
Differential hemodynamics between autogenous arteriovenous fistulas in forearm created by modified no-touch technique and conventional surgery: four-dimensional flow magnetic resonance imaging approach
Introduction
In China, autogenous arteriovenous fistula (AVF) is the vascular access method of choice for delivering hemodialysis to patients undergoing maintenance hemodialysis. An AVF is a surgically created connection between an artery and a vein, typically in the arm, which allows for efficient blood flow during dialysis. This preference is not only due to the lower incidence of complications associated with AVFs but also because of the underlying physiological mechanisms that make AVFs effective. An AVF operates on the principle of creating a direct connection between an artery and a vein, which leads to a persistent and high-flow state in the vein. This increased blood flow rate enhances the dilatation and thickening of the vein walls, a process known as maturation, which in turn creates a stable and reliable vessel access for hemodialysis. The high flow rate through the AVF also helps to prevent thrombosis and stenosis, common complications in vascular accesses. Among all types of AVFs, forearm AVFs are the most commonly used vascular access in hemodialysis patients in China, accounting for more than 90% of AVFs in Suzhou Hospital (1,2).
Nevertheless, juxta-anastomotic stenosis often occurs in the forearm AVF, especially in anastomotic outflow vein vessels, hindering AVF maturation and leading to loss of function (3). Although juxta-anastomotic stenosis is a result of multiple factors, a good prognosis has been obtained clinically by reducing vascular damage caused by surgical operations (4), such as no-touch and radial artery deviation and reimplantation (RADAR) technology (5,6). In order to further optimize surgery and expand the scope of surgical indications, we introduced a novel modified no-touch technique (MNTT) aimed at preventing devascularization of the venous and arterial walls and radial artery transection. In our initial small sample size and short-term follow-up study, MNTT can significantly reduce juxta-anastomotic stenosis and improve the primary patency rate of AVFs compared with conventional techniques (CT) (7).
In recent studies, the theoretical basis of patency rate and maturity of AVF has been deeply explored through different methods. Sigovan et al. (8) combined magnetic resonance imaging (MRI) with computational fluid dynamics (CFD) to analyze vascular remodeling in AVFs, highlighting the relationship between hemodynamics and structural changes in the fistula. Remuzzi et al. (9) discussed the pivotal role of shear stress in AVF maturation, emphasizing its importance in the optimization of AVF creation and maintenance. Based on these findings, we hypothesize that the MNTT enhances AVF outcomes for two primary reasons: it optimizes the hemodynamics of the anastomotic swing segment and minimizes surgical trauma to the blood vessels. Hence, this study focuses on investigating the hemodynamics near the anastomotic swing segment of AVFs. While ultrasound is a straightforward and recommended method for monitoring AVF maturation and complications according to guidelines (1,10), it lacks the ability to provide wall shear stress (WSS), oscillatory shear index (OSI) and other critical hemodynamic parameters for in-depth analysis. Four-dimensional (4D) flow MRI (11-13) addresses this limitation by offering a comprehensive view of AVF hemodynamics. Studies such as those by Li et al. (14) applied 4D-flow MRI to assess AVF hemodynamics in dialysis patients, finding that flow patterns, distribution, and WSS are closely associated with AVF patency. Northrup et al. (15) utilized 4D-flow MRI to discern differential hemodynamics in AVFs before and after intervention, revealing significant flow pattern differences that may correlate with AVF success. These studies inspire us to further explore differential hemodynamics between AVFs created by MNTT and CT, and we analyzed AVF blood flow rate, velocity, vorticity, relative helicity, WSS and OSI in the artery and vein using 4D-flow MRI data.
The paper is structured in the subsequent sections: section 2, titled “Methods”, describes the study design, MRI protocol and data analysis details; section 3, titled “Results”, presents the statistical results of hemodynamics parameters; section 4, titled “Discussion”, interprets the related results and compares them with existing literature; section 5, titled “Conclusions” summarizes the main findings and our contributions in this study. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1815/rc).
Methods
Study design and patients
We conducted a retrospective review of our prospectively maintained dataset, focusing on patients who underwent radio-cephalic arteriovenous fistula (RC-AVF) creation for hemodialysis. The study compared two groups: those who received the modified no-touch technique (MNTT group) between January 1, 2021, and December 23, 2023, and those who underwent the conventional surgical approach (CT group) between January 1, 2018, and December 23, 2023. The inclusion criteria for RC-AVF included the following criteria: (I) no previous history of AVF surgery in the ipsilateral arm; (II) radial artery diameter ≥1.5 mm, cephalic vein diameter ≥2.0 mm (tourniquet use) and continuous upper arm vein run-off; (III) the distance between the artery and vein was <3 cm for patients in the MNTT group. To further explore whether MNTT improved the hemodynamics in the swing segment of the AVFs, we used 4D-flow MRI to visualize and quantify the hemodynamics of AVFs created by MNTT and CT.
The surgical procedures in the MNTT group were exclusively performed by the same vascular surgeon (G.H.). In the CT group, the operation involved standard vein devascularization and mobilization, followed by an end-to-side anastomosis (vein-to-artery) using a running 7-0 or 8-0 polypropylene monofilament suture. The MNTT group’s surgical techniques were carried out as previously outlined (Figure 1A-1C). Briefly, a 4–5 cm skin incision was made at a distance of less than 3 cm between the radial artery and cephalic vein in the forearm. Subcutaneous adipose tissue was carefully dissected using curved hemostatic forceps to expose the superficial fascia, which was left intact. Hemostasis was achieved without using an electric knife. The tissue was systematically incised to expose the radial artery pedicle, including the radial artery and its adjacent veins. Micro-scissors or a sharp knife were used to create an 8–10 mm incision in the superficial fascia and cephalic vein wall, with a corresponding 8–10 mm incision in the artery pedicle (Figure 1A). A side-to-side anastomosis was established using a 7-0 non-absorbable single-strand suture (Figure 1B). The distal cephalic vein was ligated to form a functional end-to-side anastomosis (Figure 1B,1C). A total of 35 patients were enrolled in this study and underwent 4D-flow MRI examination (Figure 1D).
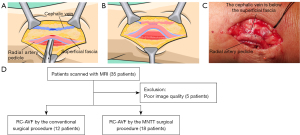
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and approved by the Institutional Review Board of Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University (No. IRB2021029). All patients provided informed consent.
Doppler ultrasound examination protocol
Patients should be positioned supine, with the upper arm exposed, at room temperature. The examination was performed on the same day as the 4D-flow MRI scan. Doppler Ultrasound (DUS) should use a high-frequency transducer probe (7–20MHz) to obtain precise images of the target vessel’s wall, lumen, and surrounding tissues. The ultrasound protocol included: (I) measurement sites: the cephalic vein and radial artery at 1.5 cm from the anastomosis; (II) measurement parameters: internal diameter, peak systolic velocity, end-diastolic velocity, and blood flow volume.
MR protocol
4D-flow MRI, derived from three-dimensional (3D) phase contrast MRI, enables comprehensive and individualized evaluation of real hemodynamics in vascular structures. In this research, 4D-flow MRI was employed to visualize and quantify the hemodynamic characteristics of AVFs created via MNTT and CT. MRI data was acquired using a 3.0T scanner (Magnetom Prisma, Siemens, Germany) equipped with a 16-channel flexible surface coil. The imaging protocol involved a free-breathing, 3D radio-frequency-spoiled, multi-shot turbo field echo sequence, with patients positioned supine and arms extended. The scan parameters included repetition time/echo time =5.5/3 ms, field of view =160×90×60 mm3, voxel size =1.0×1.0×1.0 mm3, and flip angle =20°. Velocity encoding (VENC) value should be set higher than the maximum blood flow velocity at the imaging site to avoid aliasing. According to the pre-4D-flow MRI ultrasound examination results of AVFs, the maximum blood flow velocities in the radial arteries and cephalic veins of the AVFs ranged from 70 to 400 cm/s, indicating a wide velocity span. Therefore, for each patient undergoing 4D-flow MRI, we individualized the VENC values based on their pre-examination ultrasound results, setting them within a range of 100 to 450 cm/s.
Data analysis
For 4D-flow MRI data analysis, a workflow (Figure 2) reported by Fu et al. (16,17) was used to obtain quantified values (flow velocity, flow volume, and WSS) of the AVF. As shown in Figure 2A-2D, velocity-weighted masks were utilized to obtain better segmentation of 3D lumen geometry, which is considered an recommended standard inconsensus statement (18). We employed various algorithms to obtain vessel geometry, including threshold segmentation, region growth, connected domain selection, and surface smoothing. Velocity filed calculated from 4D-flow MRI was used for calculation of hemodynamic parameters. Velocity, vorticity and relative helicity distribution inside the vessel were plotted in Figure 2E-2G. WSS and OSI mapping were shown in Figure 2H,2I.
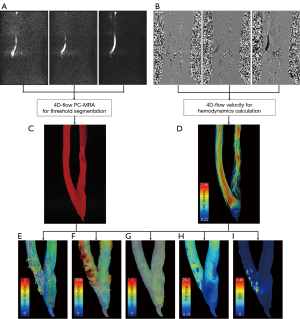
Vorticity is a measure of the local rotation in a fluid flow. It is computed from the velocity vector u using the curl operator, as given by Eq. [1]. Here, represents the curl of the velocity vector u, and the Ω is the vorticity vector.
Relative helicity is a measure of the alignment between the velocity and vorticity vectors. It is defined by Eq. [2] as the dot product of the velocity and vorticity vectors divided by the product of their magnitudes:
WSS was defined as the velocity gradient along the perpendicular direction of the vessel wall. As described in Eq. [3], µ means the dynamic viscosity and y means the distance to the wall:
OSI is a measure of the fluctuation of WSS over time. It is calculated using Eq. [4], which compares the absolute value of the time-averaged WSS to the average of the absolute WSS over a time period:
All time-averaged hemodynamic parameters were calculated using the average of obtained value at all timepoints during a cardiac cycle.
For hemodynamic analysis, a total of 35 participants agreed to undergo 4D-flow MRI examination, including 18 in the MNTT group and 12 in the CT group, 5 people were excluded due to poor image quality.
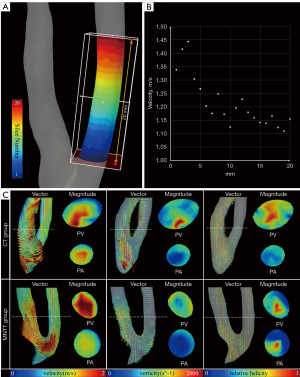
Similar to the methods of previous study by Northrup (15), the hemodynamic change is explored along the vascular axis. Taking the anastomosis as the starting point, the area with a length of 2 cm on the side of the radial artery is called the proximal artery (PA), and the area with a length of 2 cm on the cephalic venous side is called the proximal vein (PV). As shown in Figure 3, data points were collected for every 1 mm thick slice located along the PA and PV, totaling 20 data points per PA or PV. Side view and radial slices for CT and MNTT groups are shown in Figure 3C. Intergroup comparisons for continuous data were made using Student’s t-test.
All statistical analyses were performed using IBM SPSS software version 19.0 and EmpowerStats software version 6.0. Continuous data were presented as mean ± standard deviation. Histograms were used to examine data distribution, while interquartile ranges summarized non-normally distributed data. Categorical variables were presented as frequencies (n) and percentages (%). All P values ≤0.05 were considered statistically significant, and all P values were two-tailed.
Results
Detailed baseline characteristics of the study cohort are shown in Table 1. The ultrasound results revealed that the peak systolic velocity in the radial artery was higher in the MNTT group than in the CT group, though without statistical significance (P=0.063). No statistically significant differences were observed between the two groups in either the cephalic vein or radial artery regarding vessel diameter or blood flow volume (Table 2).
Table 1
| Factors | Control group (n=12) | MNTT group (n=18) | P value |
|---|---|---|---|
| Age (years) | 51.5 (46.5, 69.75) | 53 (40.5, 67.5) | 0.912 |
| Male sex | 10 [83] | 16 [89] | >0.99 |
| Primary renal disease | |||
| Diabetes | 4 [33] | 6 [33] | >0.99 |
| Glomerulonephritis | 7 [58] | 11 [61] | 0.879 |
| Unknown or others | 1 [8] | 1 [6] | – |
Data are presented as n [%], or median (range). MNTT, modified no-touch method.
Table 2
| Category | Control group | MNTT group | P value |
|---|---|---|---|
| Cephalic vein (1.5 cm from the anastomotic site) | |||
| Diameter (mm) | 5.84±2.44 | 5.59±1.13 | 0.746 |
| PSV (cm/s) | 243.92±105.43 | 228.33±81.45 | 0.652 |
| EDV (cm/s) | 127.24±60.20 | 105.44±51.46 | 0.297 |
| Flow (mL/min) | 1,118.17±1,292.74 | 1,001.83±467.67 | 0.728 |
| Radial artery (1.5 cm from the anastomotic site) | |||
| Diameter (mm) | 4.08±0.86 | 4.13±0.65 | 0.868 |
| PSV (cm/s) | 150.92±38.13 | 192.83±67.93 | 0.063 |
| EDV (cm/s) | 97.76±21.30 | 122.85±53.32 | 0.134 |
| Flow (mL/min) | 563.67±330.55 | 738.06±430.62 | 0.245 |
Data are presented as mean ± standard deviation. 4D-flow MRI, four-dimensional flow magnetic resonance imaging; EDV, end diastolic velocity; MNTT, modified no-touch method; PSV, peak systolic velocity.
Figure 4A-4D displays the velocity for radial slices and overall region of interest in PV and PA. The averaged velocity magnitude was not uniform along the axis apparently in the MNTT groups in either PV or PA. PA velocity decreased farther away from the anastomosis and exhibited a fluctuating pattern. In contrast, the averaged velocity of the CT group was similar along the axis in both PA and PV. The averaged velocity was found higher in the MNTT group (0.771±0.029 m/s in PA and 0.955±0.055 m/s in PV) than in the CT group (0.577±0.011 m/s in PA and 0.611±0.014 m/s in PV) (P<0.001). Meanwhile, the maximum and minimum velocity of the MNTT group (1.312±0.092 m/s and 0.260±0.029 m/s in PA, 1.735±0.109 m/s and 0.411±0.051 m/s in PV) were significantly higher than those of the CT group (1.151±0.023 m/s and 0.176±0.021 m/s in PA, 1.593±0.083 m/s and 0.139±0.015 m/s in PV) (the P values are all less than 0.001, Figure S1).
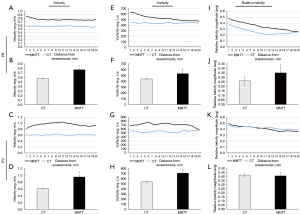
Vorticity is a vector field that gives a microscopic measure of the fluid rotation over a cardiac cycle. Figure 4E-4H shows the averaged vorticity magnitude over the period of the cardiac cycle. The trend in vorticity changes was similar to that of velocity along the vascular axis in both PA and PV. In the MNTT group, the averaged vorticity over 2-cm vessel was 529.115±50.006 1/s in PA and 709.895±43.180 1/s in PV, which was significantly different from CT group (438.028±6.819 1/s, P<0.001 and 539.670±19.891 1/s, P<0.001). However, the maximum and minimum vorticity values were not significantly different between the two groups (Figure S2).
Relative helicity represents the degree of linkage of the vortex lines of the blood flow. The magnitude of relative helicity exhibited non-uniformity along the axis for both groups in both PV and PA, which decreased progressively as distance from the anastomosis increased. As shown in Figure 4I-4L, the averaged relative helicity magnitude of PA in MNTT group was significantly higher than CT group (0.349±0.067 vs. 0.261±0.052, P<0.001). However, it was not significantly different between the two groups in PV (0.409±0.041 vs. 0.419±0.021, P<0.381). Figure S3 illustrates the maximum and minimum value of relative helicity.
WSS refers to the frictional force exerted by the blood on the endothelial surface. Figure 5A-5D displays the time-averaged WSS along the vascular axis. WSS in the MNTT group was significantly higher than in the CT group, regardless of the blood vessel (PA: 7.885±0.239 vs. 5.611±0.213 Pa, P<0.001; PV: 9.378±0.738 vs. 5.314±0.279 Pa, P<0.001). The maximum and minimum WSS in PA and PV were similar in the two groups (Figure S4).
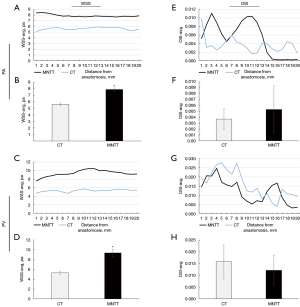
OSI characterizes WSS changes other than the direction of the mean shear stress vector. Figure 5E-5H displays OSI in radial slides and averaged over a 2-cm segment. In general, OSI showed a decreasing trend as distance from the anastomosis increased in both vessels and the two groups. However, the averaged OSI in whole PA and PV was not significantly different between the two groups (P=0.105). The maximum and minimum value of OSI are shown in Figure S5.
Discussion
Modification of the fistula surgical technique aimed at reducing neointimal hyperplasia and increasing patency rates, including no-touch and RADAR, are among the most promising strategies (4). In 2021, we proposed a new MNTT (19). The key point of this technique is to avoid devascularization of the venous and arterial walls and severing of the radial artery. We previously reported a study on 40 patients using the MNTT that demonstrated short-term improvements in reducing juxta-anastomotic stenosis and enhancing the primary patency rate of AVFs (7). Previous studies have speculated that improved hemodynamics may be one of the important reasons for the higher patency of RC-AVF. Multiple computational and experimental studies have been conducted to analyze the hemodynamics of vascular anastomoses, using both idealized (20) and patient-specific models (21). However, these works were primarily performed hemodynamic analysis based on simulations of blood flow. Additionally, the differences among surgical techniques should be explored by advanced methods. In this study, 4D-flow MRI was first used to investigate the hemodynamic parameters in artery and vein of AVFs created by MNTT and CT. Statistical results showed that PA and PV had higher velocity, vorticity and WSS in MNTT group. Our findings provided a reasonable explanation for the higher primary patency rate and lower juxta-anastomotic stenosis rate in the RC-AVF by MNTT group.
Although ultrasound has been widely used in clinical studies related to AVF, this two-dimensional method still makes it difficult to extract hemodynamic parameters from a 3D perspective. Furthermore, the pressure exerted by the probe on the forearm AVF vessels during ultrasound may affect the measurement of hemodynamic information. In addition to ultrasound method, AVF hemodynamic measurements are also conducted using MRI based CFD (8,15,22). These studies have not considered the flexibility of blood vessel walls. In recent year, 4D-flow MRI has been reported to be an efficient method for blood flow measurement within an acquired 3D volume (17,23,24) and has reached good consistency with ultrasound for evaluating AVF (14). Our study utilized 4D-flow MRI to better understand the relationships between hemodynamics and AVF prognosis. The post-processing program can reconstruct the blood flow within the fistula based on 3D volume and analyze various hemodynamic parameters. To our knowledge, this was the first study to evaluate the hemodynamic parameters of AVF across different surgical techniques using 4D-flow MRI.
Disturbed flow plays a crucial role in vascular remodeling after AVF creation (25). In our study, higher AVF flow velocity was observed in the MNTT group. Hou et al. (7) investigated postoperative evaluations of MNTT using duplex ultrasound and found that the peak systolic velocity in the brachial artery increased compared to the conventional surgical procedure. A study on the RADAR technique in a rat model showed that this configuration was also associated with increased peak systolic velocity (6). Northrup et al. (15) analyzed hemodynamics between with or without intervention before successful AVF use and found that larger venous velocity after AVF creation surgery may be important for AVF maturation. Therefore, we speculate that the better patency rate in MNTT group may be related to the higher AVF velocity.
In addition to flow velocity, vorticity and relative helicity were also measured in our study. These two parameters have been characterized and explored in many studies of cardiovascular disease (26-28). Research about pulmonary arterial found that reduced relative helicity and vorticity are associated with increased pulmonary stiffness (28). Our results showed that the averaged vorticity was significantly larger in the MNTT group than in the CT group. Similar results were reported in Northrup’s (15) study, which found venous vorticity was greater in AVFs without interventions than with interventions before successful use. As for relative helicity in the present study, the average, maximum and minimum values of arterial relative helicity, but not venous relative helicity, were significantly greater in MNTT group. Thus, the level of relative helicity appears to be important for the artery after AVF creation, while vorticity is important for both PA and PV.
WSS and OSI are believed to stimulate the vascular endothelial cells and result in intimal hyperplasia (9,16,29). Some studies have suggested that high levels of WSS are associated with decreased expression of adhesion molecules, increased expression of endothelial nitric oxide synthase, and reduced oxidative stress, which result in the survival of the endothelium and decreased intima-media thickening (30,31). For the creation of AVFs, the arteriovenous anastomosis will immediately increase blood flow and elevate venous blood pressure. Previous theoretical modeling (32) approaches have shown that changes in hemodynamics result in structural transformations in the vessel wall. These changes aim to accommodate the new hemodynamic conditions by restoring basal levels of WSS. Research on arterial vessels reveal that the relatively high WSS triggers morphological adaptations in endothelial cells, such as elongation, alignment and cytoskeletal organization (33,34). In this study, the time-averaged value of the area-averaged WSS over 2 cm was calculated, enabling the prediction of intimal thickening (35). The results showed that WSS values of both PA and PV were significantly greater in MNTT group, which has a better patency rate of AVF. Northrup et al. reported that the successful use AVF had higher WSS without intervention, indicating that AVF maturation benefited from lager WSS (7,15). Thus, a high WSS value may be key to the maturation of AVFs following the MNTT surgical procedure. Meanwhile, similar to the results of previous studies (7,15), the area-averaged OSI levels in our study were relatively small. However, high OSI signals were still located in the relatively narrow vessel and the heel (Figure 2I), which indicated the reciprocating blood flow area. The maximum OSI value can represent disturbed flow patterns acting on endothelial cells in AVFs. Studies (36,37) have shown that when local flow conditions expose endothelial cells to unidirectional WSS in PA and PV, it is expected that the cell expression of nitric oxide (NO) will increase, along with the induction of stable arterial and venous remodeling. As shown in Figure S5, the OSI-max value of the CT group was relatively greater than that of the MNTT group, suggesting that stable WSS may beneficial for the higher patency rate.
There are several limitations in this study that must be considered. Firstly, our study is a retrospective study without randomization, so selection bias could have influenced the results. A randomized controlled trial of MNTT is currently ongoing in Suzhou hospital (https://www.chictr.org.cn; ChiCTR2300068320). Secondly, this study focused on a single time point after AVF creation with no longitudinal data. While the majority of patients received MRI examinations within 4–6 weeks postoperatively, a small number of patients had intervals exceeding 12 months between surgical creation and MRI examination. Prognostic models (10,15) for AVF creation surgery pointed out that hemodynamic parameters would vary with AVF maturation. Future studies will focus more on the temporal relationship between hemodynamics and AVF remodeling. Thirdly, this study’s retrospective design lacked preoperative vascular ultrasound findings and cardiac echocardiography data for AVFs, which may have influenced the hemodynamic parameter analysis. This limitation necessitates methodological refinement in future research endeavors. Fourthly, although the resolution of the 4D-flow MRI sequence employed in this study aligns with the typical resolutions in clinical 3T MRI systems, numerous studies has reported that higher field strengths substantially enhance both signal-to-noise ratio and spatial resolution (38,39). Future investigations could explore high-resolution protocols to optimize hemodynamic characterization. Finally, current hemodynamic analysis methods mainly focus on starting from the anastomosis to the ends of the PV and PA, and determining the measurement range and developing methods to accurately compute hemodynamic parameters at the anastomosis remain important directions for future research.
Conclusions
Previous small sample size study has shown that RC-AVF created through MNTT surgical technique achieved greater patency and decreased juxta-anastomotic stenosis. Our study further explored the hemodynamics between MNTT and CT through 4D-flow MRI. Patients in the MNTT group had good prognosis related to the improved fistula hemodynamics. The paper provides a deep insight into the mechanisms of AVF remodeling with a parameterized model.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1815/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1815/coif). G.H. reports grants from Suzhou Gusu Health Talent Plan (No. GSWS2022129), Suzhou Science & Technology Plan Project (Nos. SYS20200077 and SKY2021030) and Suzhou Science and Education Strengthening Health Project (No. MSXM2024078). R.L. reports grant from the National Natural Science Foundation of China (No. 81971604). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in conformity with the Declaration of Helsinki and its subsequent amendments and approved by the institutional review board of Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University (No. IRB2021029). All patients provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RPNational Kidney Foundation. 2019 Update. Am J Kidney Dis 2020;75:S1-S164. [Crossref] [PubMed]
- Almasri J, Alsawas M, Mainou M, Mustafa RA, Wang Z, Woo K, Cull DL, Murad MH. Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. J Vasc Surg 2016;64:236-43. [Crossref] [PubMed]
- Gunawardena T, Ridgway D. Swing segment stenosis: An unresolved challenge. Semin Dial 2022;35:307-16. [Crossref] [PubMed]
- Shenoy S. Surgical technique determines the outcome of the Brescia/Cimino AVF. J Vasc Access 2017;18:1-4.
- Hörer TM, Skoog P, Quell R, Nilsson KF, Larzon T, Souza DR. No-touch technique for radiocephalic arteriovenous fistula--surgical technique and preliminary results. J Vasc Access 2016;17:6-12.
- Bai H, Sadaghianloo N, Gorecka J, Liu S, Ono S, Ramachandra AB, Bonnet S, Mazure NM, Declemy S, Humphrey JD, Dardik A. Artery to vein configuration of arteriovenous fistula improves hemodynamics to increase maturation and patency. Sci Transl Med 2020; [Crossref] [PubMed]
- Hou G, Fu M, Wang X, Liu Z, Zhang Y, Zhu D, Pang H, Li R, Shen L. Modified no-touch technique for radio-cephalic arteriovenous fistula increases primary patency and decreases juxta-anastomotic stenosis. J Vasc Access 2024;25:904-13.
- Sigovan M, Rayz V, Gasper W, Alley HF, Owens CD, Saloner D. Vascular remodeling in autogenous arterio-venous fistulas by MRI and CFD. Ann Biomed Eng 2013;41:657-68. [Crossref] [PubMed]
- Remuzzi A, Bozzetto M, Brambilla P. Is shear stress the key factor for AVF maturation? J Vasc Access 2017;18:10-4.
- Robbin ML, Greene T, Cheung AK, Allon M, Berceli SA, Kaufman JS, Allen M, Imrey PB, Radeva MK, Shiu YT, Umphrey HR, Young CJHemodialysis Fistula Maturation Study Group. Arteriovenous Fistula Development in the First 6 Weeks after Creation. Radiology 2016;279:620-9. [Crossref] [PubMed]
- Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 2012;36:1015-36. [Crossref] [PubMed]
- Han N, Wang J, Ma Y, Ma L, Zheng Y, Fan F, Wu C, Yue S, Li J, Liang J, Zhang H, Zhou Y, Yang T, Zhang J. The hemodynamic and geometric characteristics of carotid artery atherosclerotic plaque formation. Quant Imaging Med Surg 2024;14:4348-61. [Crossref] [PubMed]
- Dai C, Zhao P, Wang G, Ding H, Lv H, Qiu X, Tang R, Xu N, Huang Y, He K, Yang Z, Gong S, Wang Z. Hemodynamic assessments of unilateral pulsatile tinnitus with jugular bulb wall dehiscence using 4D flow magnetic resonance imaging. Quant Imaging Med Surg 2024;14:684-97. [Crossref] [PubMed]
- Suqin L, Mingli Z, Shiteng S, Honglan M, Lan Z, Qihong N, Qing L. Assessment of the Hemodynamics of Autogenous Arteriovenous Fistulas With 4D Phase Contrast-Based Flow Quantification MRI in Dialysis Patients. J Magn Reson Imaging 2020;51:1272-80. [Crossref] [PubMed]
- Northrup H, He Y, Le H, Berceli SA, Cheung AK, Shiu YT. Differential hemodynamics between arteriovenous fistulas with or without intervention before successful use. Front Cardiovasc Med 2022;9:1001267. [Crossref] [PubMed]
- Fu M, Peng F, Zhang M, Chen S, Niu H, He X, Xu B, Liu A, Li R. Aneurysmal wall enhancement and hemodynamics: pixel-level correlation between spatial distribution. Quant Imaging Med Surg 2022;12:3692-704. [Crossref] [PubMed]
- Fu M, Peng F, Niu H, He X, Chen S, Zhang M, Xia J, Wang Y, Xu B, Liu A, Li R. Inflow Angle Impacts Morphology, Hemodynamics, and Inflammation of Side-wall Intracranial Aneurysms. J Magn Reson Imaging 2023;57:113-23. [Crossref] [PubMed]
- Bissell MM, Raimondi F, Ait Ali L, Allen BD, Barker AJ, Bolger A, et al. 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J Cardiovasc Magn Reson 2023;25:40. [Crossref] [PubMed]
- Xiaohe W, Yuanyuan Z, Zhen L, Guocun H. A modified no-touch technique for anastomosis to create a radiocephalic arteriovenous fistula. J Vasc Surg Cases Innov Tech 2021;7:686-90. [Crossref] [PubMed]
- Gunasekera S, Ng O, Thomas S, Varcoe R, de Silva C, Barber T. Tomographic PIV analysis of physiological flow conditions in a patient-specific arteriovenous fistula. Exp Fluids 2020;61:253. [Crossref]
- Jodko D, Obidowski D, Reorowicz P, Józwik K. Blood flows in end-to-end arteriovenous fistulas: Unsteady and steady state numerical investigations of three patient-specific cases. Biocybernetics and Biomedical Engineering 2017;37:528-39. [Crossref]
- Acuna A, Berman AG, Damen FW, Meyers BA, Adelsperger AR, Bayer KC, Brindise MC, Bungart B, Kiel AM, Morrison RA, Muskat JC, Wasilczuk KM, Wen Y, Zhang J, Zito P, Goergen CJ. Computational Fluid Dynamics of Vascular Disease in Animal Models. J Biomech Eng 2018;140:0808011-08080114. [Crossref] [PubMed]
- Zhang M, Peng F, Li Y, He L, Liu A, Li R. Associations between morphology and hemodynamics of intracranial aneurysms based on 4D flow and black-blood magnetic resonance imaging. Quant Imaging Med Surg 2021;11:597-607. [Crossref] [PubMed]
- Chen W, Song X, Chen S, Chen Z, Fu M, Wei C, Zheng Z, Wu J, Li R. Blood flow and perfusion lateralization in border zone infarct using 4D flow and arterial spin labeling. Neuroradiology 2022;64:2145-52. [Crossref] [PubMed]
- Ene-Iordache B, Semperboni C, Dubini G, Remuzzi A. Disturbed flow in a patient-specific arteriovenous fistula for hemodialysis: Multidirectional and reciprocating near-wall flow patterns. J Biomech 2015;48:2195-200. [Crossref] [PubMed]
- Fenster BE, Browning J, Schroeder JD, Schafer M, Podgorski CA, Smyser J, Silveira LJ, Buckner JK, Hertzberg JR. Vorticity is a marker of right ventricular diastolic dysfunction. Am J Physiol Heart Circ Physiol 2015;309:H1087-93. [Crossref] [PubMed]
- Kheyfets VO, Schafer M, Podgorski CA, Schroeder JD, Browning J, Hertzberg J, Buckner JK, Hunter KS, Shandas R, Fenster BE. 4D magnetic resonance flow imaging for estimating pulmonary vascular resistance in pulmonary hypertension. J Magn Reson Imaging 2016;44:914-22. [Crossref] [PubMed]
- Schäfer M, Barker AJ, Kheyfets V, Stenmark KR, Crapo J, Yeager ME, Truong U, Buckner JK, Fenster BE, Hunter KS. Helicity and Vorticity of Pulmonary Arterial Flow in Patients With Pulmonary Hypertension: Quantitative Analysis of Flow Formations. J Am Heart Assoc 2017;6:e007010. [Crossref] [PubMed]
- Lee T, Qian JZ, Zhang Y, Thamer M, Allon M. Long-Term Outcomes of Arteriovenous Fistulas with Unassisted versus Assisted Maturation: A Retrospective National Hemodialysis Cohort Study. J Am Soc Nephrol 2019;30:2209-18. [Crossref] [PubMed]
- Krishnamoorthy MK, Banerjee RK, Wang Y, Zhang J, Sinha Roy A, Khoury SF, Arend LJ, Rudich S, Roy-Chaudhury P. Hemodynamic wall shear stress profiles influence the magnitude and pattern of stenosis in a pig AV fistula. Kidney Int 2008;74:1410-9. [Crossref] [PubMed]
- Fitts MK, Pike DB, Anderson K, Shiu YT. Hemodynamic Shear Stress and Endothelial Dysfunction in Hemodialysis Access. Open Urol Nephrol J 2014;7:33-44. [Crossref] [PubMed]
- Remuzzi A, Dewey CF Jr, Davies PF, Gimbrone MA Jr. Orientation of endothelial cells in shear fields in vitro. Biorheology 1984;21:617-30. [Crossref] [PubMed]
- Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 2002;225:59-64. [Crossref] [PubMed]
- Lee T, Misra S. New Insights into Dialysis Vascular Access: Molecular Targets in Arteriovenous Fistula and Arteriovenous Graft Failure and Their Potential to Improve Vascular Access Outcomes. Clin J Am Soc Nephrol 2016;11:1504-12. [Crossref] [PubMed]
- Lee SW, Antiga L, Steinman DA. Correlations among indicators of disturbed flow at the normal carotid bifurcation. J Biomech Eng 2009;131:061013. [Crossref] [PubMed]
- Brahmbhatt A, Remuzzi A, Franzoni M, Misra S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int 2016;89:303-16. [Crossref] [PubMed]
- Remuzzi A, Bozzetto M. Biological and Physical Factors Involved in the Maturation of Arteriovenous Fistula for Hemodialysis. Cardiovasc Eng Technol 2017;8:273-9. [Crossref] [PubMed]
- Hess AT, Bissell MM, Ntusi NA, Lewis AJ, Tunnicliffe EM, Greiser A, Stalder AF, Francis JM, Myerson SG, Neubauer S, Robson MD. Aortic 4D flow: quantification of signal-to-noise ratio as a function of field strength and contrast enhancement for 1.5T, 3T, and 7T. Magn Reson Med 2015;73:1864-71. [Crossref] [PubMed]
- Gottwald LM, Töger J, Markenroth Bloch K, Peper ES, Coolen BF, Strijkers GJ, van Ooij P, Nederveen AJ. High Spatiotemporal Resolution 4D Flow MRI of Intracranial Aneurysms at 7T in 10 Minutes. AJNR Am J Neuroradiol 2020;41:1201-8. [Crossref] [PubMed]


