
Preoperative ultrasonography-guided core-needle biopsy-based factors for predicting the upgrade of axillary lymph nodes in breast cancer
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer-related death in women (1). The presence of axillary metastatic disease influences clinical decision-making and treatment planning (2). The research in this field has focused on using less invasive techniques to accurately predict the presence of the disease. We reviewed the literature on the various methods for the preoperative evaluation of axillary lymph node (ALN) status, and axillary ultrasonography (US) and subsequent US-guided core-needle biopsy (CNB) targeting suspicious lymph nodes (LNs) were found to be the most commonly used method in clinical practice (3).
However, the accuracy of biopsy of ALN varies, with previous studies reporting sensitivities ranging from 21% to 95% (4). A sentinel node (SN) may be avoided when metastatic disease is identified preoperatively on biopsy. Previous studies have shown that the false-negative rate of preoperative axillary biopsy is as high as 28% (5).
The purpose of this study was to evaluate the rate of false-negative results for the US-guided CNB of ALN and determine the related factors associated with such false-negative results to predict the preoperative upgrading in diagnosis among patients with BC. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1257/rc).
Methods
Study population
This retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital (approval No. bc2023027). As we retrospectively examined data from a hospital database, the requirement for informed consent was waived. The clinical data and US features of patients with BC who underwent surgical treatment in in Tianjin Medical University Cancer Institute and Hospital from January 2022 to January 2023 were retrospectively analyzed. Initially, 382 patients were included. The inclusion criteria for the study included were as follows: (I) female sex and clinical stage T1–T2; (II) maximum axial diameter of breast lesions <5 mm; and (III) complete clinicopathological data and US imaging. Meanwhile, the exclusion criteria were as follows: (I) male sex and clinical stage beyond T2; (II) maximum axial diameter of breast lesions ≥5 mm; (III) neoadjuvant chemotherapy (NATC); and (IV) axillary biopsy performed for other primary tumors.
CNB procedure and sentinel lymph node biopsy (SLNB)
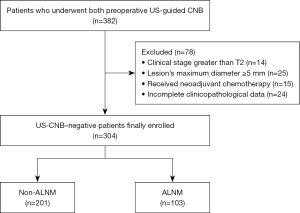
Ipsilateral ALN US was performed for patients diagnosed with primary BC. An LN was considered abnormal when the cortical thickness was >3 mm and when there was a loss of fatty hilum and a round shape on US. US-guided CNB was performed by for the most suspected LNs. Aspirates were immediately fixed in 95% ethanol after the CNB procedure. All CNB slides underwent Papanicolaou staining and reviewed by experienced pathologists. SLNB was performed using a dual-tracer method consisting of radioisotope and blue dye. The day before surgery or on the day of after injection of 99m-technetium, lymphoscintigraphy was performed. Indigo carmine was injected in subareolar area before the surgery. SNs were stained with hematoxylin and eosin and anticytokeratin after intraoperative evaluation and examination by pathologists. Ultimately, a total of 304 patients were grouped into those with and without axillary lymph node metastasis (ALNM) according to the postoperative pathological results (Figure 1).
Clinicopathologic findings
US images of primary BC and the axillary region were systematically stored and subsequently subjected to retrospective analysis. All US examinations were performed on a Logiq 9 device (GE Healthcare, Chicago, IL, USA) with a high-frequency linear array transducer (L12-5 MHz). The imaging features of the primary lesions included maximum diameter of mass, location, multifocality or unifocality, bump or nonbump, and the presence of calcification. The largest lesion was included in the study if the primary tumor presented with multiple suspicious lesions. The clinical features of tumor included age and the number of metastatic LNs. The pathological features included estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, Ki-67 index, and lymphovascular invasion (LVI) and histological grading. Molecular type conducted according to the Gallen International Breast Cancer Conference [2013] Expert Panel criteria and included luminal A (PR ≥20% positive, ER >1% positive, Ki-67% ≤14%, and HER2 negative), luminal B (luminal B1: HER2 negative, ER >1% positive, PR ≥20% positive, ER >1% positive and/or PR <20% positive, HER2 negative, and Ki-67 >14%; luminal B2: HER2 positive, ER >1% positive and/or PR >1% positive, and Ki-67% ≤14%), HER2 (HER2 positive and ER and PR negative), and triple-negative breast cancer (TNBC; ER, PR, and HER2 negative). All patients underwent immunohistochemical examination at the department of pathology.
Node Reporting and Data System 1.0 (Node-RADS 1.0) scoring
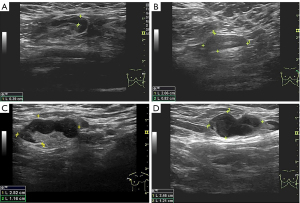
Node-RADS 1.0 (6) was used to reflect the level of suspicion for involvement by malignancy. A score 1 indicated “very low”; 2, “low”; 3, “equivocal”; 4, “high”; and 5, “very high”. Radiologists were guided to score suspicious LN according to size and configuration as per the Node-RADS 1.0. Size was divided into three categories: normal, enlarged, and bulk as follows: normal size, short-axis diameter <10 mm; bulk size, longest diameter ≥30 mm measured in the short axis or long axis; and enlarged size, diameter between 10 and 15 mm without meeting either the definitions of normal or bulk. The configuration criterion consisted of the sum of the three subcategories of texture, border, and shape. The category “texture” refers to the internal structure of LN, represents the degree of destruction by malignancy, and was classified as homogeneous (0 points), heterogeneous (1 point), focal necrosis (2 points), or gross necrosis or any new necrosis (3 points). The category “border” refers to the extent of disease destruction beyond the LN and was classified as smooth (0 points) or irregular or ill-defined (1 point). The category “shape” refers to two features of delineation of the fatty hilum and geometric shape and was classified as any shape with preserved fatty hilum (0 points) or kidney bean-like or oval without fatty hilum (1 point). Node-RADS seeks not to overinterpret the size of LN, and the criterion of configuration is of critical importance for the final Node-RADS scoring. When an enlarged LN in the size criterion is not accompanied by abnormal morphologic features according to the configuration criteria, the Node-RADS score is 2 (low suspicion). The features of the LN of the given subcategory are then translated into a minimum achievable configuration score ranging from 0 to 5 points. All suspected LNs were preoperatively scored on the basis of Node-RADS 1.0 by three breast radiologists (with 6 to 10 years of experience in breast US) (Figure 2). All suspicious nodes underwent US-guided CNB with an adjustable automatic Bard biopsy gun (ranging from 15 to 22 mm; BD Biosciences, San Jose, CA, USA), paired with a disposable 14-G trough puncture cutting biopsy needle. An experienced physician performed CNB under US guidance, inserting the needle parallel to the long axis of the probe. Final diagnosis was determined by the surgical pathologic results.
Statistical analysis
Data are expressed as numbers and percentages for categorical variables and as the median and interquartile range for continuous variables (age). The chi-squared test or Fisher exact test was used to comparatively evaluate data from categorical variables, while the Mann-Whitney test was used to analyzed continuous variables. A P value <0.05 was considered statistically significant. Univariate analyses and multivariate logistic regression were used to identify the independent predictors. The performance of the model for predicting preoperative ALN upgrade after surgery was evaluated via the area under the curve (AUC), sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). The Hosmer-Lemeshow goodness-of-fit test was used to evaluate the calibration and discrimination. Statistical analysis was performed with R 4.3.1 (R Development Core Team, Vienna, Austria).
Results
Patient characteristics and nodal burden
A total of 304 patients were enrolled in this study, among whom 103 patients had ALNM and 201 did not. All patients were female, with an average age of 53 (age range, 25–79) years. The average size of the primary BC was 16.8 (range, 1–50) mm. There were 103 cases of pathologically positive LNs and 201 cases of pathologically negative LNs. The false-negative rate of US-guided CNB of ALN was 33.8% (103/304). The majority of patients had invasive ductal carcinoma (IDC) (n=272; 89.4%). Among the 103 patients with a false-negative result, 81.5% (n=84) underwent axillary lymph node dissection (ALND), and the average number of positive LNs was 3 (range, 1–11). Nineteen patients with a false-negative result shad SLNB alone. Of these patients,16 cases were due to only micrometastases in SLNB, which was one of the key factors contributing to false-negative results in CNB, and the false negatives in the other 3 cases were attributed to physical and age-related factors that precluded the patients from undergoing ALND. A comparison of the US and clinicopathological features of patients between the ALNM group (n=103) and non-ALNM group (n=201) is provided in Table 1.
Table 1
| Variables | Overall (n=304), n (%) | Non-ALNM (n=201), n (%) | ALNM (n=103), n (%) | P value |
|---|---|---|---|---|
| Pathological grade | <0.001 | |||
| I | 144 (47.4) | 76 (37.8) | 68 (66.0) | |
| II | 68 (22.4) | 54 (26.9) | 14 (13.6) | |
| III | 92 (30.3) | 71 (35.3) | 21 (20.4) | |
| Molecular type | <0.001 | |||
| Luminal A | 57 (18.8) | 17 (8.5) | 40 (38.8) | |
| Luminal B | 130 (42.8) | 76 (37.8) | 54 (52.4) | |
| TNBC | 88 (28.9) | 81 (40.3) | 7 (6.8) | |
| HER2 enriched | 29 (9.5) | 27 (13.4) | 2 (1.9) | |
| Ki-67 | 0.098 | |||
| Negative | 53 (17.4) | 35 (17.4) | 18 (17.5) | |
| Positive | 251 (82.6) | 166 (82.6) | 85 (82.5) | |
| P53 | 0.407 | |||
| Negative | 120 (39.5) | 76 (37.8) | 44 (42.7) | |
| Positive | 184 (60.5) | 125 (62.2) | 59 (57.3) | |
| LVI | <0.001 | |||
| Negative | 265 (87.2) | 189 (94.0) | 76 (73.8) | |
| Positive | 39 (12.8) | 12 (6.0) | 27 (26.2) | |
| Multicenter | 0.132 | |||
| Negative | 242 (79.6) | 155 (77.1) | 87 (84.5) | |
| Positive | 62 (20.4) | 46 (22.9) | 16 (15.5) | |
| Lesion type | 0.504 | |||
| Lump | 199 (65.5) | 124 (61.7) | 75 (72.8) | |
| Nonlump | 105 (34.5) | 77 (38.3) | 28 (27.2) | |
| Location | 0.106 | |||
| Outer upper | 95 (31.2) | 69 (34.5) | 26 (25.2) | |
| Other | 209 (68.8) | 132 (65.7) | 77 (74.8) | |
| Maximum diameter | 0.157 | |||
| <3 cm | 48 (15.8) | 36 (17.9) | 12 (11.7) | |
| ≥3 cm | 256 (84.2) | 165 (82.1) | 91 (88.3) | |
| Calcification | 0.345 | |||
| Negative | 162 (53.3) | 111 (55.2) | 51 (49.5) | |
| Positive | 142 (46.7) | 90 (44.7) | 52 (50.4) | |
| Scoring | <0.001 | |||
| 1 | 2 (0.7) | 2 (0.9) | 0 (0.0) | |
| 2 | 104 (34.2) | 89 (44.2) | 15 (14.6) | |
| 3 | 119 (39.1) | 85 (42.2) | 34 (33.0) | |
| 4 | 64 (21.1) | 22 (10.9) | 42 (40.8) | |
| 5 | 15 (4.9) | 3 (1.4) | 12 (11.7) |
ALNM, axillary lymph node metastasis; BC, breast cancer; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; TNBC, triple-negative breast cancer.
Univariate analysis
The results of the univariate and multivariate analyses are summarized in Table 2. Histological grades II and III were significantly associated with upgrade in diagnosis as compared with grade I (P<0.001). However, there was no statistical difference between grades II and III in this regard. Regarding Node-RADS 1.0 score, as compared with ALN score <4, a score ≥4 was significantly associated with an upgrade in diagnosis (P<0.001), but there was no statistical difference between scores of 4 and 5.
Table 2
| Feature | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Scoring ≥4 | 7.758 (4.436, 13.913) | <0.001 | 9.459 (4.504, 21.365) | <0.001 | |
| Calcification | 1.258 (0.781, 2.027) | 0.345 | – | – | |
| Ki-67 | 0.996 (0.538, 1.893) | 0.989 | – | – | |
| Lesion type | 0.601 (0.354, 1.002) | 0.055 | – | – | |
| Location | 1.548 (0.918, 2.665) | 0.107 | – | – | |
| LVI | 5.595 (2.753, 11.998) | 0.001 | 3.675 (1.462, 9.655) | 0.006 | |
| Maximum diameter | 1.655 (0.841, 3.461) | 0.16 | – | – | |
| Luminal B | 0.302 (0.152, 0.580) | <0.001 | 0.266 (0.118, 0.578) | 0.001 | |
| TNBC | 0.037 (0.013, 0.091) | <0.001 | 0.035 (0.011, 0.101) | <0.001 | |
| HER2 | 0.031(0.005, 0.121) | <0.001 | 0.022 (0.003, 0.106) | <0.001 | |
| Multicenter | 0.620 (0.323, 1.139) | 0.134 | – | – | |
| P53 | 0.815 (0.503, 1.325) | 0.408 | – | – | |
| Pathological grade II | 0.290 (0.144, 0.555) | <0.001 | 0.338 (0.140, 0.763) | 0.011 | |
| Pathological grade III | 0.512 (0.219, 1.159) | <0.001 | 0.512 (0.219, 1.159) | 0.113 | |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; LN, lymph node; LVI, lymphovascular invasion; OR, odds ratio; TNBC, triple-negative breast cancer.
Multivariate analysis
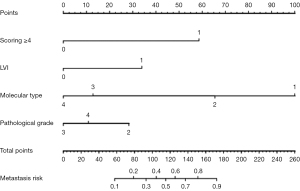
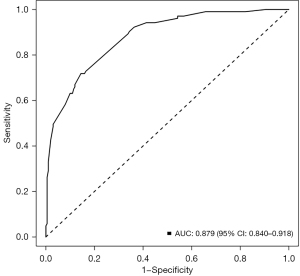
Multivariate analysis showed that luminal B (P=0.001), TNBC (P<0.001), HER2 (P<0.001), LVI (P=0.0016), and Node-RADS 1.0 score ≥4 (P<0.001) were independent influencing factors for predicting upgrade in diagnosis via preoperative US combined with biopsy. According to the results of multivariate logistic regression analysis, a nomogram was constructed to predict upgrade in diagnosis of ALN, and each variable was scored to obtain the total points (Figure 3). The nomogram demonstrated excellent performance, achieving an AUC of 0.879 [95% confidence interval (CI): 0.840–0.918] (Figure 4). The sensitivity, specificity, accuracy, PPV, and NPV of preoperative US-guided CNB of ALNs combined with clinicopathological factors and LN scoring were 85.6%, 71.8%, 80.9%, 85.6%, and 71.8%, respectively.
Discussion
ALN status in BC is important for determining the overall staging and prognosis and for selecting the appropriate treatment option. US, magnetic resonance imaging (MRI), and other imaging examinations are useful for the preoperative evaluation of ALNs, which is helpful for the clinical development of the optimal treatment plan (7,8). US and US-guided CNB of ALNs have attracted considerable attention in the evaluation of axillary status of patients with BC due to the advantages of minimal invasiveness. However, the false-negative rates for US-guided CNB vary substantially (25–40%) (9,10). Therefore, the aim of this study was to identify predictive factors and develop models based on relevant clinicopathological parameters to reduce unnecessary invasive procedures by preoperatively predicting an upgrade in diagnosis. In this study, histological grades II and III, luminal B, TNBC, HER2, presence of LVI, and Node-RADS 1.0 score ≥4 were risk factors for an upgrade in the diagnosis of ALNs. We developed and validated a nomogram using the related risk factors in order to realize the possibility of the preoperative prediction of ALNM. It demonstrated excellent performance, achieving an AUC of 0.879 (95% CI: 0.840–0.918). Finally, univariate analysis and multivariate logistic regression analysis revealed that luminal B, TNBC, HER2, LVI presence, and Node-RADS 1.0 score ≥4 were independent predictors for predicting an upgrade in the diagnosis of ALN.
In published studies on predictors of ALNM (11-13), age, tumor size, histologic subtype, and LVI have been reported to be independently associated with ALNM. The most widely used and validated nomogram (14) for predicting ALNM is from the Memorial Sloan Kettering Cancer Center (MSK) and includes ER status and PR status as independent predictive factors. In addition, MSK study indicated tumor location was found to be an independent predictor, which suggests the possibility of alternative routes of lymphatic flow, especially to the internal mammary chain, which was different from the findings from our study. The AUC in our study was 0.879, which was superior to 0.754 of the MSK study. Nevertheless, the MSK nomogram is an accurate, accessible, and multivariate predictive tool that can significantly improve the preoperative prediction of LN.
As for histological grade, it remains a controversial variable for ALNM in BC. In our study, as compared with grade I, grade II–III was more significantly associated with ALNM. Histological grade was also found to be significant risk factor for ALMN in previous research (15,16), in which a higher tumor histological grade, a lower the degree of differentiation, and a higher the degree of malignancy were associated with relapse and metastasis.
Molecular subtype was also an important factor for the upgrade in diagnosis in our study, which is consistent with other work (17). The upgrade rate of patients with luminal B and HER2-positive status was significantly higher than that for the other subtypes. This may be because HER2 positivity and the luminal B subtype are highly correlated with ALNM. The synergistic interaction between the luminal B subtype and HER2 positivity further elevates the risk of LNs. However, whether there is a connection between molecular subtype and ALNM remains controversial. Some studies have suggested that the luminal A subtype is associated with a lower risk of ALNM as compared to other subtypes. However, Jones et al. (18) reported that molecular subtype in BC was not associated with nodal positivity, N stage. However, other studies have found a lower incidence of LN metastases in patients with BC and nonluminal subtypes (19,20).
In this study, the presence of LVI was also an important variable associated with the prognosis of patients with node-positive and node-negative BC. Another study found that the presence of LVI is an important predictor of ALNM (21) regardless of the size of the metastatic deposit in ALN, which is consistent with our research. More rigorous US and CNB can reduce the detection rate of SLNB if LVI is detected preoperatively.
The morphological changes of LN are an important means to evaluating ALNM via US. Spiculated margin is one of the key characteristics of tumor invasive growth and is more prone to ALNM (22). Our study found that the postoperative ALNM rate of patients with an LN score ≥4 who were US-guided CNB negative was significantly higher than that of those with an ALN score <4. This suggest that patients with ALN score ≥4, a biopsy-negative status, and other ALNM risk factors may be considered for CNB. In addition, we found that there was no difference in the rate of ALNM among patients with different degrees of suspicion related to morphology, suggesting that abnormal ultrasound findings of LN could be caused not only by BC metastasis but also by the reactive hyperplasia of LNs (23). In addition, other research (24) suggests that false-negative CNB findings may also be caused by the wrong selection of LN for biopsy. Therefore, in biopsy examination, it is necessary to increase not only the number of punctures of the most suspicious LN but also the number of punctured LNs to reduce the false-negative rate of preoperative diagnosis.
We also evaluated the possibility of predicting ALNM based on clinical factors. Location and size of primary lesions were also significant factors in determining LN status in BC (25,26). Several studies have reported that the incidence of ALNM increases with larger tumor size. However, in our study, there were no statistical differences between location and size of BC lesions and upgrade in diagnosis, which may be attributed to the small sample size. In addition, the relationship between tumor size and ALNM is challenging to determine as it may be influenced by various factors, including molecular type, histological grade, and the expression of molecules determine tumor growth and lymphatic metastasis (27). One study demonstrated that younger patients were more likely to develop regional LN and distant metastases as compared with older patients (28). Other studies also suggest age to be an independent predictor of ALNM (11). In our study, there was no statistical difference between age and false negatives, and the false-negative rate was associated with the pathological type and multifocal nature of the primary tumor, which may be due to the small number of cases with invasive lobular carcinoma and multifocal disease.
Other research (13,29) indicates that patients with BC after NATC may have reduced SLN identification rates and false-negative rates of biopsy, and thus our study excluded patients who received NATC. In five patients with false-negative lesions, there were isolated metastatic deposits smaller than 3 mm, which could not be avoided by US-guided CNB. Biopsy techniques cannot be expected to routinely detect such small metastases. Only four patients with false-negative CNB results had clinically important LN involvement, which the retrospective analysis revealed was due to incorrect target selection.
Conclusions
The accurate preoperative assessment and prediction the status of ALN can facilitate the determination of the optimal treatment strategy. The results of our study highlight the difficulty in management when the result of biopsy is benign for patients with BC. Our study developed and validated a nomogram for the preoperative prediction of ALNM based on US-guided CNB of ALNs and associated clinicopathological factors.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1257/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1257/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital (approval No. bc2023027), which waived the requirement for informed consent due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. Axillary Nodal Evaluation in Breast Cancer: State of the Art. Radiology 2020;295:500-15. [Crossref] [PubMed]
- Houssami N, Diepstraten SC, Cody HS 3rd, Turner RM, Sever AR. Clinical utility of ultrasound-needle biopsy for preoperative staging of the axilla in invasive breast cancer. Anticancer Res 2014;34:1087-97.
- Fung AD, Collins JA, Campassi C, Ioffe OB, Staats PN. Performance characteristics of ultrasound-guided fine-needle aspiration of axillary lymph nodes for metastatic breast cancer employing rapid on-site evaluation of adequacy: analysis of 136 cases and review of the literature. Cancer Cytopathol 2014;122:282-91. [Crossref] [PubMed]
- Leenders MW, Broeders M, Croese C, Richir MC, Go HL, Langenhorst BL, Meijer S, Schreurs WH. Ultrasound and fine needle aspiration cytology of axillary lymph nodes in breast cancer. To do or not to do? Breast 2012;21:578-83. [Crossref] [PubMed]
- Elsholtz FHJ, Asbach P, Haas M, Becker M, Beets-Tan RGH, Thoeny HC, Padhani AR, Hamm B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): a concept for standardized assessment of lymph nodes in cancer. Eur Radiol 2021;31:6116-24. Erratum in: Eur Radiol 2021;31:7217. [Crossref] [PubMed]
- Marino MA, Avendano D, Zapata P, Riedl CC, Pinker K. Lymph Node Imaging in Patients with Primary Breast Cancer: Concurrent Diagnostic Tools. Oncologist 2020;25:e231-42. [Crossref] [PubMed]
- Baruah BP, Goyal A, Young P, Douglas-Jones AG, Mansel RE. Axillary node staging by ultrasonography and fine-needle aspiration cytology in patients with breast cancer. Br J Surg 2010;97:680-3. [Crossref] [PubMed]
- Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, Jones PA. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 2011;196:251-6. [Crossref] [PubMed]
- Diepstraten SC, Sever AR, Buckens CF, Veldhuis WB, van Dalen T, van den Bosch MA, Mali WP, Verkooijen HM. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:51-9. [Crossref] [PubMed]
- Dihge L, Bendahl PO, Rydén L. Nomograms for preoperative prediction of axillary nodal status in breast cancer. Br J Surg 2017;104:1494-505. [Crossref] [PubMed]
- Zhao YX, Liu YR, Xie S, Jiang YZ, Shao ZM. A Nomogram Predicting Lymph Node Metastasis in T1 Breast Cancer based on the Surveillance, Epidemiology, and End Results Program. J Cancer 2019;10:2443-9. [Crossref] [PubMed]
- Chen K, Liu J, Li S, Jacobs L. Development of nomograms to predict axillary lymph node status in breast cancer patients. BMC Cancer 2017;17:561. [Crossref] [PubMed]
- Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol 2007;25:3670-9. [Crossref] [PubMed]
- Guo Q, Dong Z, Zhang L, Ning C, Li Z, Wang D, Liu C, Zhao M, Tian J. Ultrasound Features of Breast Cancer for Predicting Axillary Lymph Node Metastasis. J Ultrasound Med 2018;37:1354-3. [Crossref] [PubMed]
- Akissue de Camargo Teixeira P, Chala LF, Shimizu C, Filassi JR, Maesaka JY, de Barros N. Axillary Lymph Node Sonographic Features and Breast Tumor Characteristics as Predictors of Malignancy: A Nomogram to Predict Risk. Ultrasound Med Biol 2017;43:1837-45. [Crossref] [PubMed]
- Zhou W, He Z, Xue J, Wang M, Zha X, Ling L, Chen L, Wang S, Liu X. Molecular subtype classification is a determinant of non-sentinel lymph node metastasis in breast cancer patients with positive sentinel lymph nodes. PLoS One 2012;7:e35881. [Crossref] [PubMed]
- Jones T, Neboori H, Wu H, Yang Q, Haffty BG, Evans S, Higgins S, Moran MS. Are breast cancer subtypes prognostic for nodal involvement and associated with clinicopathologic features at presentation in early-stage breast cancer? Ann Surg Oncol 2013;20:2866-72. [Crossref] [PubMed]
- Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [Crossref] [PubMed]
- Mattes MD, Bhatia JK, Metzger D, Ashamalla H, Katsoulakis E. Breast Cancer Subtype as a Predictor of Lymph Node Metastasis according to the SEER Registry. J Breast Cancer 2015;18:143-8. [Crossref] [PubMed]
- Colleoni M, Rotmensz N, Maisonneuve P, Sonzogni A, Pruneri G, Casadio C, Luini A, Veronesi P, Intra M, Galimberti V, Torrisi R, Andrighetto S, Ghisini R, Goldhirsch A, Viale G. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol 2007;18:1632-40. [Crossref] [PubMed]
- Ran Z, Hou L, Guo H, Wang K, Li X. Expression of VEGF, COX-2 and MMP-9 in breast cancer and their relationship with ultrasound findings. Int J Clin Exp Pathol 2018;11:4264-9.
- Garcia-Reyes K, Greenwood HI, Price ER. Outcomes of ultrasound-guided axillary lymph node sampling in the absence of primary breast cancer. Clin Imaging 2017;44:92-6. [Crossref] [PubMed]
- Ding J, Jiang L, Wu W. Predictive Value of Clinicopathological Characteristics for Sentinel Lymph Node Metastasis in Early Breast Cancer. Med Sci Monit 2017;23:4102-8. [Crossref] [PubMed]
- Siotos C, McColl M, Psoter K, Gilmore RC, Sebai ME, Broderick KP, Jacobs LK, Irwin S, Rosson GD, Habibi M. Tumor Site and Breast Cancer Prognosis. Clin Breast Cancer 2018;18:e1045-52. [Crossref] [PubMed]
- Phung MT, Tin Tin S, Elwood JM. Prognostic models for breast cancer: a systematic review. BMC Cancer 2019;19:230. [Crossref] [PubMed]
- Min SK, Lee SK, Woo J, Jung SM, Ryu JM, Yu J, Lee JE, Kim SW, Chae BJ, Nam SJ. Relation Between Tumor Size and Lymph Node Metastasis According to Subtypes of Breast Cancer. J Breast Cancer 2021;24:75-84. [Crossref] [PubMed]
- Erić I, Petek Erić A, Koprivčić I, Babić M, Pačarić S, Trogrlić B. Independent factors FOR poor prognosis in young patients with stage I-III breast cancer. Acta Clin Croat 2020;59:242-51. [Crossref] [PubMed]
- Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M, McCready DR, Karp SE, Trop I, Lisbona A, Wright FC, Younan RJ, Provencher L, Patocskai E, Omeroglu A, Robidoux A. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015;33:258-64. [Crossref] [PubMed]


