
Development of a nomogram model using the dual-energy computed tomography angiography parameters of carotid plaque, the vascular lumen, and perivascular fat to predict acute stroke events
Introduction
Stroke is the second leading cause of death globally (1). From 1990 to 2019, the incidence of stroke increased by 70%, while stroke-related mortality increased by 43% (1). In 2019, China recorded 3.94 million new stroke cases and 2.19 million stroke-related fatalities (2). Carotid plaque is a major risk factor for stroke, and approximately 25% of strokes result from ipsilateral carotid artery stenosis (3-5).
Imaging is crucial in the assessment of carotid plaque risk (6). Multiparametric ultrasound of the carotid arteries has several advantages, including that it is quick, non-invasive, and cost effective; however, it also has limitations, such as high operator dependence and a limited ability to assess the intracranial segments of the internal carotid artery (7). Magnetic resonance imaging (MRI) of the carotid vessel wall can detect features of high-risk plaques, including intraplaque hemorrhage (IPH), plaque ulceration, lipid-rich necrotic core (LRNC), and thinning/disruption of the fibrous cap (8-10); however, long scan times and high costs limit its clinical utility.
Some scholars have attempted to use computed tomography angiography (CTA) for research related to carotid plaque. For example, Sheahan et al. investigated the correlation between carotid CTA plaque components and pathological findings, and found a strong association between the volumes of various plaque components and the corresponding pathological results (11). Other studies have found that assessing the computed tomography (CT) values of perivascular adipose tissue (PVAT) surrounding the carotid artery via CTA may serve as a predictor for the risk of acute cerebrovascular events (12-14). Although dual-energy computed tomography (DECT) technology has been used to assess plaque characteristics, previous studies have mostly focused on individual aspects of plaque composition or vascular lumen assessment (15,16). Thus, a more comprehensive approach is needed that integrates multiple dual-energy computed tomography angiography (DECTA) parameters, including carotid plaque, the vascular lumen, and PVAT.
A multidimensional analysis would provide a clearer understanding of carotid plaque vulnerability, offering insights into plaque burden, composition, vascular stenosis, and the inflammatory response. By evaluating a combination of these features, this study sought to enhance our understanding of carotid plaque assessment and improve the predictive modeling of acute cerebrovascular events, something that previous studies, which have focused on single-dimensional indicators, have not fully addressed.
This study aimed to examine the quantitative DECTA parameters of plaque, the vascular lumen, and PVAT associated with acute cerebrovascular events, and to develop a risk prediction model for their occurrence. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2765/rc).
Methods
Patients
A retrospective analysis of clinical, laboratory, and imaging data collected from patients who underwent DECTA and cranial MRI at Xuzhou Medical University Affiliated Jiawang District People’s Hospital between January 2023 and September 2024 was conducted. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (approval No. XYFY2024-KL456), and the requirement of informed consent from patients was waived due to the retrospective nature of the study.
Patients were included in the study if they met the following criteria: (I) had a time interval between the carotid CTA and cranial MRI examinations of no more than 3 days; (II) had measurable carotid plaque; and (III) had no atherosclerotic plaque in the ipsilateral anterior or middle cerebral artery. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had poor-quality images or significant image artifacts that made analysis impossible; (II) had anterior circulation vascular diseases (e.g., aneurysm, dissection, or vascular malformation); (III) had an autoimmune disease, hematological disease, malignant tumor, or systemic disease requiring intensive treatment; and/or (IV) had undergone stent placement or vascular dissection surgery in the common carotid, internal carotid, or intracranial arteries.
Clinical data, including gender, age, body mass index (BMI), a history of hypertension, diabetes, stroke [transient ischemic attack (TIA)], smoking, alcohol consumption, and serum biomarkers (total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein), were retrospectively collected by a cardiovascular radiologist. The time interval between the laboratory tests and the CTA examination was no more than 7 days.
DECTA scanning protocol
Head and neck DECTA was performed using a third-generation dual-source CT scanner (SOMATOM Drive, Siemens, Forchheim, Germany). The scanning range extended from the aortic arch to the cranial vault (Table S1). A non-ionic iodinated contrast agent, iopamidol (Qinglida, 100 mg/mL, produced by Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd., Nanjing, China), was administered. A high-pressure injector (Medrad Stellant, Bayer AG, Leverkusen, Germany) delivered the contrast agent at a rate of 4.0 mL/s and a dosage of 1.0–1.2 mL/kg of body weight, followed by a 30-mL saline flush.
Cranial MRI-diffusion-weighted imaging (DWI) sequence
The cranial MRI-DWI sequence scanning protocol is detailed in Table S2.
PVAT quantitative parameter analysis
The post-processing of the DECTA images was performed by two senior radiologists, each with over 10 years of expertise in head and neck CTA, using a Siemens (Syngo. ViaVB40B) workstation. Regions of interest (ROIs) were defined on the most prominent axial slice of the carotid plaque PVAT, using liver virtual non-contrast (VNC), electron density (Rho)/effective atomic number (Zeff), and Monochromatic Energy (mono E) modes. The ROIs were placed at least 1 mm away from the carotid artery edges and surrounding tissues, following the methodology established by Baradaran et al. (12), ensuring careful exclusion of perivascular soft tissue and small traversing vessels. Both radiologists independently reviewed the images and defined the ROIs. If any discrepancies arose between their measurements or interpretations, a final decision was made by direct consultation. This collaborative approach allowed for the resolution of conflicting views based on both radiologists’ extensive experience and expertise. The average value of the two ROIs was calculated for the statistical analysis.
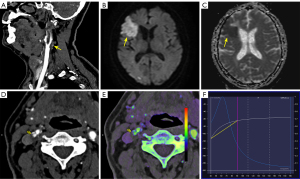
The DECTA parameters for PVAT included the fat fraction (FF), VNC values, iodine concentration (IC), Rho, effective atomic number (Zeff), dual-energy index (DEI), energy spectrum curve, as well as CT values at 40 keV (40KH) and 90 keV (90KH), and the slope of the energy spectrum curve (K) (Figure 1).
Plaque quantitative parameter analysis
Non-linear blending data were acquired using a Siemens post-processing workstation (Syngo. ViaVB40B) and subsequently imported into United Imaging software (UAI.OCR 1.0.8962.1961) to extract the quantitative parameters of the carotid plaques. The extracted parameters comprised the total plaque volume (TPV), calcified plaque volume (CV), and non-calcified plaque volume (NCV). NCV was further categorized into lipid-rich volume (LRV), fibrotic volume (FV), and lipid-fiber volume (LFV) (17) (Figure S1).
In this study, plaque components with a CT value of ≥350 Hounsfield units (HU) were classified as calcified, while those with a CT value <30 HU were classified as lipid. Components with CT values of 30–130 HU were categorized as lipid-fibrous, while those with CT values of 130–350 HU were classified as fibrous (Figure 2).
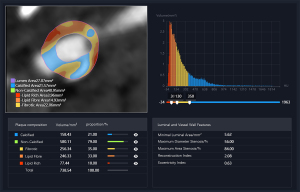
The following quantitative parameter ratios were calculated for the analysis: lipid-rich volume to total plaque volume (LRV/TPV), lipid-rich volume to non-calcified plaque volume (LRV/NCV), fibrous volume to total plaque volume (FV/TPV), fibrous volume to non-calcified plaque volume (FV/NCV), lipid-fiber volume to total plaque volume (LFV/TPV), lipid-fiber volume to non-calcified plaque volume (LFV/NCV), and non-calcified plaque volume to total plaque volume (NCV/TPV).
Quantitative parameters of the vascular lumen
The quantitative vascular lumen parameters included the maximum diameter stenosis (MDS) rate, maximum area stenosis (MAS) rate, reconstruction index (RI), and eccentricity index (EI) (Figure S2). The RI was calculated as follows: RI = (Total external membrane area of the plaque – Reference external membrane area)/Reference external membrane area. The reference external membrane area was calculated as follows: Reference external membrane area = (Proximal plaque-free external membrane area + Distal plaque-free external membrane area)/2 (18). The EI was calculated as follows: EI = (Maximum wall thickness – Minimum wall thickness)/Maximum wall thickness (19).
Evaluation of cerebrovascular events
Two senior neuroimaging radiologists reviewed the cranial MRI images using the Picture Archiving and Communication System. They were blinded to all the clinical data and predictive model results. Acute cerebral infarction was identified as a lesion showing hyperintensity on DWI and hypointensity on apparent diffusion coefficient (ADC) sequences (20). Patients with acute cerebral infarction in the ipsilateral anterior circulation were categorized as the symptomatic (STA) group, while those without infarction were classified as the asymptomatic (ATA) group (Figure 1).
Statistical analysis
Cases with incomplete or missing data were excluded from the analysis (complete-case analysis). The data analysis was conducted using SPSS 23.0 and the R packages pROC, rms, and ggplot2. The Kolmogorov-Smirnov test was used to assess data normality. For normally distributed data, the results are reported as the mean ± standard deviation (), while for non-normally distributed data, the results are expressed as the median (interquartile range) [M (Q1, Q3)]. The categorical variables are presented as the percentage (%). The qualitative data were compared using the chi-square (χ2) test, and the quantitative data were analyzed using the t-test. For the non-normally distributed data, the Mann-Whitney U test was applied. Univariate variables with a P value <0.05 were included in a multivariate logistic regression, and a nomogram for predicting STA was then constructed. The predictive performance of the univariate and combined variables was evaluated by receiver operating characteristic (ROC) curve analysis, and differences between the ROC curves were assessed using the DeLong test. Statistical significance was defined as a P value <0.05.
Results
Initially, 81 participants were enrolled in this study. However, two participants were excluded due to severe imaging artifacts, three due to prior intracranial or carotid artery stenting, one due to missing clinical data, and six due to missing plaque analysis data. Thus, ultimately, 69 participants were included in the final analysis.
Comparison of demographic characteristics between the STA and ATA groups
A total of 69 patients were included in this study, with 20 in the STA group (29.0%) and 49 in the ATA group (71.0%). No statistically significant differences were found between the two groups in terms of the demographic characteristics (P>0.05) (Table 1).
Table 1
| Demographic characteristics | STA group (n=20) | ATA group (n=49) | P value |
|---|---|---|---|
| Male | 16 (80.00) | 34 (69.40) | 0.371 |
| Age (years) | 67.5 (61.0, 73.5) | 71.0 (64.0, 76.0) | 0.375 |
| BMI (kg/m2) | 23.73±2.38 | 25.21±3.40 | 0.081 |
| History of hypertension | 0.139 | ||
| Yes | 12 (60.0) | 38 (77.6) | |
| No | 8 (40.00) | 11 (22.4) | |
| History of diabetes | 0.433 | ||
| Yes | 5 (25.0) | 17 (34.7) | |
| No | 15 (75.0) | 32 (65.3) | |
| History of coronary heart disease | 0.932 | ||
| Yes | 2 (10.0) | 7 (14.3) | |
| No | 18 (90.0) | 42 (85.7) | |
| History of TIA | 0.400 | ||
| Yes | 6 (30.0) | 20 (40.8) | |
| No | 14 (70.0) | 29 (59.2) | |
| Smoking history | 0.355 | ||
| Yes | 8 (40.0) | 14 (28.6) | |
| No | 12 (60.0) | 35 (71.4) | |
| Alcohol consumption history | 0.474 | ||
| Yes | 3 (15.0) | 13 (26.5) | |
| No | 17 (85.0) | 36 (73.5) | |
| Total cholesterol (mmol/L) | 4.46±1.17 | 4.40±1.23 | 0.870 |
| Triglycerides (mmol/L) | 1.22 (0.82, 1.96) | 1.37 (1.10, 1.73) | 0.324 |
| High-density lipoprotein (mmol/L) | 1.16±0.27 | 1.09±0.22 | 0.266 |
| Low-density lipoprotein (mmol/L) | 2.83±0.93 | 2.69±1.01 | 0.597 |
Data are presented as n (%), mean ± standard deviation or median (interquartile range). ATA, asymptomatic; BMI, body mass index; STA, symptomatic; TIA, transient ischemic attack.
Comparison of dual-energy PVAT parameters between the STA and ATA groups
The STA group had significantly lower FF and K values than the ATA group, but significantly higher VNC, Rho, and 40KH values, and all the differences were statistically significant (P≤0.001) (Table 2).
Table 2
| Dual-energy PVAT parameters | STA group (n=20) | ATA group (n=49) | P value |
|---|---|---|---|
| FF (%) | 76.00±13.74 | 91.36±15.58 | <0.001* |
| VNC (HU) | –75.05±26.61 | –100.96±18.98 | <0.001* |
| IC (mg/mL) | –0.13 (–0.50, 0.08) | 0.00 (0.00, 0.05) | 0.091 |
| Rho (HU) | –56.50 (–66.45, –50.25) | –74.30 (–84.50, –64.80) | 0.001* |
| Zeff | 6.78 (6.60, 7.00) | 6.70 (6.40, 6.98) | 0.272 |
| DEI | –0.01±0.01 | –0.01±0.01 | 0.345 |
| 40KH (HU) | –90.92±44.22 | –168.76±58.62 | <0.001* |
| 90KH (HU) | –73.10±26.90 | –74.96±23.38 | 0.775 |
| K (HU/keV) | 0.36±0.81 | 1.88±1.27 | <0.001* |
Data are presented as mean ± standard deviation or median (interquartile range). *, P<0.05. ATA, asymptomatic; DEI, dual-energy index; FF, fat fraction; IC, iodine concentration; PVAT, perivascular adipose tissue; STA, symptomatic; VNC, virtual non-contrast; Rho, electron density; Zeff, effective atomic number; 40KH, computed tomography values at 40 keV; 90KH, computed tomography values at 90 keV; K, the slope of the energy spectrum curve.
Comparison of plaque quantitative parameters between the STA and ATA groups
The LRV/NCV ratio was significantly higher in the STA group than the ATA group (P=0.045). However, no other plaque quantitative parameters displayed significant differences between the two groups (Table 3).
Table 3
| Plaque quantitative parameters | STA group (n=20) | ATA group (n=49) | P value |
|---|---|---|---|
| TPV (mm3) | 544.91 (340.87, 926.47) | 448.55 (236.55, 799.35) | 0.296 |
| CV (mm3) | 21.20 (0.09, 315.36) | 70.61 (11.77, 158.90) | 0.605 |
| NCV (mm3) | 375.72 (262.73, 611.84) | 308.60 (180.29, 578.98) | 0.296 |
| LRV (mm3) | 68.22 (31.29, 188.95) | 42.39 (19.05, 84.26) | 0.107 |
| FV (mm3) | 112.75 (49.59, 194.23) | 94.43 (48.51, 159.21) | 0.543 |
| LFV (mm3) | 211.20 (122.87, 309.93) | 169.22 (102.14, 346.37) | 0.368 |
| LRV/TPV (%) | 0.18±0.12 | 0.12±0.10 | 0.051 |
| LRV/NCV (%) | 0.20±0.11 | 0.14±0.11 | 0.045* |
| FV/TPV (%) | 0.20±0.06 | 0.23±0.08 | 0.098 |
| FV/NCV (%) | 0.20 (0.17, 0.37) | 0.31 (0.21, 0.41) | 0.190 |
| LFV/TPV (%) | 0.46±0.18 | 0.44±0.18 | 0.634 |
| LFV/NCV (%) | 0.53±0.12 | 0.53±0.12 | 0.949 |
| NCV/TPV (%) | 0.96 (0.73, 1.00) | 0.86 (0.66, 0.97) | 0.228 |
Data are presented as mean ± standard deviation or median (interquartile range). *, P<0.05. ATA, asymptomatic; CV, calcified plaque volume; LRV, lipid-rich volume; FV, fibrotic volume; LFV, lipid-fiber volume; LRV/TPV, lipid volume to total plaque volume; LRV/NCV, lipid volume to non-calcified plaque volume; FV/TPV, fibrous volume to total plaque volume; FV/NCV, fibrous volume to non-calcified plaque volume; LFV/TPV, lipid-fiber volume to total plaque volume; LFV/NCV, lipid-fiber volume to non-calcified plaque volume; NCV, non-calcified plaque volume; NCV/TPV, non-calcified plaque volume to total plaque volume; STA, symptomatic; TPV, total plaque volume.
Comparison of vascular lumen quantitative parameters between the STA and ATA groups
No significant differences were observed in the vascular lumen quantitative parameters between the STA and ATA groups (P>0.05) (Table 4).
Table 4
| Parameters of the vascular lumen | STA group (n=20) | ATA group (n=49) | P value |
|---|---|---|---|
| MDS rate (%) | 29.00 (11.50, 36.50) | 19.00 (7.00, 47.00) | 0.926 |
| MAS rate (%) | 53.50 (23.50, 60.00) | 35.00 (16.00, 72.00) | 0.916 |
| RI (%) | 1.40 (1.21, 1.61) | 1.47 (1.19, 1.67) | 0.995 |
| EI (%) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 0.892 |
Data are presented as median (interquartile range). ATA, asymptomatic; EI, eccentricity index; MDS, maximum diameter stenosis; MAS, maximum area stenosis; RI, reconstruction index; STA, symptomatic.
Predictive performance of the DECTA plaque, vascular lumen, and PVAT quantitative parameters for STA
A logistic regression analysis was conducted using the significant dual-energy PVAT variables (i.e., FF, VNC, Rho, 40KH, and K) and the plaque variable (i.e., the LRV/NCV ratio) to construct a dynamic nomogram for predicting STA (Figure 3). The final logistic regression model used to calculate the predicted probability was expressed as follows: logit(P) = 9.8276 + –0.0582 × FF + 0.0284 × VNC + 0.0309 × Rho + 0.0016 × 40KH + –1.4958 × K + 3.0481 × LRV/NCV.
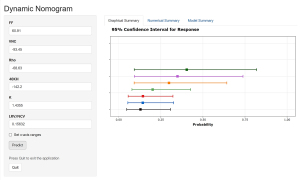
The dynamic nomogram can be accessed at https://dual-energy-carotid-cta.shinyapps.io/dynnomapp/. Users can interactively input variable values and observe real-time predictions along with confidence intervals. The predictive performance of various models is compared in Table 5.
Table 5
| Parameter | AUC | SE (%) | SP (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| FF | 0.774 (0.660–0.889) | 85.0 (70.0–100.0) | 67.3 (53.1–79.6) | 51.5 (41.0–64.3) | 91.7 (83.3–100.0) |
| VNC | 0.804 (0.691–0.917) | 75.0 (55.0–90.0) | 79.6 (67.3–89.8) | 60.0 (47.1–76.2) | 88.6 (80.4–97.4) |
| Rho | 0.761 (0.625–0.897) | 75.0 (55.0–90.0) | 77.6 (65.3–87.8) | 57.7 (45.2–75.0) | 88.4 (80.4–97.4) |
| 40KH | 0.865 (0.773–0.956) | 90.0 (75.0–100.0) | 71.4 (59.2–83.7) | 56.2 (45.7–69.6) | 94.6 (87.2–100.0) |
| K | 0.860 (0.766–0.954) | 90.0 (75.0–100.0) | 75.5 (63.3–85.7) | 60.0 (48.6–73.1) | 94.9 (87.8–100.0) |
| LRV/NCV | 0.660 (0.527–0.794) | 95.0 (85.0–100.0) | 42.9 (28.6–57.1) | 40.4 (34.6–47.6) | 95.5 (86.2–100.0) |
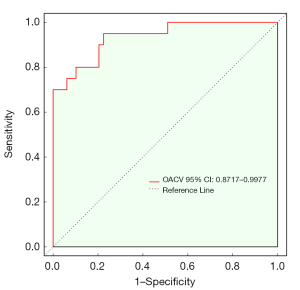
| OACV | 0.934 (0.872–0.998) | 95.0 (85.0–100.0) | 77.6 (65.3–87.8) | 63.4 (52.8–76.9) | 97.4 (92.1–100.0) |
ATA, asymptomatic; FF, fat fraction; 40KH, computed tomography values at 40 keV; K, the slope of the energy spectrum curve; LRV/NCV, lipid volume to non-calcified plaque volume; OACV, overall combination variable; SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; PVAT, perivascular adipose tissue; Rho, electron density; STA, symptomatic; VNC, virtual non-contrast.
The overall combination variable (OACV) had an AUC of 0.934, a sensitivity of 95.0%, and a specificity of 77.6% (Table 5 and Figure 4), and outperformed the individual univariate variables in predicting STA. The Delong test indicated a statistically significant difference between the predictive performance of the OACV and that of the individual univariate variables (P<0.05). The calibration curve, generated from 1,000 bootstrap iterations, demonstrated a strong concordance between the predicted and observed probabilities (Figure 5).
Discussion
A graphical flowchart summarizing the study workflow, key pathological mechanisms, quantitative imaging parameters, and the construction of the prediction model is presented in Figure 6. In this study, we identified DECTA features linked to STA and developed a multi-modal nomogram prediction model. The model showed strong predictive performance, and had an AUC of 0.934, a sensitivity of 95.0%, and a specificity of 77.6%. In contrast to previous studies (15,21-23), our approach incorporated multidimensional data from carotid plaque, the vascular lumen, and PVAT, offering a more comprehensive assessment of plaque vulnerability. This method provides detailed insights into plaque burden, composition, vascular stenosis, deformation, and inflammatory response, thereby providing clinicians with more individualized information for monitoring carotid plaques.
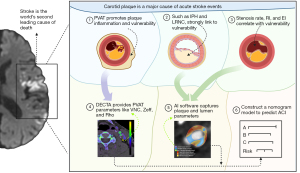
Previous research by Li et al. (21) demonstrated that DECT is superior to traditional mixed-energy CT in differentiating between LRNC and other plaque components, such as IPH, fibrous tissue, and loose matrix (AUC: 0.945). In our study, automated software delineation was used to quantify plaque components, and the LRV/NCV ratio of the STA group was significantly higher than that of the ATA group. This aligns with Li et al.’s findings. Since larger LRNCs are associated with higher risks of plaque rupture (24), these results demonstrate the utility of DECTA and artificial intelligence (AI)-based analysis in quickly and accurately quantifying LRNC, thereby expediting clinical assessments of vulnerable vessels in STA patients.
Interestingly, unlike previous studies (25), we found that the RI was lower in the STA group than the ATA group. We hypothesize that this finding is indicative of altered vascular mechanics in patients with symptomatic plaque. In clinical terms, the RI is a marker of vascular remodeling, and a lower RI could suggest the exhaustion of compensatory mechanisms in carotid plaques. Typically, plaques in earlier stages of atherosclerosis may undergo positive remodeling, with higher RI values reflecting the expansion of the vessel wall to accommodate plaque growth without significant stenosis. However, in the context of symptomatic patients with acute cerebrovascular events, a lower RI suggests that this compensatory remodeling is no longer sufficient, leading to hemodynamic instability and increasing the likelihood of plaque rupture or thrombosis. This is clinically relevant because it could help identify patients at higher risk of stroke due to the plaque’s advanced stage of progression. A lower RI may thus serve as an early marker of vulnerable plaques that are at higher risk for acute events, enabling more personalized clinical decision making.
Additionally, the EI did not differ significantly between the two groups. This may be due to the relatively small sample size and individual variability in the study population. Expanding the sample size is essential to validate the role of EI across different plaque types and to establish its clinical relevance.
By integrating plaque volume, composition, and geometric features, this study offers a multidimensional imaging framework that provides more comprehensive insights into plaque characteristics compared to earlier studies that focused on single aspects, such as plaque composition (26). This comprehensive approach extends our understanding of the relationship between plaque features and disease progression, offering valuable imaging evidence for personalized prevention strategies.
Research has shown that PVAT plays a critical role in atherosclerotic plaque progression by releasing reactive oxygen species and inflammatory factors, which can promote plaque vulnerability (27-29). Additionally, PVAT also exhibits self-regulatory and degradative mechanisms. Baradaran et al. (12) and Saba et al. (30) confirmed a strong correlation between carotid PVAT, plaque inflammation, and cerebrovascular events. However, relying solely on CT values in such studies has limitations, as the measurements can be influenced by contrast agents and may lack sufficient parameter diversity.
We found that the STA group had lower FF and K values and higher 40KH values than the ATA group, suggesting that the inflammatory response in PVAT after plaque rupture increased PVAT density in the STA patients compared to the ATA patients. These results further reinforce the relationship between PVAT, vulnerable plaques, and acute cerebrovascular events (31). Additionally, the predictive performance of our model was significantly better than that of previous studies using mixed-energy CT (15,16). This can be attributed to the use of dual-energy material decomposition technology, where VNC techniques reduce the influence of iodinated contrast agents, providing purer PVAT measurements. This enhanced multiparametric analysis of PVAT using DECT thus improves the accuracy of monitoring plaque vulnerability and offers a more stable model for individualized plaque assessment.
In constructing the nomogram, the dual-energy PVAT features were the most significant, while several plaque burden and vascular lumen parameters were excluded. This suggests that PVAT may serve as a more sensitive biomarker of plaque vulnerability, as its response to plaque rupture occurs earlier than morphological changes in the plaque or vascular lumen. This finding could potentially help clinicians to identify high-risk carotid plaques earlier, allowing for more personalized treatment strategies. For example, patients with high PVAT density and specific PVAT parameter profiles could undergo more aggressive monitoring or intervention, such as antiplatelet therapy or surgical interventions like carotid endarterectomy or stenting.
Future research should focus on validating these results in larger, multi-center cohorts to establish standardized measurement protocols for PVAT parameters. Additionally, future studies could explore the dynamic changes in PVAT over time and their correlation with plaque progression and rupture. Longitudinal studies examining the relationship between PVAT changes and clinical outcomes, such as stroke incidence or recurrent cerebrovascular events, could provide valuable insights into the prognostic utility of PVAT assessment. Further, integrating PVAT parameter analysis with other imaging modalities and biomarkers could lead to the development of a comprehensive, multi-modal risk assessment system for stroke prevention.
The study had a number of limitations. First, it had a relatively small sample size, which might have affected the statistical power. Second, it was a single-center retrospective study nature, and thus lacks multi-center external validation. Third, despite the use of AI software for plaque composition analysis, it lacked surgical specimen comparisons. Finally, it lacked a subgroup analysis based on different plaque types.
Conclusions
The quantitative assessment of PVAT, plaque, and vascular lumen parameters using carotid DECTA significantly enhances the ability to predict acute stroke events. This method offers clinicians a valuable tool for more precise risk stratification and personalized treatment planning in stroke prevention. To further validate the nomogram model, multi-center clinical trials need to be conducted. Additionally, integrating it into hospital information systems for automated risk assessment could improve clinical decision making and facilitate its broader implementation in real-world settings.
Acknowledgments
We would like to sincerely thank our colleagues Sinong Zhang, Jiahui Cai, Miaomiao Kan, Pan Yu, Li Hu, Xiancun Fan, Qingtang Geng, and He Lu for their assistance in data collection and the language editing of this manuscript.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2765/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2765/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (approval No. XYFY2024-KL456), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, Ren Y, Qian Z, Vaughn MG, McMillin SE, Hay SI, Naghavi M, Cai M, Wang C, Zhang Z, Zhou M, Lin H, Yang Y. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021;6:e897-906. [Crossref] [PubMed]
- Fu J, Deng Y, Ma Y, Man S, Yang X, Yu C, Lv J, Wang B, Li L. National and Provincial-Level Prevalence and Risk Factors of Carotid Atherosclerosis in Chinese Adults. JAMA Netw Open 2024;7:e2351225. [Crossref] [PubMed]
- Kamtchum-Tatuene J, Wilman A, Saqqur M, Shuaib A, Jickling GC. Carotid Plaque With High-Risk Features in Embolic Stroke of Undetermined Source: Systematic Review and Meta-Analysis. Stroke 2020;51:311-4. [Crossref] [PubMed]
- Messas E, Goudot G, Halliday A, Sitruk J, Mirault T, Khider L, Saldmann F, Mazzolai L, Aboyans V. Management of carotid stenosis for primary and secondary prevention of stroke: state-of-the-art 2020: a critical review. Eur Heart J Suppl 2020;22:M35-42. [Crossref] [PubMed]
- Murgia A, Balestrieri A, Francone M, Lucatelli P, Scapin E, Buckler A, Micheletti G, Faa G, Conti M, Suri JS, Guglielmi G, Carriero A, Saba L. Plaque imaging volume analysis: technique and application. Cardiovasc Diagn Ther 2020;10:1032-47. [Crossref] [PubMed]
- David E, Grazhdani H, Aliotta L, Gavazzi LM, Foti PV, Palmucci S, Inì C, Tiralongo F, Castiglione D, Renda M, Pacini P, Di Bella C, Solito C, Gigli S, Fazio A, Bella R, Basile A, Cantisani V. Imaging of Carotid Stenosis: Where Are We Standing? Comparison of Multiparametric Ultrasound, CT Angiography, and MRI Angiography, with Recent Developments. Diagnostics (Basel) 2024.
- Boussel L, Arora S, Rapp J, Rutt B, Huston J, Parker D, Yuan C, Bassiouny H, Saloner D. MAPP Investigators. Atherosclerotic plaque progression in carotid arteries: monitoring with high-spatial-resolution MR imaging--multicenter trial. Radiology 2009;252:789-96. [Crossref] [PubMed]
- Bianda N, Di Valentino M, Périat D, Segatto JM, Oberson M, Moccetti M, Sudano I, Santini P, Limoni C, Froio A, Stuber M, Corti R, Gallino A, Wyttenbach R. Progression of human carotid and femoral atherosclerosis: a prospective follow-up study by magnetic resonance vessel wall imaging. Eur Heart J 2012;33:230-7. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72. [Crossref] [PubMed]
- Sheahan M, Ma X, Paik D, Obuchowski NA, St Pierre S, Newman WP 3rd, Rae G, Perlman ES, Rosol M, Keith JC Jr, Buckler AJ. Atherosclerotic Plaque Tissue: Noninvasive Quantitative Assessment of Characteristics with Software-aided Measurements from Conventional CT Angiography. Radiology 2018;286:622-31. [Crossref] [PubMed]
- Baradaran H, Myneni PK, Patel P, Askin G, Gialdini G, Al-Dasuqi K, Kamel H, Gupta A. Association Between Carotid Artery Perivascular Fat Density and Cerebrovascular Ischemic Events. J Am Heart Assoc 2018;7:e010383. [Crossref] [PubMed]
- Zhang S, Yu X, Gu H, Kang B, Guo N, Wang X. Identification of high-risk carotid plaque by using carotid perivascular fat density on computed tomography angiography. Eur J Radiol 2022;150:110269. [Crossref] [PubMed]
- Liu X, Wu F, Jia X, Qiao H, Liu Y, Yang X, Li Y, Zhang M, Yang Q. Pericarotid adipose tissue computed tomography attenuation distinguishes different stages of carotid atherosclerotic disease: a cross-sectional study. Quant Imaging Med Surg 2023;13:8247-58. [Crossref] [PubMed]
- Zhang J, Li S, Wu L, Wang H, Wang C, Zhou Y, Sui B, Zhao X. Application of Dual-Layer Spectral-Detector Computed Tomography Angiography in Identifying Symptomatic Carotid Atherosclerosis: A Prospective Observational Study. J Am Heart Assoc 2024;13:e032665. [Crossref] [PubMed]
- Wang LJ, Zhai PQ, Xue LL, Shi CY, Zhang Q, Zhang H. Machine learning-based identification of symptomatic carotid atherosclerotic plaques with dual-energy computed tomography angiography. J Stroke Cerebrovasc Dis 2023;32:107209. [Crossref] [PubMed]
- de Weert TT, Ouhlous M, Meijering E, Zondervan PE, Hendriks JM, van Sambeek MR, Dippel DW, van der Lugt A. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26:2366-72. [Crossref] [PubMed]
- Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002;105:939-43. [Crossref] [PubMed]
- Ohara T, Toyoda K, Otsubo R, Nagatsuka K, Kubota Y, Yasaka M, Naritomi H, Minematsu K. Eccentric stenosis of the carotid artery associated with ipsilateral cerebrovascular events. AJNR Am J Neuroradiol 2008;29:1200-3. [Crossref] [PubMed]
- Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021;325:1088-98. [Crossref] [PubMed]
- Li Z, Cao J, Bai X, Gao P, Zhang D, Lu X, Sui B. Utility of Dual-Layer Spectral Detector CTA to Characterize Carotid Atherosclerotic Plaque Components: An Imaging-Histopathology Comparison in Patients Undergoing Endarterectomy. AJR Am J Roentgenol 2022;218:517-25. [Crossref] [PubMed]
- Meng X, Li F, Wu W, Wu J. Diagnostic Value of Carotid Plaque Assessment with AIS Based on Quantitative Parameters of Dual-Layer Detector Spectral CT. Int J Gen Med 2024;17:1263-72. [Crossref] [PubMed]
- Bai X, Gao P, Zhang D, Zhang S, Liang J, Lu X, Sui B. Plaque burden assessment and attenuation measurement of carotid atherosclerotic plaque using virtual monoenergetic images in comparison to conventional polyenergetic images from dual-layer spectral detector CT. Eur J Radiol 2020;132:109302. [Crossref] [PubMed]
- Saba L, Loewe C, Weikert T, Williams MC, Galea N, Budde RPJ, Vliegenthart R, Velthuis BK, Francone M, Bremerich J, Natale L, Nikolaou K, Dacher JN, Peebles C, Caobelli F, Redheuil A, Dewey M, Kreitner KF, Salgado R. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol 2023;33:1063-87. [Crossref] [PubMed]
- Hardie AD, Kramer CM, Raghavan P, Baskurt E, Nandalur KR. The impact of expansive arterial remodeling on clinical presentation in carotid artery disease: a multidetector CT angiography study. AJNR Am J Neuroradiol 2007;28:1067-70. [Crossref] [PubMed]
- Benson JC, Nardi V, Bois MC, et al. Correlation between computed tomography angiography and histology of carotid artery atherosclerosis: Can semi-automated imaging software predict a plaque's composition? Interv Neuroradiol 2022;28:332-7. [Crossref] [PubMed]
- Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 2005;25:2594-9. [Crossref] [PubMed]
- Katsiki N, Mikhailidis DP. Perivascular Adipose Tissue: Pathophysiological Links With Inflammation, Atherosclerosis, and Thrombosis. Angiology 2022;73:195-6. [Crossref] [PubMed]
- Kim HW, Shi H, Winkler MA, Lee R, Weintraub NL. Perivascular Adipose Tissue and Vascular Perturbation/Atherosclerosis. Arterioscler Thromb Vasc Biol 2020;40:2569-76. [Crossref] [PubMed]
- Saba L, Zucca S, Gupta A, Micheletti G, Suri JS, Balestrieri A, Porcu M, Crivelli P, Lanzino G, Qi Y, Nardi V, Faa G, Montisci R. Perivascular Fat Density and Contrast Plaque Enhancement: Does a Correlation Exist? AJNR Am J Neuroradiol 2020;41:1460-5. [Crossref] [PubMed]
- Chen JF, Yang J, Chen WJ, Wei X, Yu XL, Huang DD, Deng H, Luo YD, Liu XJ. Mono+ algorithm assessment of the diagnostic value of dual-energy CT for high-risk factors for colorectal cancer: a preliminary study. Quant Imaging Med Surg 2024;14:432-46. [Crossref] [PubMed]



