
Diagnostic performance of white light endoscopy, narrow band imaging, and flexible spectral imaging color enhancement for early gastric cancer: a systematic review and meta-analysis
Introduction
Gastric cancer (GC) is one of the most prevalent malignant neoplasms globally, ranking as the fifth most frequently diagnosed cancer and the third leading cause of cancer-related mortality in 2020 (1). A significant challenge in managing GC lies in its nonspecific and inconspicuous manifestations, which often lead to delayed diagnosis. As a result, a considerable proportion of GC cases are detected at advanced stages, contributing to poor survival rates (2). Screening initiatives, particularly utilizing endoscopic examination, play a pivotal role in mitigating the incidence of advanced GC (3). Commonly employed endoscopic modalities encompass white light endoscopy (WLE), narrow band imaging (NBI), and flexible spectral imaging color enhancement (FICE).
While WLE has traditionally served as the standard endoscopic assessment, its accuracy in diagnosing EGC is limited. NBI enhances image resolution and contrast by utilizing specific wavelengths of light to visualize the superficial mucosal microvasculature and pit patterns (4). Furthermore, FICE, a form of image-enhanced endoscopic technology, has demonstrated superior performance, offering clearer lesion visualization while being less invasive and less time-intensive (5).
Numerous studies have highlighted the superior diagnostic efficacy of NBI and FICE over WLE in EGC diagnosis. However, the reported accuracy, sensitivity and specificity outcomes across studies comparing NBI with WLE exhibit considerable variability. WLE accuracy rates ranged from 59.7% to 89.2%, with sensitivity varying from 40% to 76.9%, and specificity ranging from 57.0% to 94.7%. Conversely, NBI accuracy ranged from 85.9% to 98.6%, sensitivity from 60.0% to 95.0%, and the specificity from 88.0% to 99.7% (6-14). As for studies comparing FICE and CLE, the ending points were various. Consequently, the debate regarding whether NBI and FICE outperform WLE in EGC diagnosis persists. The primary objective of this systematic review and meta-analysis was to comprehensively evaluate the efficacy of NBI, FICE, and WLE in diagnosing EGC. We present this article in accordance with the PRISMA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-482/rc).
Methods
Registration
We registered a protocol of the study in PROSPERO with the registration number of CRD42023397899.
Search strategy
The meta-analysis was designed, conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (15). We systematically searched 4 key databases including PubMed, Web of Science, EMBASE, and the Cochrane Library up to February 2023. We used Medical Subject Headings (MeSH) items and free text words to construct search strategies. The article keywords were as follows: ‘early gastric cancer’ AND ‘diagnosis’ AND ((‘white light endoscopy’ AND ‘narrow band imaging’) OR (‘white light endoscopy’ AND ‘flexible spectral imaging color enhancement’) OR (‘narrow band imaging’ AND ‘flexible spectral imaging color enhancement’)). And the complete search strategies for each database are presented in detail in Table S1.
Study selection
We included the articles that met all the following criteria: (I) population: patients who might be diagnosed as ECG and received endoscopic biopsies; (II) intervention and comparison: the diagnostic efficacy between NBI and WLE, or between FICE and WLE, or between NBI and FICE; (III) study design: full-text publications of original randomized controlled trial or cohort study; (IV) study that provided true-positive (TP), false-positive (FP), true-negative (TN) and false-negative (FN) directly or with sufficient data for indirect calculation; (V) we adopted the Japanese standard: the revised Vienna classification as the standard as gold standard to recognize the lesions. According the classification system, category C4 (mucosal high-grade neoplasia) and C5 (submucosal invasion by neoplasia) were diagnosed as EGC. And noncancerous lesions were C1 (negative for neoplasia), C2 (indefinite for neoplasia) and C3 (mucosal low-grade neoplasia).
The exclusion criteria were: (I) duplication articles; (II) study types including reviews, meta-analyses, case reports, letters, comments, or conference abstracts; (III) the study did not contain the technologies we focused or technologies were not used to diagnose EGC; (IV) the study only contained patients which were pathologically diagnosed as EGC; (V) study lacking sufficient information for the calculation of TP, FP, TN, and FN. When multiple publications on the same study population, or the overlapping of patient cohort were found, study based on the largest cohort was included in the meta-analysis; (VI) studies aimed at comparing the diagnostic performance of WLE and NBI with magnification.
Data extraction and quality assessment
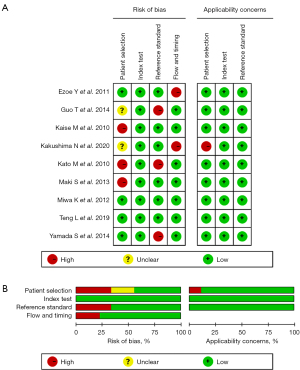
We extracted various parameters including the number of lesions, country, and lesion morphology, lesion size, among others. The primary outcomes comprised the pooled sensitivity and specificity, and secondary outcomes encompassed the positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the area under the curve (AUC). The study search, selection, and data extraction were conducted independently by two reviewers. Disagreements were resolved by a third reviewer through discussion. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) was employed to evaluate the quality of included articles. QUADAS-2 is used in identifying diagnostic meta-analysis, which basically includes four areas: patient selection, index test, reference standard and flow and time. Each item is assessed based on the three possible outcomes: yes, no, unclear (16).
Statistical analysis
A meta-analysis was conducted to evaluate accuracy of the two different technologies, namely NBI, and WLE, in differentiating EGCs from other gastric lesions. The TP, FP, FN, and TN were used to calculate the pooled sensitivity, specificity, PLR, and NLR. DOR along with its corresponding 95% confidence interval (CI) were calculated. The inconsistency index (I2) and Q statistics were used to evaluate the heterogeneity, with I2>50% or P<0.1 reflecting significant heterogeneity (17). A random-effects model (DerSimonian-Laird method) would be applied if heterogeneity was significant. Subgroup analyses and influence analyses were performed subsequently. Otherwise, If the heterogeneity was not significant, a fixed-effects model (Mantel-Haenszel model) would be used. The covariates in subgroup analyses included country (China or Japan), the number of lesions (n>200 or n≤200), the type of studies (prospective or retrospective), and the source of biopsies (acquired via endoscopy or both surgery and endoscopy). We conducted sensitivity analyses using a leave-one-out-method to verify our results. A summary receiver operating characteristic (SROC) curve was plotted (18). The AUC was calculated to estimate the diagnostic accuracy of the technologies, with an AUC exceeding 0.75 indicating great diagnostic efficacy. Deeks’ asymmetry was employed to evaluate the publication bias by constructing a funnel plot of diagnostic log odds ratio vs 1/sqrt (effective sample size), using a significance level of P<0.1. Stata version 14.2 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
Results
Included studies
After systematically searching PubMed, Web of Science, EMBASE, and the Cochrane Library databases, a total of 444 articles were identified. The selection process and reasons for exclusion are summarized in Figure 1. Ultimately, 16 studies were incorporated into our research. Among these studies,9were deemed suitable for meta-analyses comparing the diagnostic efficacy of NBI and WLE in detecting EGC (6-14), while others were considered eligible for evaluating the performance of FICE versus WLE (5,19-24).
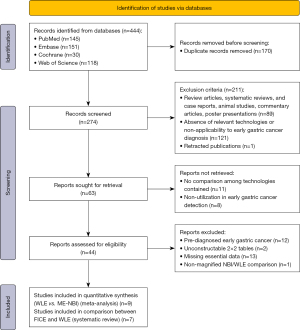
Study characteristics and quality assessment
The characteristics of studies comparing NBI and WLE are shown in Table 1. Among the included studies, 7 researches were conducted in Japan and 2 were from China. Additionally, 4 studies included less than 200 lesions, while others were not. Besides, 6 researches were prospective while others were retrospective. Most studies used irregular microvascular pattern (IMVP)/irregular micro-surface pattern (IMSP) plus demarcation line (DL) with NBI to differentiate EGC from gastric lesions, and only two studies used other criteria to diagnose EGC.
Table 1
| Characteristics | Yamada 2014 (6) | Maki 2013 (7) | Kakushima 2020 (8) | Guo T 2014 (9) | Ezoe 2011 (10) | Teng 2019 (11) | Kato 2010 (12) | Miwa 2012 (13) | Kaise 2010 (14) |
|---|---|---|---|---|---|---|---|---|---|
| Number of lesions | 353; WLE/NBI: 176/177 | 93 | 870; WLE/NBI: 480/390 | 643 | 353; WLE/NBI: 176/177 | 301 | 201 | 135 | 201 |
| Sex (male/female) | 278/75 | 73/20 | 761/107 | 316/192 | 278/75 | 189/112 | NA | 77/58 | 98/13 |
| Age (years, mean) | 69 | LGA/EGC: 69.4/72.2 | WLE/NBI: 70.8/71.2 | 63 | 69 | LGA/EGC: 63.0/63.2 | 66.3 | 70.1 | 66.3 |
| Country | Japan | Japan | Japan | China | Japan | China | Japan | Japan | Japan |
| Morphology type of EGC | |||||||||
| 0–I | 0 | 0 | NA | 0 | 0 | 2 | NA | 12 | NA |
| 0–IIa | 0 | 61 | 303 | 0 | 29 | 97 | |||
| 0–IIb | 0 | 0 | 148 | 0 | 4 | 0 | |||
| 0–IIc | 20 | 0 | 192 | 20 | 95 | 26 | |||
| Lesion size (mm) | 6 | LGA/EGC: 16.3/22.1 | NA | 7 | 5.6 | LGA/EGC: 16.7/26.8 | 7 | NA | 7 |
| Number of endoscopists | 31 | 2 | More than 30 | 4 | 1 | 2 | NA | NA | NA |
| Type of assessment | Prospective | Retrospective | Prospective | Retrospective | Prospective | Prospective | Prospective | Retrospective | Prospective |
| Sensitivity (%) (WLE/NBI) | 40.0/60.0 | 64.0/95.0 | 55.2/65.4 | 75.0/70.8 | 40.0/60.0 | 76.9/89.2 | 42.9/92.9 | 69.6/87.7 | 42.9/92.9 |
| Specificity (%) (WLE/NBI) | 68.0/92.0 | 94.0/88.0 | 94.7/91.0 | 89.5/99.7 | 67.9/94.3 | 71.9/90.6 | 61.0/94.7 | 57.0/97.5 | 61.0/94.7 |
| Accuracy (%) (WLE/NBI) | 65.0/89.0 | 74.0/92.0 | 89.2/85.9 | 89.0/98.6 | 64.8/90.4 | 74.1/90.0 | 59.7/91.0 | 62.2/93.3 | 59.7/94.5 |
| TP (WLE/NBI) | 8/12 | 39/58 | 37/51 | 18/17 | 8/12 | 100/116 | 6/12 | 39/49 | 6/13 |
| FP (WLE/NBI) | 50/13 | 2/4 | 22/28 | 65/2 | 50/9 | 48/16 | 73/16 | 34/2 | 73/10 |
| FN (WLE/NBI) | 12/8 | 22/3 | 30/27 | 6/7 | 12/8 | 30/14 | 8/2 | 17/7 | 8/1 |
| TN (WLE/NBI) | 106/144 | 30/28 | 391/284 | 554/617 | 106/148 | 123/155 | 114/171 | 45/77 | 114/177 |
EGC, early gastric cancer; LGA, low-grade adenoma; NA, not available; NBI, narrow band imaging; TP, true-positive; FP, false-positive; TN, true-negative; FN, false-negative; WLE, white light endoscopy.
The results of QUADAS-2 quality scale are presented in Table S2 and Figure 2. If the risk of bias and concerns about applicability were assessed as low, articles were considered to be of high quality. If not, it might affect the accuracy of the results and contribute to heterogeneity (16). It is noteworthy that all included articles were assessed high quality relatively.
Diagnostic performance of NBI and WLE
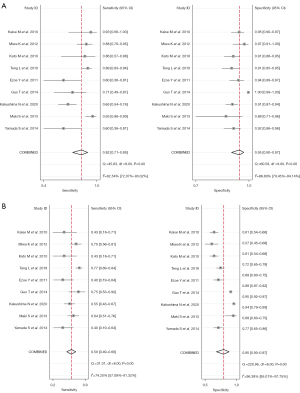
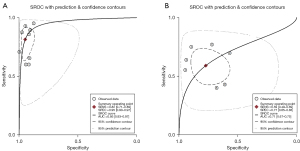
The details of extracted or calculated sensitivity, specificity, accuracy, TP, FP, TN, and FN are delineated in Table 1. Our analysis revealed that patients in NBI group exhibited superior sensitivity (0.82; 95% CI: 0.71–0.89, significant heterogeneity, I2=82.54%, P<0.01) and specificity (0.95; 95% CI: 0.90–0.97, significant heterogeneity, I2=86.80%, P<0.01) compared to those diagnosed with WLE (sensitivity: 0.59; 95% CI: 0.49–0.69; significant heterogeneity, I2=74.20%, P<0.01; specificity: 0.77; 95% CI: 0.65–0.86; significant heterogeneity, I2=96.38%, P<0.01 respectively) (Figure 3). The AUC for WLE was 0.71 (95% CI: 0.67–0.75), whereas for NBI, it was 0.95 (95% CI: 0.93–0.97) (Figure 4). Because of significant heterogeneities observed in both sensitivity (WLE: I2=74.20%, NBI: I2=82.54%) and specificity (WLE: I2=96.38%, NBI: I2=86.80%), random effect models were chosen for calculation. The pooled sensitivity, specificity, PLR, NLR, and DOR in random effect models are as presented in Table S3. As for the sensitivity analysis (Figure S1), we could see that Kakushima’s study might have influence on the results of NBI (8). However, after eliminating Kakushima’s study, the heterogeneity persisted. We also performed subgroup analyses (Table S4) to find out the potential sources of heterogeneity. Number of lesions, derivation of biopsies, study type and diagnostic criteria might be the sources of heterogeneity in the analyses of NBI’s specificity. Besides, derivation of biopsies and diagnostic criteria also appeared to contribute to the heterogeneity of NBI’s sensitivity. Additionally, the country, derivation of biopsies and the study type were implicated as sources of heterogeneity in WLE’s sensitivity. And derivation of biopsies is the source of the heterogeneity of WLE’s specificity. The results of funnel plot asymmetry texts for WLE and NBI were insignificant with the P value of 0.11 and 0.52, respectively (Figure S2).
Diagnostic performance of FICE and WLE
We only systematically reviewed the full studies on comparison between FICE and WLE instead of conducting meta-analyses due to the challenges in extracting information, variations in endpoints, and overall low quality of the available researches. However, our research indicated that FICE exhibited superior diagnostic performance compared to WLE, especially in terms of the improvement the quality of images. We summarized the characteristics of these researches in Table 2. We had to admit that our research was unable to fully integrate the comparison between FICE and WLE due to a dearth of data, which in turn compromised the robustness of our conclusions regarding FICE. It is imperative to conduct targeted analyses to bridge this gap.
Table 2
| Study (ref.) | Country | Study type | Number of gastric lesions | Results |
|---|---|---|---|---|
| Jung 2011 (19) | Korea | Prospective | 171 | (I) PA: WLME, 0.85; FIME, 0.91 |
| (II) AEP: WLME, 0.76; FIME, 0.86 | ||||
| Dohi 2016 (20) | Japan | Retrospective | 40 | The mean visibility scores: WLI, 3.04; FICE, 3.14 |
| Nakamura 2013 (21) | Japan | Retrospective | 22 | Three-point scale (FICE image was superior/comparable, or inferior to the WL image): 8/13/1 |
| Osawa 2012 (22) | Japan | Retrospective | 82 | The median (first quartile to third quartile) color difference: FICE images:27.2; conventional images:18.7 |
| Yokoyama 2019 (5) | Japan | Prospective | 36 | The color difference: W-CE, 20.38±7.69; F-UE, 21.92±11.68 |
| Tanioka 2011 (23) | Japan | Retrospective | 50 | Five-point scale: |
| Grade 1/2/3/4/5: 0/0/11/17/0 | ||||
| Grade 1, 2: inferior to conventional images | ||||
| Grade 3: comparable to conventional images | ||||
| Grade 4, 5: superior to conventional images | ||||
| Osawa 2008 (24) | Japan | Prospective | 27 | Demarcation of the depressed-type early gastric cancer was easily identified by optimal band images without magnification in 26 of 27 cases (96%) |
AEP, the degree of agreement between endoscopic and pathological diagnosis; FICE, flexible spectral imaging color enhancement; FIME, magnifying endoscopy with flexible spectral imaging color enhancement system; F-CE, endoscopic images by FICE ultrathin endoscopy; PA, the proportion of agreement; WLME, magnifying endoscopy with white light; WLI, white light imaging; WL, white light; W-CE, endoscopic images by white light endoscopy; WLE, white light endoscopy.
Discussion
GC, one of the most common malignant tumors, remains the leading cause of cancer-related mortality globally. The 5-year survival rate of GC is poor for most patients are diagnosed at advanced stages, which was reported to be only 30% (2). However, studies have demonstrated the 5-year survival rate of EGC was around 90% (25). In order to decrease the mortality rate of GC, it is imperative for clinicians to diagnose GC early. It is acknowledged that endoscopy is a precise examination. There are a lot of endoscopic technologies applied in clinical practice to help doctors diagnose EGC at early stage. Thanks to the implementation of these advanced technologies including WLE, NBI, and FICE, GC could be diagnosed earlier.
There were studies concluding that NBI and FICE could improve the diagnostic rate of EGC and had overcome several limitations of WLE, just as Manfredi summarized (26). And some meta-analyses mainly focused on one endoscopic technology, such as magnifying narrow band imaging (M-NBI) (27,28). However, there were also some original studies focusing on the difference of diagnostic performance between NBI and WLE, or FICE and WLE. Therefore, it is necessary to conduct a study that focused on identifying the diagnostic performance of NBI and FICE compared with WLE.
After full-text reading, we found all the articles chose pathologic results as gold standard. However, the standard of pathology varies between the Western and Japan (29,30). In our study, we adopted the Japanese standard: the revised Vienna classification as the standard. As mentioned above, category C4 and C5 were diagnosed as EGC, while noncancerous were C1, C2 and C3.
The results of two meta-analyses which aimed at the diagnostic performance of NBI were consistent with ours, demonstrating NBI is a powerful tool in detecting EGC. The pooled sensitivity, specificity, and AUC of NBI in our study were 0.82, 0.95, and 0.95, respectively. Likewise, the pooled sensitivity, specificity, and AUC of Huang’s study were 0.84, 0.95, and 0.96, respectively. The pooled sensitivity, specificity, and AUC of Hu’s study were 0.96, 0.86, and 0.9623, respectively (27,28). Additionally, Hu’s study mentioned that six of included articles also compared the diagnostic performance between NBI and WLE (28). The pooled sensitivity and specificity were 0.57 and 0.79, respectively, which were similar with our results for WLE (the pooled sensitivity and specificity were 0.59 and 0.77, respectively). Until now, there was only one diagnostic meta-analysis comparing the diagnostic efficacy of WLE and M-NBI (31). Their diagnostic indicators of WLE were poor (the pooled sensitivity, specificity, and AUC was 0.48, 0.67, and 0.62, respectively) than ours (the pooled sensitivity, specificity, and AUC was 0.59, 0.77 and 0.71, respectively). However, the results of NBI were alike, affirming its potential as a promising tool in EGC detection.
The I2 values of our results were more than 50%, which indicated the existence of heterogeneity. Similarly, Zhang’s study also demonstrated high grade of heterogeneity (31). They performed further sensitivity analyses for the studies of small lesions and studies with a score of 12 points or greater using QUADAS criteria in the literature quality assessment. To investigate potential sources of heterogeneity and confirm the robustness of our results, we conducted sensitivity analyses using a leave-one-out method and undertook subgroup analyses.
As for the articles comparing FICE and WLE, most of them highlighted the superior quality of color differentiation achieved through FICE compared to conventional imaging techniques (5,20-23). It indicated that FICE could help endoscopists identify EGC from other lesions precisely.
There are several mechanisms which can explain why NBI and FICE performed better than WLE in diagnosing EGC. NBI, an advanced endoscopic imaging tool, enhances the resolution and contrast of images by visualizing the superficial mucosal microvasculature and pit patterns clearly (4). This capability enables the identification of intrapapillary capillary loops, which are pivotal in the detection of early-stage cancer. Therefore, many studies have adopted the VS classification system proposed by Yao et al. to diagnose EGC: the presence of a DL and an IMVP or an IMSP (32). In addition, FICE is one of image-enhanced endoscopic tools which can improve the diagnostic accuracy of gastrointestinal cancer. In contrast to WLE, FICE captures images by each wavelength and reconstitute virtual images according to the choice of different combinations of wavelength to provide high-contrast images (5). In essence, the enhanced imaging capabilities of NBI and FICE, facilitated by their respective mechanisms, enable clinicians to identify EGC more accurately.
We evaluated the publication bias through constructing funnel plots. The P value of WLE and NBI in funnel plot asymmetry tests were 0.11 and 0.52, respectively, which demonstrated that the publication bias was insignificant. Considering the AUC, which were 0.71 (95% CI: 0.67–0.75) for WLE and 0.95 (95% CI: 0.93–0.97) for NBI, it is evident that the diagnostic performance of NBI surpasses that of WLE.
There were several advantages in our study. Firstly, it was the first study aimed at comparing the diagnostic performance of WLE, NBI, and FICE, which can supply more evidence to support the conclusion that NBI and FICE play an important role in the detection of EGC and guide further studies. Secondly, we performed sensitivity analyses and subgroup analyses to elucidate the sources of heterogeneity.
However, our study also had several limitations. Firstly, all the included articles were conducted in China and Japan. Therefore, it might not represent cases in other regions worldwide. Further studies conducted in diverse populations are necessary to validate our conclusions across different demographic and clinical contexts. Secondly, high heterogeneity was observed in our study. Although we conducted sensitivity analyses using a leave-one-out-method and sub-group analyses to eliminate it, the heterogeneity was still existed. Maybe other unreported factors could affect the overall estimation. Thirdly, we also attempted to clarify whether FICE was more accurate than WLE in the detection of EGC. However, we found that only seven articles were included and the information supplied was insufficient, indicating that we could not perform meta-analyses between FICE and WLE. Thirdly, many articles included were retrospective, which may introduce potential biases. Therefore, it is crucial to highlight this concern alongside the pressing need for well-designed prospective studies. Lastly, the detection rate of EGC varies significantly depending on the endoscopists. The variation is influenced by factors such as the duration of the examination and the implementation of Image Enhanced Endoscopy (IEE) (33-36). We only tried to find out whether the implementation of IEE have impact on the detection rate of EGC for the duration of the examination was unrecorded.
Conclusions
Our study demonstrated that NBI is a powerful and promising endoscopic tool which can be used in the diagnosis of EGC, surpassing the performance of WLE. Additionally, our results suggest that FICE hold promise as a reliable endoscopic tool which can improve EGC diagnosis. Further high-quality studies are needed to identify the diagnostic performance of NBI and FICE. By continuing to advance our understanding of the diagnostic capabilities of NBI and FICE, we can improve the detection rates of EGC, ultimately leading to better patient outcomes and reduced mortality from GC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-482/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-482/coif). Q.Y. receives funding from Qingdao Science and Technology Demonstration and Guidance Special Fund for the Benefit of the People (No. 21-1-4-rkjk-7-nsh). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Degu A, Karimi PN, Opanga SA, Nyamu DG. Predictors of survival outcomes among patients with gastric cancer in a leading tertiary, teaching and referral hospital in Kenya. Cancer Med 2023;12:4147-60. [Crossref] [PubMed]
- Mabe K, Inoue K, Kamada T, Kato K, Kato M, Haruma K. Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig Endosc 2022;34:412-9. [Crossref] [PubMed]
- Cho JH, Jeon SR, Jin SY. Clinical applicability of gastroscopy with narrow-band imaging for the diagnosis of Helicobacter pylori gastritis, precancerous gastric lesion, and neoplasia. World J Clin Cases 2020;8:2902-16. [Crossref] [PubMed]
- Yokoyama T, Miyahara R, Funasaka K, Furukawa K, Yamamura T, Ohno E, Nakamura M, Kawashima H, Watanabe O, Hirooka Y, Hirakawa A, Goto H. The utility of ultrathin endoscopy with flexible spectral imaging color enhancement for early gastric cancer. Nagoya J Med Sci 2019;81:241-8. [PubMed]
- Yamada S, Doyama H, Yao K, Uedo N, Ezoe Y, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Saito Y, Muto M. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc 2014;79:55-63. [Crossref] [PubMed]
- Maki S, Yao K, Nagahama T, Beppu T, Hisabe T, Takaki Y, Hirai F, Matsui T, Tanabe H, Iwashita A. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between low-grade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer 2013;16:140-6. [Crossref] [PubMed]
- Kakushima N, Yoshida N, Doyama H, Yano T, Horimatsu T, Uedo N, Yamamoto Y, Kanzaki H, Hori S, Yao K, Oda I, Tanabe S, Yokoi C, Ohata K, Yoshimura K, Ishikawa H, Muto M. Near-focus magnification and second-generation narrow-band imaging for early gastric cancer in a randomized trial. J Gastroenterol 2020;55:1127-37. [Crossref] [PubMed]
- Guo T, Lu XH, Yang AM, Zhou WX, Yao F, Wu X, Wang LY, Lu CM, Fei GJ, Shu HJ, Wu DS, Li Y, Li XQ, Qian JM. Enhanced magnifying endoscopy for differential diagnosis of superficial gastric lesions identified with white-light endoscopy. Gastric Cancer 2014;17:122-9. [Crossref] [PubMed]
- Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology 2011;141:2017-2025.e3. [Crossref] [PubMed]
- Teng L, Zhang Q, Zhang X, Chen J, Wang Q, Zhou J, Li X. Diagnostic Value of White Light Endoscopy and Magnifying Endoscopy With Narrow-band Imaging for Distinguishing Intestinal-type Gastric Adenoma and Early Gastric Cancer. Chinese Journal of Gastroenterology 2019;24:389-94.
- Kato M, Kaise M, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc 2010;72:523-9. [Crossref] [PubMed]
- Miwa K, Doyama H, Ito R, Nakanishi H, Hirano K, Inagaki S, Tominaga K, Yoshida N, Takemura K, Yamada S, Kaneko Y, Katayanagi K, Kurumaya H, Okada T, Yamagishi M. Can magnifying endoscopy with narrow band imaging be useful for low grade adenomas in preoperative biopsy specimens? Gastric Cancer 2012;15:170-8. [Crossref] [PubMed]
- Kaise M, Kato M, Tajiri H. High-definition endoscopy and magnifying endoscopy combined with narrow band imaging in gastric cancer. Gastroenterol Clin North Am 2010;39:771-84. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [Crossref] [PubMed]
- Jung SW, Lim KS, Lim JU, Jeon JW, Shin HP, Kim SH, Lee EK, Park JJ, Cha JM, Joo KR, Lee JI. Flexible spectral imaging color enhancement (FICE) is useful to discriminate among non-neoplastic lesion, adenoma, and cancer of stomach. Dig Dis Sci 2011;56:2879-86. [Crossref] [PubMed]
- Dohi O, Terasaki K, Nakano T, Ueda T, Iwai N, Majima A, Gen Y, Okayama T, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Handa O, Konishi H, Itoh Y. Improved visibility of early gastric cancer using image-enhanced laser endoscopy. United European Gastroenterology Journal 2016;4:A196.
- Nakamura M, Nishikawa J, Goto A, Nishimura J, Hashimoto S, Okamoto T, Sakaida I. Usefulness of ultraslim endoscopy with flexible spectral imaging color enhancement for detection of gastric neoplasm: a preliminary study. J Gastrointest Cancer 2013;44:325-8. [Crossref] [PubMed]
- Osawa H, Yamamoto H, Miura Y, Ajibe H, Shinhata H, Yoshizawa M, Sunada K, Toma S, Satoh K, Sugano K. Diagnosis of depressed-type early gastric cancer using small-caliber endoscopy with flexible spectral imaging color enhancement. Dig Endosc 2012;24:231-6. [Crossref] [PubMed]
- Tanioka Y, Yanai H, Sakaguchi E. Ultraslim endoscopy with flexible spectral imaging color enhancement for upper gastrointestinal neoplasms. World J Gastrointest Endosc 2011;3:11-5. [Crossref] [PubMed]
- Osawa H, Yoshizawa M, Yamamoto H, Kita H, Satoh K, Ohnishi H, Nakano H, Wada M, Arashiro M, Tsukui M, Ido K, Sugano K. Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc 2008;67:226-34. [Crossref] [PubMed]
- Waki K, Shichijo S, Uedo N, Takeuchi Y, Maekawa A, Kanesaka T, Takeuchi Y, Higashino K, Ishihara R, Tanaka Y, Michida T. Long-term outcomes after endoscopic resection for late-elderly patients with early gastric cancer. Gastrointest Endosc 2022;95:873-83. [Crossref] [PubMed]
- Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JT, Murad FM, Siddiqui UD, Wallace MB, Banerjee S. Electronic chromoendoscopy. Gastrointest Endosc 2015;81:249-61. [Crossref] [PubMed]
- Huang W, Wang L, Du J, Yang JM. The value of narrow-band imaging with magnifying endoscopy in diagnosis of early gastric cancer: a meta-analysis. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015;44:435-42. [PubMed]
- Hu YY, Lian QW, Lin ZH, Zhong J, Xue M, Wang LJ. Diagnostic performance of magnifying narrow-band imaging for early gastric cancer: A meta-analysis. World J Gastroenterol 2015;21:7884-94. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IAWHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Zhang Q, Wang F, Chen ZY, Wang Z, Zhi FC, Liu SD, Bai Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer 2016;19:543-52. [Crossref] [PubMed]
- Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009;41:462-7. [Crossref] [PubMed]
- Dohi O, Ono S, Kawada K, Kitamura S, Hatta W, Hori S, et al. Linked color imaging provides enhanced visibility with a high color difference in upper gastrointestinal neoplasms. J Gastroenterol Hepatol 2023;38:79-86. [Crossref] [PubMed]
- Kawamura T, Wada H, Sakiyama N, Ueda Y, Shirakawa A, Okada Y, Sanada K, Nakase K, Mandai K, Suzuki A, Kamaguchi M, Morita A, Nishioji K, Tanaka K, Mochizuki N, Uno K, Yokota I, Kobayashi M, Yasuda K. Examination time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig Endosc 2017;29:569-75. [Crossref] [PubMed]
- Murakami D, Yamato M, Amano Y, Nishino T, Arai M. Variation in the rate of detection of minute and small early gastric cancers at diagnostic endoscopy may reflect the performance of individual endoscopists. BMJ Open Gastroenterol 2023;10:e001143. [Crossref] [PubMed]
- Park JM, Huo SM, Lee HH, Lee BI, Song HJ, Choi MG. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology 2017;153:460-469.e1. [Crossref] [PubMed]


