
- Martín-Noguerol T, Santos-Armentia E, Ramos A, Luna A. An update on susceptibility-weighted imaging in brain gliomas. Eur Radiol 2024;34:6763-75. [Crossref] [PubMed]
- Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY. Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol 2009;30:1402-8. [Crossref] [PubMed]
- Kim HS, Jahng GH, Ryu CW, Kim SY. Added value and diagnostic performance of intratumoral susceptibility signals in the differential diagnosis of solitary enhancing brain lesions: preliminary study. AJNR Am J Neuroradiol 2009;30:1574-9. [Crossref] [PubMed]
- Schwarz D, Bendszus M, Breckwoldt MO. Clinical Value of Susceptibility Weighted Imaging of Brain Metastases. Front Neurol 2020;11:55. [Crossref] [PubMed]
- Li C, Ai B, Li Y, Qi H, Wu L. Susceptibility-weighted imaging in grading brain astrocytomas. Eur J Radiol 2010;75:e81-5. [Crossref] [PubMed]
- Radbruch A, Graf M, Kramp L, Wiestler B, Floca R, Bäumer P, Roethke M, Stieltjes B, Schlemmer HP, Heiland S, Bendszus M. Differentiation of brain metastases by percentagewise quantification of intratumoral-susceptibility-signals at 3Tesla. Eur J Radiol 2012;81:4064-8. [Crossref] [PubMed]
- Pinker K, Noebauer-Huhmann IM, Stavrou I, Hoeftberger R, Szomolanyi P, Weber M, Stadlbauer A, Grabner G, Knosp E, Trattnig S. High-field, high-resolution, susceptibility-weighted magnetic resonance imaging: improved image quality by addition of contrast agent and higher field strength in patients with brain tumors. Neuroradiology 2008;50:9-16. [Crossref] [PubMed]
- El-Koussy M, Schenk P, Kiefer C, Osman OM, Mordasini P, Ozdoba C, Schroth G, Gönner F. Susceptibility-weighted imaging of the brain: does gadolinium administration matter? Eur J Radiol 2012;81:272-6. [Crossref] [PubMed]
- Hori M, Ishigame K, Kabasawa H, Kumagai H, Ikenaga S, Shiraga N, Aoki S, Araki T. Precontrast and postcontrast susceptibility-weighted imaging in the assessment of intracranial brain neoplasms at 1.5 T. Jpn J Radiol 2010;28:299-304. [Crossref] [PubMed]
- Zhang W, Ma XX, Ji YM, Kang XS, Li CF. Haemorrhage detection in brain metastases of lung cancer patients using magnetic resonance imaging. J Int Med Res 2009;37:1139-44. [Crossref] [PubMed]
- Franceschi AM, Moschos SJ, Anders CK, Glaubiger S, Collichio FA, Lee CB, Castillo M, Lee YZ. Use of Susceptibility-Weighted Imaging (SWI) in the Detection of Brain Hemorrhagic Metastases from Breast Cancer and Melanoma. J Comput Assist Tomogr 2016;40:803-5. [Crossref] [PubMed]
- Chang EL, Hassenbusch SJ 3rd, Shiu AS, Lang FF, Allen PK, Sawaya R, Maor MH. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery 2003;53:272-80; discussion 280-1. [Crossref] [PubMed]
- Ranjan T, Abrey LE. Current management of metastatic brain disease. Neurotherapeutics 2009;6:598-603. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4-6. [Crossref] [PubMed]
- Mohammed W, Xunning H, Haibin S, Jingzhi M. Clinical applications of susceptibility-weighted imaging in detecting and grading intracranial gliomas: a review. Cancer Imaging 2013;13:186-95. [Crossref] [PubMed]
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 2010;16:116-22. [Crossref] [PubMed]
- Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2002;87:694-701. [Crossref] [PubMed]
- Fontanini G, Vignati S, Bigini D, Lucchi M, Mussi A, Basolo F, Angeletti CA, Bevilacqua G. Neoangiogenesis: a putative marker of malignancy in non-small-cell lung cancer (NSCLC) development. Int J Cancer 1996;67:615-9. [Crossref] [PubMed]
- Chen L, Chen M, Han Z, Jiang F, Xu C, Qin Y, Ding N, Liu Y, Zhang T, An Z, Guo C. Clinical significance of FAP-α on microvessel and lymphatic vessel density in lung squamous cell carcinoma. J Clin Pathol 2018;71:721-8. [Crossref] [PubMed]
- Haller S, Haacke EM, Thurnher MM, Barkhof F. Susceptibility-weighted Imaging: Technical Essentials and Clinical Neurologic Applications. Radiology 2021;299:3-26. [Crossref] [PubMed]
- Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging 2015;33:1-25. [Crossref] [PubMed]


