
Clinical and CT imaging features of different types of thoracic complex lymphatic anomaly
Introduction
The latest guideline of the International Society for the Study of Vascular Anomalies in 2018 classifies lymphatic malformations into common (cystic) lymphatic malformations, generalized lymphatic anomaly (GLA), kaposiform lymphangiomatosis (KLA), Gorham-Stout disease (GSD), central conducting lymphatic anomaly (CCLA), “acquired” progressive lymphatic anomaly, primary lymphedema, and others (1). Among them, GLA, KLA, GSD, and CCLA are called complex lymphatic anomaly (CLA) due to their extensive involvement, diverse clinical symptoms, and complex treatment strategies. CLA is a developmental anomaly of the lymphatic vessels involving multiple organs and systems. The disease is caused by congenital structural and functional abnormalities, which lead to lymphatic obstruction, dilatation, and hyperplasia. On static computed tomography (CT), lymphangiectasia means that the lymphatic vessels show tortuous tubular or cystic changes. Lymphangiosis refers to the structural abnormality of lymphatic vessels, accompanied by the surrounding trabecular changes and the blurring of the fat space. This eventually leads to lymphedema, chylous leakage, or lymphatic hyperplasia of tissues. Secondary lymphatic malformations caused by infection, surgery, trauma, or tumors are not included (2-4).
GLA is a multiorgan or tissue disease characterized by proliferation, dilation, or infiltration of lymphatic vessels. GLA is common in children and adolescents, with no significant gender differences (2,5). KLA is a more aggressive subtype of GLA with a 5-year survival rate of 51%. KLA has a poor prognosis, which is characterized by local spindle lymphatic endothelial cells, thrombocytopenia, and coagulation disorders (1,6). Since the imaging manifestations of KLA and GLA are similar, they are merged into GLA for discussion in this paper (7). GSD is characterized by progressive osteolysis, involving both cortical and cancellous bone. The symptoms of GSD include bone pain, limited mobility, deformity, and chylous pleural effusion. The mortality rate of GSD is about 13.3%, and the prognosis is worse when the spine is involved or accompanied by chylous pleural effusion (1,8-10). CCLA is characterized by dilation, obstruction, or dysmotility of the central lymphatic vessels, thereby affecting lymphatic drainage. CCLA can be accompanied by chylous pleural effusion, chylous ascites, protein-losing enteropathy, or lymphedema (11).
CLA is usually most likely to be present in the chest. Ozeki et al. (10) reported 85 patients with CLA, among whom 56 (65.9%) had chest lesions. When CLA involves the chest, especially the lung, the prognosis is usually poor. In mild cases, it can lead to chest tightness, cough, and expectoration; in severe cases, it can lead to respiratory failure and death (12,13). Therefore, it is very important to clarify the manifestations of different types of thoracic CLA for accurate diagnosis and treatment guidance (13). One of the key points of this study was to retrospectively analyze the clinical and imaging features of thoracic CLA in order to improve the understanding of this kind of disease. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1252/rc).
Methods
Patients
Between January 2017 and December 2020, the clinical and imaging data of 119 patients diagnosed with thoracic CLA were retrospectively reviewed at Beijing Shijitan Hospital, Capital Medical University (Peking University Ninth School of Clinical Medicine). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Ethics Board of Beijing Shijitan Hospital (No. IIT2024-059-001) and the requirement for individual consent for this retrospective analysis was waived. A total of 67 patients with GLA, 21 patients with GSD, and 31 patients with CCLA were evaluated.
The inclusion criteria were as follows: (I) thoracic CLA diagnosed by a combination of clinical and radiological findings; GLA refers to diffuse lymphatic vessel abnormalities in multiple organs or tissues. The grouping criteria were as follows: patients with two or more lymphatic malformations were defined as GLA group A. Patients with GSD with extensive and continuous lymphatic lesions involving cortical bone were allocated to group B. Those whose central lymphatic vessels (including the thoracic duct and cisterna) were significantly tortuous and dilated comprised group C. (II) Conventional CT and computed tomography lymphangiography (CTL) were performed.
The exclusion criteria were as follows: (I) common (cystic) lymphatic malformations; (II) lymphatic abnormalities caused by secondary factors (surgery, tumor, infection, and trauma); (III) no chest involvement; and (IV) without CTL and incomplete clinical data (Figure 1).
Of the 119 patients, 47 were male and 72 were female. The mean age at onset was 26 years, and the median age was 25 years. The clinical manifestations of cases are shown in Table 1. Thoracic duct outlet exploration was performed in 93 of 119 patients. This procedure involves releasing the thoracic duct if adhesion is found during exploration.
Table 1
| Characteristic or symptom | Group A (n=67) | Group B (n=21) | Group C (n=31) | P value |
|---|---|---|---|---|
| Male:female | 25:42 | 9:12 | 13:18 | 0.856 |
| Age (years) | 30.5 [30]† | 12 [17]† | 24 [22] | 0.005 |
| General symptoms | ||||
| Coagulopathy | 52 (77.6) | 17 (81.0) | 25 (80.6) | 0.953 |
| High D-dimer | 38 (56.7) | 16 (76.2) | 20 (64.5) | 0.262 |
| Thrombocytopenia | 3 (4.5) | 1 (4.8) | 5 (16.1) | 0.143 |
| Hypofibrinogenemia | 33 (49.3) | 9 (42.9) | 17 (54.8) | 0.696 |
| Fever | 10 (14.9) | 2 (9.5) | 4 (12.9) | 0.933 |
| Primary limb swelling | 9 (13.4)‡ | 5 (23.8) | 13 (41.9)‡ | 0.009 |
| Respiratory symptom | ||||
| Cough | 18 (26.9) | 1 (4.8) | 5 (16.1) | 0.070 |
| Chest tightness | 30 (44.8) | 11 (52.4) | 10 (32.3) | 0.317 |
| Chest pain | 3 (4.5) | 4 (19.0) | 1 (3.2) | 0.071 |
| Abdominal symptoms | ||||
| Abdominal distention | 8 (11.9) | 2 (9.5) | 6 (19.4) | 0.573 |
| Diarrhea | 1 (1.5)‡ | 1 (4.8) | 8 (25.8)‡ | <0.001 |
| Abdominal pain | 5 (7.5) | 3 (14.3) | 2 (6.5) | 0.594 |
Categorical variables are presented as n (%) or n. Continuous variables are presented as median [interquartile range]. The same superscript symbols represent a statistically significant difference. †, group A was compared with group B, P<0.0167; ‡, group A was compared with group C, P<0.0167. CLA, complex lymphatic anomaly.
Conventional CT and CTL
Conventional chest CT
All cases were examined by Siemens SOMATOM Sensation 16 (Somatom Sensation Cardiac 16, Siemens Healthineers, Erlangen, Germany) or Philips iCT (Brilliance iCT, Philips Healthcare, Best, the Netherlands). The CT scanning range was from the level of the inferior border of the thyroid cartilage in the neck to the base of the lung, and scan parameters were set as follows: tube voltage of 80–120 kV, tube current of 250–300 mA, and pitch of 1. After scanning, the raw data were transferred to the workstation for thin-layer reconstruction with a layer thickness of 2 mm and a layer spacing of 2 mm. After scanning, the raw data were transferred to the CT postprocessing workstation for postprocessing reconstruction, such as multiplanar reformation (MPR), maximum intensity projection (MIP), and volume rendering (VR).
CTL
The direct lymphangiography (DLG) was performed using a GE Innova 2000-IQ DSA machine (AXIOM; Siemens Healthineers). The skin and subcutaneous area between the 1st ~3rd toes of the relatively healthy side of the foot were punctured, 2 mL of methylene blue stain (2.5% Patent Blue V dye; 1 mL Guerbet Laboratories, Aulnay-sous-Bois, France) mixed with 2% lidocaine 1:1 was injected. Ultra-fluid iodized oil (Lipiodol UF, Guerbet, France) was injected with a fine needle puncture of 8–20 mL, when superficial lymphatic vessels were found under microscope. The DLG was terminated when (I) the contrast agent could flow into the blood through the venous angular gyrus when the patient is calm or breathing deeply, and (II) lipiodol could not enter the thoracic duct but was visible in the peripheral blood vessels. In this case, due to the lack of hydrostatic pressure from the large vessels of the jugular vein, the lipiodol entered the bloodstream rapidly, and excessive amounts can lead to pulmonary embolism, requiring the operation to be stopped.
All cases were examined by unenhanced chest CT scans for 30 minutes to 2 hours on a Siemens SOMATOM Sensation Cardiac 16 or Philips iCT. The CT scanning range was from the level of the inferior border of the thyroid cartilage in the neck to the base of lung, and scan parameters were set as follows: tube voltage of 80–120 kV, tube current of 250–300 mA, and pitch of 1. After scanning, the raw data were transferred to the workstation for thin-layer reconstruction with a layer thickness of 2 mm and a layer spacing of 2 mm. After scanning, the raw data were transferred to the CT postprocessing workstation for postprocessing reconstruction, such as MPR, MIP, and VR.
Image analysis
Two radiologists with more than 5 years of experience evaluated the CT imaging features. A consensus was reached under the guidance of a senior physician in cases of inconsistent observations.
Conventional chest CT
- Lesion location: (i) chest: lung, mediastinum, axilla; (ii) abdomen and pelvis: abdominopelvic wall, abdominopelvic cavity, retroperitoneum, spleen; (iii) bone; (iv) chylothorax, chylous ascites, and chylopericardium.
- CT types of thoracic CLA: (i) cystic type: single or multiple cystic lesions with thin-walled, low density fluid, different sizes, round, round-like, or irregular shape; (ii) cystic-solid type: single or multiple lesions with heterogeneous density and visible solid components; (iii) diffuse swelling type: diffuse tortuous tubular appearance, diffuse lymphatic hyperplasia, with or without mass effect; (iv) mixed type: combining two or more types.
- Abnormal CT findings in the lung: (i) ground-glass opacity (GGO): parenchymal GGO refers to diffuse GGO caused by aspiration or lymph infusion, which can be divided into acinar GGO, central GGO, and patchy GGO. Interstitial GGO refers to GGO caused by uneven thickening of alveolar wall due to interstitial lymphatic reflux disorder, which is mostly distributed in the periphery of the lung. (ii) Peripheral interstitial changes: thickening of the interlobular septum, thickening of the intralobular interstitium, and mixed changes. (iii) Axial interstitial changes: thickening of the bronchovascular bundle, thickening of the lobular nucleus, and mixed changes (14). (iv) Lung consolidation: localized lung consolidation is a patchy, non-characteristic consolidation caused by the backflow of chylous fluid into the alveoli. Central lung consolidation is a central consolidation of both lungs caused by the growth and spread of mediastinal lymphatic tissue proliferation to the bilateral hilum. Compression lung consolidation refers to the compression of adjacent lung tissue by pleural effusion and pericardial effusion. (v) Pulmonary nodules: the cystic type is a round or round-like low-density fluid nodule. The diameter ranges from 5 to 10 mm. The acinar pulmonary nodules are diffuse and multiple, about 5 to 10 mm in diameter, with blurred margins. The tree-in-bud nodules are located in the peripheral or the oblique fissure, with multiple clusters of tiny nodules, less than 3 mm in diameter. (vi) Other signs: a) Frog-spawn sign refers to the diffuse distribution of multiple small nodules in the background of poor lung transmittance. It is so named because it resembles frog spawn in jelly-like mucus. b) The crazy paving sign.
- Skeletal changes: (i) involved location: spine, ribs, upper limb bones (sternum, clavicle, scapula, and humerus), pelvic bones (ilium, ischium, and pubis), and lower limb bones (femur, tibia, and fibula). (ii) cystic changes: single or multiple round-like cystic hypodense shadows in bone. Single or multiple round cystic low-density shadows in bone with clear borders, with or without hardened edges, and maximum diameter <5 cm. Canal-like or honeycomb-like changes: tortuous tube-shaped low-density shadows with clear edges and continuous distribution at multiple levels. The bone cortex was smooth or irregularly fractured in a worm-like pattern. Osteoporosis-like changes: diffuse decrease in bone density and thinning of bone trabeculae. Osteosclerosis-like changes: the cortical bone was smooth and thickened, with or without an increase in the density of cancellous bone in the medullary cavity. Mixed changes: Two or more types were combined. (iii) Complications: soft tissue hyperplasia, thoracic or pelvic collapse. Soft tissue hyperplasia is defined as soft tissue density shadow in the area of abnormal bone density, and thoracic or pelvic collapse refers to abnormal shape of the chest and pelvis.
CTL
Abnormal distribution location of lipiodol: end of the thoracic duct, end of the right lymphatic duct, peribronchovascular bundle, hilum, mediastinum (anterior mediastinum, middle mediastinum, posterior mediastinum), pericardium, pleura, axilla, bowel or mesentery, renal pelvis, retroperitoneum, perineum, abdominopelvic wall, and abdominopelvic cavity. If lipiodol is deposited in an area other than the central lymphatic vessel, lymphatic reflux is considered; if the lipiodol deposits appear as clumps or beads, lymphangiectasia is considered.
Statistical analysis
The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used. Measurement data conforming to normal distribution were expressed as mean ± standard deviation, and differences between groups were analyzed by analysis of variance (ANOVA). Measurement data that did not meet the normal distribution were expressed as median (interquartile range), and the difference between groups was analyzed by rank sum test. The age of the patients in this study was continuous data and did not conform to the normal distribution, and the rank sum test was used for statistical analysis. Either the chi-square test or Fisher’s exact test was used to compare the clinical and imaging differences among the three groups, with P<0.05 considered statistically significant. Bonferroni test was used for pairwise comparison, with P<0.0167 considered statistically significant.
Results
Patients’ clinical data
There was a significant difference in age among the three groups (P=0.005), and patients in group B were the youngest. There were more patients with limb swelling (P=0.009) and diarrhea (P<0.001) in group C than there were in group A. No significant differences between the 3 groups were observed for gender, high D-dimer, thrombocytopenia, hypofibrinogenemia, fever, cough, chest tightness, chest pain, abdominal distention, and abdominal pain.
CT signs
The differences between the 3 groups were statistically significant for involvement of the lungs (P=0.004), mediastinum (P=0.026), abdominopelvic wall (P=0.014), spleen (P=0.020), and bone (P<0.001). The lung involvement in group A was more than that in group B (P=0.011) and group C (P=0.016). The mediastinum involvement in group A was more than that in group B (P=0.007). The involvement of the abdominopelvic wall in group B was more than that in group A (P=0.011), and the involvement of the spleen in group B was more than that in group C (P=0.006), and the difference was statistically significant. In addition, all patients in group B had bone involvement, so the differences were statistically significant when compared with group A and group C (P<0.001) (Table 2, Figure 2). There were significant differences in the types of cystic (P=0.001) and diffuse swelling (P=0.023) among the 3 groups. The cystic changes in group A were more than those in group B (P=0.014) and group C (P=0.002), and the difference was statistically significant. The changes of diffuse swelling type in group C were more than those in group A (P=0.006), and the difference was statistically significant (Table 3).
Table 2
| Lesion locations | Group A (n=67) | Group B (n=21) | Group C (n=31) | P value |
|---|---|---|---|---|
| Lung | 63 (94.0)†‡ | 15 (71.4)† | 23 (74.2)‡ | 0.004 |
| Mediastinum | 50 (74.6)† | 9 (42.9)† | 21 (67.7) | 0.026 |
| Axilla | 13 (19.4) | 2 (9.5) | 2 (6.5) | 0.225 |
| Abdominopelvic wall | 4 (6.0)† | 6 (28.6)† | 2 (6.5) | 0.014 |
| Abdominopelvic cavity | 12 (17.9) | 6 (28.6) | 7 (22.6) | 0.527 |
| Retroperitoneum | 13 (19.4) | 5 (23.8) | 9 (29.0) | 0.549 |
| Spleen | 23 (34.3) | 10 (47.6)§ | 4 (12.9)§ | 0.020 |
| Bone | 30 (44.8)†‡ | 21 (100)†§ | 3 (9.7)§‡ | <0.001 |
| Chylothorax | 33 (49.3) | 14 (66.7) | 11 (35.5) | 0.087 |
| Chylopericardium | 9 (13.4) | 1 (4.8) | 3 (9.7) | 0.619 |
| Chylous ascites | 11 (16.4) | 3 (14.3) | 10 (32.3) | 0.165 |
Data are presented as n (%). The same superscript symbols represent a statistically significant difference. †, group A was compared with group B, P<0.0167; ‡, group A was compared with group C, P<0.0167; §, group B was compared with group C, P<0.0167.
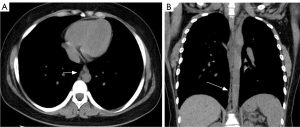
Table 3
| Lesion types | Group A (n=67) | Group B (n=21) | Group C (n=31) | P value |
|---|---|---|---|---|
| Cystic type | 33 (49.3)†‡ | 4 (19.0)† | 5 (16.1)‡ | 0.001 |
| Cystic-solid type | 4 (6.0) | 0 (0.0) | 0 (0.0) | – |
| Diffuse swelling type | 9 (13.4)‡ | 5 (23.8) | 8 (25.8)‡ | 0.023 |
| Mixed type | 2 (3.0) | 0 (0.0) | 1 (3.2) | >0.999 |
Data are presented as n (%). The same superscript symbols represent a statistically significant difference. †, group A was compared with group B, P<0.0167; ‡, group A was compared with group C, P<0.0167.
There were significant differences in GGO (P=0.011) (Figure 3), peripheral interstitial changes (P=0.013), compression pulmonary consolidation (P=0.012) (Figure 4), and cystic pulmonary nodules (P=0.017) (Figure 5) among the 3 groups. The GGO (P=0.003) and peripheral interstitial changes (P=0.016) in group A were more than those in group B. Cystic pulmonary nodules (P=0.014) and compression pulmonary consolidation (P=0.012) in group A were more than those in group C, and the difference was statistically significant (Table 4).
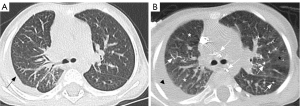
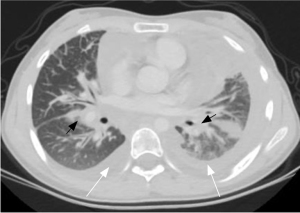
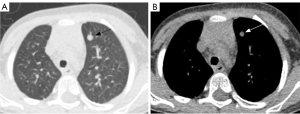
Table 4
| CT features of lung | Group A (n=67) | Group B (n=21) | Group C (n=31) | P value |
|---|---|---|---|---|
| GGO | 44/67 (65.7)† | 6/21 (28.6)† | 17/31 (54.8) | 0.011 |
| Parenchymal GGO | 15/44 (34.1) | 2/6 (33.3) | 5/17 (29.4) | 0.385 |
| Acinar GGO | 5/15 (33.3) | 1/2 (50.0) | 1/5 (20.0) | 0.866 |
| Central GGO | 7/15 (46.7) | 1/2 (50.0) | 2/5 (40.0) | 0.734 |
| Patchy GGO | 3/15 (20.0) | 0/2 (0.0) | 2/5 (40.0) | 0.699 |
| Interstitial GGO | 29/44 (65.9) | 4/6 (66.7) | 12/17 (70.6) | 0.135 |
| Peripheral interstitial changes | 25/67 (37.3)† | 2/21 (9.5)† | 5/31 (16.1) | 0.013 |
| Thickening of the interlobular septum | 20/25 (80.0) | 2/2 (100.0) | 5/5 (100.0) | 0.091 |
| Thickening of the intralobular interstitium | 2/25 (8.0) | 0/2 (0.0) | 0/5 (0.0) | - |
| Mixed changes | 3/25 (12.0) | 0/2 (0.0) | 0/5 (0.0) | - |
| Axial interstitial changes | 29/67 (43.3) | 7/21 (33.3) | 11/31 (35.5) | 0.624 |
| Thickening of the bronchovascular bundle | 16/29 (55.2) | 6/7 (85.7) | 6/11 (54.5) | 0.740 |
| Thickening of the lobular nucleus | 1/29 (3.4) | 0/7 (0.0) | 1/11 (9.1) | 0.685 |
| Mixed changes | 12/29 (41.4) | 1/7 (14.3) | 4/11 (36.4) | 0.424 |
| Lung consolidation | 38/67 (56.7)‡ | 12/21 (57.1) | 8/31 (25.8)‡ | 0.012 |
| Localized | 2/38 (5.3) | 1/12 (8.3) | 2/8 (25.0) | 0.577 |
| Central | 8/38 (21.1) | 1/12 (8.3) | 1/8 (12.5) | 0.394 |
| Compression | 28/38 (73.7)‡ | 10/12 (83.3) | 5/8 (62.5)‡ | 0.023 |
| Pulmonary nodules | 29/67 (43.3) | 5/21 (23.8) | 9/31 (29.0) | 0.291 |
| Cystic type | 19/29 (65.5)‡ | 2/5 (40.0) | 2/9 (22.2)‡ | 0.017 |
| Acinar type | 5/29 (17.2) | 1/5 (20.0) | 1/9 (11.1) | 0.866 |
| Tree-bud-like type | 5/29 (17.2) | 2/5 (40.0) | 6/9 (66.7) | 0.220 |
| Other signs | 9/67 (13.4) | 2/21 (9.5) | 6/31 (19.4) | 0.593 |
| Frog-spawn sign | 5/9 (55.6) | 2/2 (100.0) | 6/6 (100.0) | 0.220 |
| Crazy paving sign | 4/9 (44.4) | 0/2 (0.0) | 0/6 (0.0) | – |
Data are presented as n (%). The same superscript symbols represent a statistically significant difference. †, group A was compared with group B, P<0.0167; ‡, group A was compared with group C, P<0.0167. CT, computed tomography; GGO, ground-glass opacity.
Bone was involved in 30 of 67 patients in group A and all 21 patients in group B. One patient in group B had skull involvement (Figure 6). No skull involvement was found in group A. In group A and group B, the spine was the most involved, followed by pelvic bones, limb bones (including upper limb bones and lower limb bones), and ribs. The bone involvement in group B was more than that in group A, and the difference was statistically significant. In group A, the cystic type was the most common type (P=0.008) (Figure 7). In group B, mixed type was the most common type (P<0.001). In addition, compared with group A, group B had more soft tissue hyperplasia around the bone lesion (P<0.001) (Figure 8) and the thoracic or pelvic collapse (P=0.017) (Table 5).
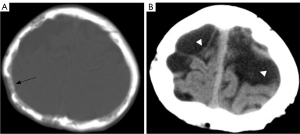
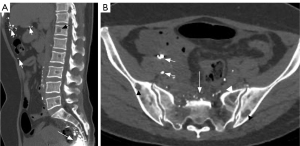
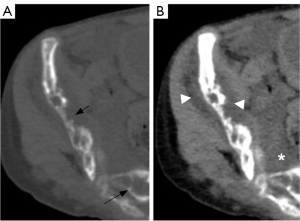
Table 5
| Abnormalities | Group A (n=67) | Group B (n=21) | P value |
|---|---|---|---|
| Lesion location | |||
| Spine | 25 (37.3) | 20 (95.2) | 0.001 |
| Rib | 8 (11.9) | 12 (57.1) | <0.001 |
| Upper limb bones | 13 (19.4) | 11 (52.4) | 0.003 |
| Pelvic bones | 25 (37.3) | 17 (81.0) | <0.001 |
| Lower limb bones | 13 (19.4) | 13 (61.9) | <0.001 |
| Lesion type | |||
| Cystic changes | 23 (34.3) | 1 (4.8) | 0.008 |
| Canal-like changes | 0 (0.0) | 1 (4.8) | 0.239 |
| Osteoporosis-like changes | 2 (3.0) | 1 (4.8) | 0.563 |
| Osteosclerosis-like changes | 0 (0.0) | 1 (4.8) | 0.239 |
| Mixed changes | 5 (7.5) | 17 (81.0) | <0.001 |
| Complications | |||
| Soft tissue hyperplasia | 7 (10.4) | 14 (66.7) | <0.001 |
| Thoracic or pelvic collapse | 3 (4.5) | 5 (23.8) | 0.017 |
Data are presented as n (%). P value level of significance for pairwise comparisons =0.05.
CTL signs
Abnormal deposition sites of lipiodol in 3 groups (Table 6, Figure 3, and Figure 7)
Table 6
| Location | Group A (n=67) | Group B (n=21) | Group C (n=31) | P value |
|---|---|---|---|---|
| The end of the thoracic duct | 49 (73.1) | 11 (52.4) | 16 (51.6) | 0.058 |
| The end of the right lymphatic duct | 8 (11.9) | 4 (19.0) | 6 (19.4) | 0.528 |
| Bronchovascular bundle | 3 (4.5) | 1 (4.8) | 4 (12.9) | 0.357 |
| Hilus of lung | 17 (25.4) | 5 (23.8) | 7 (22.6) | 0.957 |
| Lung | 8 (11.9) | 1 (4.8) | 2 (6.5) | 0.629 |
| Mediastinum | 35 (52.2) | 16 (76.2) | 15 (48.4) | 0.102 |
| Anterior mediastinum | 27 (40.3) | 6 (28.6) | 13 (41.9) | 0.572 |
| Middle mediastinum | 28 (41.8) | 5 (23.8) | 13 (41.9) | 0.306 |
| Posterior mediastinum | 19 (28.4) | 5 (23.8) | 13 (41.9) | 0.293 |
| Pericardium | 13 (19.4) | 3 (14.3) | 6 (19.4) | 0.861 |
| Pleura | 28 (41.8) | 9 (42.9) | 9 (29.0) | 0.439 |
| Axilla | 6 (9.0) | 1 (4.8) | 3 (9.7) | 0.908 |
| Intestine or mesentery | 3 (4.5)† | 1 (4.8) | 7 (22.6)† | 0.020 |
| Renal pelvis | 3 (4.5) | 3 (14.3) | 5 (16.1) | 0.103 |
| Retroperitoneum | 13 (19.4) | 8 (38.1) | 9 (29.0) | 0.193 |
| Perineum | 5 (7.5) | 3 (14.3) | 2 (6.5) | 0.594 |
| Abdominopelvic wall | 6 (9.0) | 3 (14.3) | 3 (9.7) | 0.712 |
| Abdominopelvic cavity | 15 (22.4) | 7 (33.3) | 4 (12.9) | 0.214 |
Data are presented as n (%). The same superscript symbols represent a statistically significant difference. †, group A was compared with group C, P<0.0167.
In 8 of the patients across the 3 groups, direct lymphography revealed that the lipiodol did not continue up the lymphatic vessels from the 10th thoracic vertebra to the 4th lumbar vertebra. The abnormal deposition of lipiodol was most common in the end of the thoracic duct in the 3 groups (P=0.058). The abnormal deposition of lipiodol in the intestine or mesentery of the 3 groups was statistically significant (P=0.020). After pairwise comparison, it was found that group C was more common than group A, and the difference was statistically significant (P=0.010).
Discussion
In this study, we compared and analyzed the clinical and imaging data of 67 patients with GLA, 21 patients with GSD, and 31 patients with CCLA to investigate the differences in clinical and imaging findings in different types of thoracic CLA. The lungs, mediastinum, and bone were the most frequently involved sites across the 3 groups of patients. There were differences among the 3 groups in the age, clinical symptoms, types of lung lesions, morphology of bone lesions, and surrounding soft tissue hyperplasia. In addition, CTL can indirectly reflect the dilatation and reflux of lymphatic vessels, which is an important reference value for the diagnosis and evaluation of CLA.
According to the literature (10,12), CLA can occur at all ages, with a predominance among neonates, children, and adolescents. There is no significant difference in the incidence between males and females. In this study, there were 47 males and 72 females, with slightly more females than males. The median age was 25 years, but the disease occurred across a wide range of ages and was not limited to children, which differs from previous reports. There was no statistically significant difference between the 3 groups in terms of gender, and the age was younger in group B than in groups A and C, unlike previous literature which reported a younger age in group C (14). The difference in chest symptoms between the 3 groups was not statistically significant. However, patients in group C had significantly more limb swelling and diarrhea symptoms than the other two groups, which was consistent with the literature (10,15,16).
CLA can occur in any part of the body. However, chest involvement usually means a poor prognosis, which can lead to respiratory failure and death in severe cases (12,13). The reported abnormal pulmonary imaging findings of CLA include GGO and diffuse interstitial thickening (5). In this study, the lung was involved in all 3 groups. The lung involvement in group A was more than that in groups B and C. There are many types of GGO, among which interstitial GGO is the most common. In 44 patients in this study, “cloudy” interstitial GGO was seen, with clear or blurred margins. They were diffusely distributed in one or both lungs. This is not the same as a substantial GGO caused by the filling of the alveolar or glandular cavity. We speculate that interstitial GGO is a specific manifestation of CLA. It may be caused by pulmonary interstitial abnormalities, such as alveolar wall interstitial thickening and lymphangiectasia. In addition, 27 of the 44 patients in this study who presented with interstitial GGO had a transient change, presenting with this sign after DLG. We speculate that the imaging findings of interstitial GGO are the result of a combination of increased lymph flow and lymphatic reflux disorder. The increased lymphatic flow, lymphangiectasis, and uneven thickening of the alveolar wall lead to interstitial GGO, which is consistent with the views of Itkin et al. and Nitschké et al. (17,18). DLG results in a transient increase in lymphatic flow. In the presence of large molecules of lipiodol, the increased lymphatic hydrostatic pressure leads to an increased degree of impaired reflux. When the factors are relieved, the lymphatic flow gradually returns to its preoperative homeostasis. At this time, the range and density of interstitial GGO in the lung decrease or disappear.
In this study, lung consolidation was the second most common sign after GGO, of which compression was the most common type. Compression consolidation was most common in group A. We speculated that this is due to the fact that patients in group A had more pleural and pericardial effusions than those in the other 2 groups. Secondly, central consolidation was also common, and there was no significant difference among the 3 groups. It presented as diffuse soft tissue in the mediastinum infiltrating the lung along the bilateral bronchovascular bundles. The consolidation extended radially from the hilum to the periphery of the lung, which was consistent with the literature (8). In addition, cystic lung nodules have not been reported in the literature. There were differences between the 3 groups, among which patients in group A were the most commonly affected by cystic lung nodules. We speculated that the nodules may be caused by abnormal enlargement of the lymphatic capillaries or the precollector lymphatic vessels, resulting in lymph retention. These lung nodules were all less than 1 cm in diameter and were considered related to gene regulation. It has been reported that the size of the cyst in patients with CLA is related to the gene expression profile in the PI3K-AKT-MTOR pathway (19). In addition, Aviv (20) found that dilated lymphatic vessels extending from the visceral pleura along the interlobular septum and pulmonary veins to the hilum could be observed in patients with lymphatic interstitial changes, which may be the pathological basis of pulmonary interstitial changes in CLA. In this study, peripheral interstitial changes (such as thickening of the interlobular septum and intralobular interstitium) and central interstitial changes (such as thickening of the bronchovascular bundle and centrilobular intestitium) were observed, which was consistent with previous literature (21).
In this study, chest CLA patients mostly involved the bone. A total of 30 of 67 group A patients had skeletal involvement. All patients in group B had bone involvement. Whether patients in group C will have bone involvement is currently controversial (2,12). There were 3 patients in group C who had skeletal involvement. The skeletal involvement is characterized by round hypodensity with well-defined margins in the spine, ribs, pelvic bones, and upper limb bones. The lesions were located only in the cancellous bone and did not involve the cortical bone. According to Lala et al., Steiner, and Winterberger (22-24), CLA-associated bone lesions can occur in all bones, especially in the tibia, humerus, ilium, and spine, and rarely in the skull. The most common sites of involvement in groups A and B were the spine, followed by the pelvic bones, the appendicular bones, and the ribs. There were more patients in group B than in group A, and the difference was statistically significant. In addition, the skull was involved in one patient in group B. It presented as multiple cystic hypodense lesions in the frontal, parietal, occipital, and clivus. Multiple cystic foci were seen bilaterally in the frontal, parietal subdural, bilateral posterior horn of lateral ventricles, and bilateral orbit. The common sites of involvement in this study were slightly different from previous literature. It may be that the imaging scan is mainly confined to the chest. In this study, osteolysis was classified into cystic type, canal-like type, osteoporosis-like type, osteosclerosis-like type, and mixed types according to the morphology of osteolytic changes. The most common type in group A was cystic type. In group B, the mixed type and canal-like type were more common. In addition, there was more periskeletal soft tissue hyperplasia and bone deformity in group B than in group A, and the difference was statistically significant. The above findings are consistent with previous reports in the literature (2,10).
The cystic type was the most common type of abnormal lymphatic morphology in group A. In group C, the diffuse swelling type was the most common. This result is related to the definition and formation mechanism of the disease. Group A is characterized by multifocal lymphatic abnormalities, including microcystic and macrocystic lymphatic malformations in multiple locations. Therefore, the cystic type was more common in group A. In group C, central lymphatic vessels (thoracic duct or cisterns) were dysfunctional, resulting in lymphatic flow disorders and multiple collateral branches. Therefore, patients in group C were mainly of diffuse swelling type.
CTL is a method for CT examination at a certain time after DLG (25). It has a high density resolution and powerful image post-processing function, which can clearly show the abnormal deposition and distribution of lipiodol. It can judge and speculate the tortuosity and dilation of lymphatic vessels and lymph reflux, which has an important reference value for the diagnosis and evaluation of lymphatic lesions. For patients with CLA, when the lipiodol shows abnormal morphology such as plaque or mass, it suggests that the lymphatic vessels are tortuous and dilated. In addition, the lipiodol injected in lymphatic vessels by direct lymphangiography normally flows along the ipsilateral iliac lymphatic vessels, the lumbar trunk, and the lymph in the cisterna into the thoracic duct and then into the venous circulation. Therefore, the presence of lipiodol at any other site, such as the end of the thoracic duct, axilla, mediastinum, renal pelvis, and mesentery, can be defined as lymphatic reflux. In this study, CTL was used to diagnose patients with thoracic CLA. It was found that abnormal deposition of lipiodol in the end of the thoracic duct was the most common. In addition, 93 of 119 patients underwent the exploratory operation of the thoracic duct outlet. Surgery confirmed that all patients had terminal thoracic duct obstruction. In combination with conventional chest CT, abnormal deposition of lipiodol, and surgical findings, we speculated that one of the reasons for the abnormal CT findings in thoracic CLA may be the obstruction of the terminal thoracic duct. In addition, abnormal deposition of lipiodol in the bowel or mesentery was more prevalent in group C. Patients in group C had central lymphatic system dilation and dyskinesia. Abnormal lymphatic drainage may lead to abnormal lymph reflux in protein-losing enteropathy. When the lymphatic vessels of the intestinal wall dilate and rupture, lymph can leak from the intestinal wall into the intestinal cavity or abdominal cavity. At this point, lipiodol appears at the corresponding sites, as reported in the literature (26).
The limitations of this study are as follows: (I) this study is a retrospective study and the disease is rare, so there may have been selection bias; (II) this study lacked a normal control group; (III) only conventional CT and CTL were used in this study, and we will explore and study a variety of imaging methods in the future.
Conclusions
In conclusion, thoracic CLA is a lymphatic system disease involving multiple sites and organs, and its clinical and imaging manifestations are diverse. Different types of thoracic CLA have different clinical and imaging manifestations. Lung, mediastinum, and bone are the most frequently involved sites. In addition, the patients were different in age, limb swelling, diarrhea, lesion location, abnormal manifestations of lungs, morphology of bone lesions, surrounding soft tissue hyperplasia, and lesion morphology. Conventional chest CT and CTL can provide imaging information for the diagnosis of different types of patients, which is helpful for further accurate diagnosis and treatment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1252/rc
Funding: The work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1252/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by Institutional Ethics Board of Beijing Shijitan Hospital (No. IIT2024-059-001) and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Monroe EJ. Brief Description of ISSVA Classification for Radiologists. Tech Vasc Interv Radiol 2019;22:100628. [Crossref] [PubMed]
- Solorzano E, Alejo AL, Ball HC, Magoline J, Khalil Y, Kelly M, Safadi FF. Osteopathy in Complex Lymphatic Anomalies. Int J Mol Sci 2022;23:8258. [Crossref] [PubMed]
- Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The Lymphatic System: Integral Roles in Immunity. Annu Rev Immunol 2017;35:31-52. [Crossref] [PubMed]
- Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic Vessel Network Structure and Physiology. Compr Physiol 2018;9:207-99. [Crossref] [PubMed]
- Ozeki M, Fukao T. Generalized Lymphatic Anomaly and Gorham-Stout Disease: Overview and Recent Insights. Adv Wound Care (New Rochelle) 2019;8:230-45. [Crossref] [PubMed]
- Anthony MD, Swilling A, Jiwani ZM, Heym K, Margraf LR, Fierke S, Akers LJ, Ray A. Multidisciplinary Multiagent Treatment of Complex Lymphatic Anomalies with Severe Bone Disease: A Single-Site Experience. Lymphat Res Biol 2022;20:118-24. [Crossref] [PubMed]
- Ricci KW, Iacobas I. How we approach the diagnosis and management of complex lymphatic anomalies. Pediatr Blood Cancer 2022;69:e28985. [Crossref] [PubMed]
- Goyal P, Alomari AI, Kozakewich HP, Trenor CC 3rd, Perez-Atayde AR, Fishman SJ, Greene AK, Shaikh R, Chaudry G. Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol 2016;46:1282-90. [Crossref] [PubMed]
- Rossi M, Buonuomo PS, Battafarano G, Conforti A, Mariani E, Algeri M, Pelle S, D'Agostini M, Macchiaiolo M, De Vito R, Gonfiantini MV, Jenkner A, Rana I, Bartuli A, Del Fattore A. Dissecting the mechanisms of bone loss in Gorham-Stout disease. Bone 2020;130:115068. [Crossref] [PubMed]
- Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical Features and Prognosis of Generalized Lymphatic Anomaly, Kaposiform Lymphangiomatosis, and Gorham-Stout Disease. Pediatr Blood Cancer 2016;63:832-8. [Crossref] [PubMed]
- Perschbacher SE, Perschbacher KA, Pharoah MJ, Bradley G, Lee L, Yu E. Gorham's disease of the maxilla: a case report. Dentomaxillofac Radiol 2010;39:119-23. [Crossref] [PubMed]
- Mäkinen T, Boon LM, Vikkula M, Alitalo K. Lymphatic Malformations: Genetics, Mechanisms and Therapeutic Strategies. Circ Res 2021;129:136-54. [Crossref] [PubMed]
- Manevitz-Mendelson E, Leichner GS, Barel O, Davidi-Avrahami I, Ziv-Strasser L, Eyal E, Pessach I, Rimon U, Barzilai A, Hirshberg A, Chechekes K, Amariglio N, Rechavi G, Yaniv K, Greenberger S. Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis 2018;21:287-98. [Crossref] [PubMed]
- Passarello L, Lau C, McCahon E, Popat H. A neonatal case of central conducting lymphatic anomaly successfully treated with sirolimus. Pediatr Blood Cancer 2022;69:e29752. [Crossref] [PubMed]
- Byrne AB, Brouillard P, Sutton DL, Kazenwadel J, Montazaribarforoushi S, Secker GA, et al. Pathogenic variants in MDFIC cause recessive central conducting lymphatic anomaly with lymphedema. Sci Transl Med 2022;14:eabm4869. [Crossref] [PubMed]
- Taghinia AH, Upton J, Trenor CC 3rd, Alomari AI, Lillis AP, Shaikh R, Burrows PE, Fishman SJ. Lymphaticovenous bypass of the thoracic duct for the treatment of chylous leak in central conducting lymphatic anomalies. J Pediatr Surg 2019;54:562-8. [Crossref] [PubMed]
- Itkin M, Rabinowitz DA, Nadolski G, Stafler P, Mascarenhas L, Adams D. Abnormal Pulmonary Lymphatic Flow in Patients With Lymphatic Anomalies and Respiratory Compromise. Chest 2020;158:681-91. [Crossref] [PubMed]
- Nitschké M, Bell A, Karaman S, Amouzgar M, Rutkowski JM, Scherer PE, Alitalo K, McDonald DM. Retrograde Lymph Flow Leads to Chylothorax in Transgenic Mice with Lymphatic Malformations. Am J Pathol 2017;187:1984-97. [Crossref] [PubMed]
- Gomez-Acevedo H, Dornhoffer JR, Stone A, Dai Y, Richter GT. Gene Expression Differences in Pediatric Lymphatic Malformations: Size Really Matters. Lymphat Res Biol 2018;16:347-52. [Crossref] [PubMed]
- Aviv R, McHugh K. Mechanisms of chylous effusion in lymphangiomatosis. AJR Am J Roentgenol 2000;175:1191. [Crossref] [PubMed]
- Zhang Y, Sun X, Liu M, Li X, Zhang M, Duan Y, Wang R. Association between lymphatic abnormalities in the neck and thorax in primary chylopericardium and surgical outcomes evaluated by non-enhanced magnetic resonance (MR) lymphangiography. Quant Imaging Med Surg 2024;14:5961-72. [Crossref] [PubMed]
- Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly--clinical, radiologic, and histologic differentiation. Skeletal Radiol 2013;42:917-24. [Crossref] [PubMed]
- Steiner GM, Farman J, Lawson JP. Lymphangiomatosis of bone. Radiology 1969;93:1093-8. [Crossref] [PubMed]
- Winterberger AR. Radiographic diagnosis of lymphangiomatosis of bone. Radiology 1972;102:321-4. [Crossref] [PubMed]
- Sun X, Shen W, Xia S, Wen T, Wang R. Diffuse Pulmonary Lymphangiomatosis: MDCT Findings After Direct Lymphangiography. AJR Am J Roentgenol 2017;208:300-5. [Crossref] [PubMed]
- Clemens RK, Pfammatter T, Meier TO, Alomari AI, Amann-Vesti BR. Combined and complex vascular malformations. Vasa 2015;44:92-105. [Crossref] [PubMed]


