
Noninvasive assessment of glymphatic dysfunction and IDH mutation in glioma with DTI-ALPS and DTI metrics
Introduction
About 30% of all primary brain tumors and 80% of malignant brain tumors are gliomas, which are the most prevalent primary brain tumor and the leading cause of primary brain tumor death (1). Based on histological analysis, gliomas are typically divided into two categories: low-grade gliomas (LGG) and high-grade gliomas (HGG) (2). LGG patients have a better prognosis than HGG patients, with a median survival of 5.2 years (3). However, LGG heavily impacts patient health-related quality of life. Patients experience a broad range of symptoms, including cognitive impairment, seizures, and even recurrence or progression (4). In contrast, patients with HGG have a worse median survival of 9–10 months, primarily as a result of aggressive metastases and resistance to treatment (5). All gliomas need to be carefully assessed, closely watched, and treated according to the right protocol, regardless of the tumor grade or other characteristics.
The occurrence of glioma is closely related to neuro-immunity, which is affected by the tumor microenvironment (TME). The TME is a dynamic environment that is greatly impacted by alterations in cellular composition, cell-to-cell contact, and metabolic products produced by cells, in addition to other chemical elements (6). Numerous substances found in the TME, including transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), contribute to immunosuppression and immune evasion in order to facilitate tumor fast invasion, migration, and treatment resistance (7).
Before Louveau et al.’s discovery of meningeal lymphatic vessels (MLVs), the central nervous system (CNS) was thought to be an immune-privileged organ protected by the blood-brain barrier. These MLVs are linked to the deep cervical lymph nodes and can transport immunological cells from the cerebrospinal fluid (CSF) (8). Some studies have found that the MLV may play an important role in brain tumor immunology (6-10).
Apart from MLVs, researchers have discovered that the glymphatic system (GS), which is essentially a periarterial CSF inflow pathway and peripheral venous clearance pathway, is functional via aquaporin-4 (AQP4) water channels in astrocytic end-feet (11). The GS is the central nervous drainage system, which is important for excretion of the metabolic waste products in the brain. The connection between the GS and MLV drainage has been demonstrated in Aspelund et al.’s research (12). When paired with the MLVs, the GS-associated brain-wide pathway preserved tissue homeostasis, which is necessary for regular immune response and monitoring; this provides a plausible route to study the CNS-immune connection and can be a candidate strategy for treating glioma.
Magnetic resonance imaging (MRI) is a promising non-invasive technique in the exploration of gliomas (13), and there have been many reports about diffusion tensor imaging (DTI) in glioma grading and IDH mutation (14,15). The DTI-derived ALPS-index, which reflects the diffusivity along the perivascular spaces of medullary veins at the level of the lateral ventricle body, is thought to be a possible biomarker of glymphatic function (16,17). The DTI-ALPS index and cerebral glymphatic clearance as determined by glymphatic MRI were shown to be significantly correlated in a study that examined and compared glymphatic clearance function using the DTI-ALPS method and the traditional intrathecal injection of gadolinium (18). Specifically, a low ALPS-index represents a decline in GS function. The method has been applied to a range of neurological disorders, including Parkinson’s disease (19), Alzheimer’s disease (20), migraine (21), epilepsy (22), and so on. Initial research has also looked into alterations in GS function in glioma patients and animal models (23-26). However, there have been few studies examining the ALPS-index and DTI metrics simultaneously to reveal the pathological changes of the gliomas and alterations in the intracranial GS.
This study aimed to assess glioma-induced GS damage, investigate the utility of the ALPS-index and DTI metrics in evaluating IDH mutations and predicting glioma patient survival, and examine how tumor location affects ALPS-index and DTI metrics changes. We hypothesized that the GS function would be reduced in glioma patients compared to healthy controls (HC), and that both the ALPS-index and DTI metrics would be lower in HGG compared to LGG. Furthermore, when predicting IDH mutations, the combined diagnostic efficacy of the ALPS-index and other tensor metrics is superior to that of either index alone. Both the ALPS-index and DTI metrics can serve as prognostic markers influencing patient survival time. Additionally, the ALPS-index and DTI metrics may vary depending on glioma location. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2710/rc).
Methods
Participants
The demographic and imaging data of glioma patients were derived from the University of California San Francisco Preoperative Diffuse Glioma MRI (UCSF-PDGM) from the Cancer Imaging Archive (TCIA) database (https://www.cancerimagingarchive.net/collection/ucsf-pdgm/) (27) and HC were derived from volunteers from Consortium for Reliability and Reproducibility (CoRR) (28) (https://fcon1000.projects.nitrc.org/indi/CoRR/html/upsm1.html). Therefore, this study was exempt from ethical approval; the informed consent of the participants had already been obtained from the above public databases. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
The clinical data extracted included sex, age, tumor grade, tumor location, IDH mutation status, and survival time.
The inclusion criteria were as follows: (I) single glioma in the brain; (II) the tumor is limited to one cerebral hemisphere; and (III) patients who had not received chemotherapy or radiotherapy before the examination.
The exclusion criteria were as follows: (I) multiple tumors in the brain; (II) tumor extending across the midline; and (III) the lateral ventricle structure was severely damaged. A flowchart of participant inclusion is presented in Figure 1.
We randomly selected three sub-datasets of healthy volunteers from the CoRR, comprising a total of 61 cases: (I) XHCUMS datasets; (II) IPCAS datasets; and (III) NKI datasets.
MRI scanning
The patients from UCSF-PDGM datasets were performed using a 3-T scanner (Discovery 750, GE Healthcare, Waukesha, WI, USA) and a dedicated 8-channel head coil (Invivo, Gainesville, FL, USA). The MRI scanning parameters were as follows: T2: sagittal three-dimensional fast spin echo (3D-FSE) [repetition time (TR)/echo time (TE), 2,200/100 msec; section thickness, 1.2 mm; matrix, 256×256; field of view (FOV), 25.6 cm; the number of signals acquired, 1]; T2-fluid-attenuated inversion recovery (T2-FLAIR): coronal 3D-FSE (TR/TE/inversion time, 5,700/115/1,650 msec; section thickness, 1.2 mm; matrix, 256 ×256; FOV, 25.6 cm; the number of signals acquired, 1); DTI: axial echoplanar imaging (TR/TE, 8,400/73, msec; section thickness, 2 mm; matrix, 128×128; FOV, 28 cm; the number of signals acquired,1; b-value, 2,000 sec/mm2; 55 directions). The DTI scanning parameters of HC are listed in Table S1.
MRI data pre-processing
Two radiologists with more than five years of experience independently preprocessed the original image based on the DSI studio platform (2008–2024 Fang-Cheng Yeh). The specific steps were as follows: Step 1, we imported original images of DTI into the platform, the motion and eddy artifacts removed along the skull were corrected by the motion correction operation under the correction button; correction ensured that images were aligned with each other, reduced variation due to head movements, and improved data consistency and comparability. Step 2, we reconstructed the image and selected to generalized q-sampling imaging (29) with a diffusion sampling length ratio of 1.25 used to reconstruct an individual space to generate a color-coded FA map. Step 3, the regions of interest (ROI) were placed and DTI-derived tensor metrics and water diffusivity along the x, y, and z axes were calculated. Where considerable variability occurred in the outlined ROIs, final segmentation was based on consensus between the readers.
The ROI for DTI-ALPS analysis was placed on a transverse section at the level of the lateral ventricle body, approximately 2–5 mm in diameter (Figure 2A). In addition, the ROI for DTI-derived tensor metrics was placed on T2-FLAIR, carefully choosing the tumor’s solid component, and avoiding necrotic, cystic, and surrounding edema areas, with the diameter of 2–5 mm (Figure 2B). The DTI-derived tensor metrics were obtained including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD).
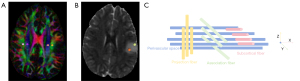
At the lateral ventricle level, the perivascular space and medullary veins are perpendicular to the projection (z-axis) and the association (y-axis) fibers. GS dysfunction accompanied by histological changes will affect projection and association fibers. Therefore, the diffusivity along the perivascular space direction can be represented by the partial diffusivity along the x-axis of the projection fiber (Dyyproj) and the association fiber (Dzzassoc) (Figure 2C). The calculation of the ALPS-index is based on the research of Taoka et al. (30). The ALPS-index is defined as follows:
Where Dxxproj and Dxxassoc represent diffusivity in the x-axis direction of the projection fiber and the association fiber, respectively; Dyyproj represents diffusivity in the y-axis direction of the projected fiber; Dzzassoc represents diffusivity in the z-axis direction of the association fiber.
Statistical analysis
The statistical analysis was conducted using SPSS 27.0 (IBM Corp., Armonk, NY, USA), and GraphPad Prism 10 (GraphPad Software, San Diego, CA, USA) was used for image visualization. To ensure the appropriateness of the statistical methods, we first assessed the distribution of continuous variables using the Kolmogorov-Smirnov test. Based on the normality test results, parametric tests were applied to normally distributed data, whereas non-parametric tests were used for data that violated the normality assumption. Interobserver reliability was evaluated using the intraclass correlation coefficient (ICC), which is a robust measure for assessing consistency between observers. For group comparisons, the paired t-test was used to compare the ALPS-index between cerebral hemispheres, as it is suitable for paired data with normal distributions. Analysis of covariance (ANCOVA) with age adjustment was employed to assess group differences in the ALPS-index and other tensor metrics, as it controls for potential confounding effects. The Kruskal-Wallis H test was applied for data that did not meet the assumptions of parametric tests, such as homogeneity of variance. To address multiple comparisons, the Bonferroni correction was used to reduce the risk of Type I errors. Receiver operating characteristic (ROC) curves were utilized to evaluate the diagnostic performance of the ALPS-index, DTI-derived tensor metrics, and their combined models. The area under the curve (AUC), sensitivity, specificity, and cutoff values were calculated to quantify their predictive accuracy. For survival analysis, the univariate Cox proportional hazards model was selected to assess the predictive value of the ALPS-index, DTI metrics, and clinical factors for overall survival (OS). OS was defined as the time interval from the start of diagnosis to the end of the follow-up period. Clinical factors included grade (LGG vs. HGG), age (median age <44 vs. ≥44 years), sex (female vs. male), and IDH mutation (wild type vs. mutant). ALPS-index (the cutoff value in ROC analysis <1.18 vs. ≥1.18), FA value (the cutoff value in ROC analysis <0.19 vs. ≥0.19), MD value (the cutoff value in ROC analysis <0.99 vs. ≥0.99), AD value (the cutoff value in ROC analysis <1.19 vs. ≥1.19), RD value (the cutoff value in ROC analysis <0.98 vs. ≥0.98). A multivariate Cox proportional hazards model was further performed. A P value <0.05 was considered significant. It should also be noted that no grade II or IV gliomas were observed in the subtentorium cerebelli; therefore, this area was excluded from tumor location analyses.
Results
Patient demographics
A total of 146 patients with glioma were included, including 53 patients with grade II, 43 patients with grade III, and 50 patients with grade IV. There were 46 IDH mutations in grade II, 29 IDH mutations in grade III, and 22 IDH mutations in grade IV. The tumor locations are listed in Table 1, and the frontal lobe was the most common site for tumor growth. The average OS was 31.92±21.97 months for grade II, 28.62±24.40 months for grade III, and 26.80±18.85 months for grade IV, respectively. The characteristics of the patients are shown in Table 1.
Table 1
| Characteristics | Grade II | Grade III | Grade IV | Healthy control |
|---|---|---|---|---|
| No. of patients | 53 | 43 | 50 | 61 |
| Age (years) | 40.42±13.16 | 46.61±13.72 | 46.68±12.40 | 46.28±13.41 |
| Gender | ||||
| Male | 29 | 26 | 36 | 41 |
| Female | 24 | 17 | 14 | 20 |
| Tumor location | ||||
| Frontal | 23 | 13 | 16 | – |
| Temporal | 11 | 9 | 18 | – |
| Parietal | 6 | 10 | 11 | – |
| Occipital | 0 | 0 | 3 | – |
| Basal | 13 | 10 | 2 | – |
| Subtentorium cerebelli | 0 | 1 | 0 | – |
| IDH mutation | ||||
| Wild type | 7 | 14 | 28 | – |
| Mutant | 46 | 29 | 22 | – |
| Overall survival (months) | 31.92±21.97 | 28.62±24.40 | 26.80±18.85 | – |
Categorical variables are shown as number. Continuous variables are shown as mean ± standard deviation. IDH, isocitrate dehydrogenase.
ALPS-index paired with t-test in both cerebral hemispheres
The ALPS-index of ipsilateral gliomas was significantly lower than that of contralateral gliomas, regardless of grade II (P=0.01) or grade III (P=0.03). However, in grade IV gliomas, the ALPS-index of ipsilateral gliomas was higher than contralateral gliomas (P=0.01). The ALPS-index of ipsilateral glioma with IDH mutation was significantly lower than that of the contralateral in grade II (P=0.01) or grade III (P=0.05). For grade IV gliomas, the ALPS-index of ipsilateral gliomas was higher than that of the contralateral gliomas with statistical significance (P=0.02). We did not find significant difference in the wild type. In grade II, the ALPS-index in the frontal/temporal/basal was significantly lower than that on the contralateral side (P<0.05). In grade III gliomas, the ALPS-index in the parietal was significantly lower than that on the contralateral side (P=0.01). Meanwhile in grade IV, the ALPS-index in the frontal was significantly higher than that on the contralateral side (P=0.04). The ALPS-index of ipsilateral glioma in alive patients was significantly lower than that of the contralateral in grade II (P=0.01) or grade III (P=0.04). In contrast, for grade IV gliomas, the ALPS-index of ipsilateral gliomas was higher than that of the contralateral gliomas, and there was statistical significance (P=0.01). However, there was no significant difference in death cases (P>0.05). The details are shown in Table 2 and Figure 3.
Table 2
| Characteristics | Grade II | Grade III | Grade IV | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | |||
| Tumor grade | 0.01 | 0.03 | 0.01 | |||||
| Ipsilateral to glioma | 1.31±0.18 | 1.30±0.22 | 1.34±0.19 | |||||
| Contralateral to glioma | 1.43±0.21 | 1.40±0.21 | 1.24±0.16 | |||||
| IDH mutation, mutant | 0.01 | 0.05 | 0.02 | |||||
| Ipsilateral to glioma | 1.33±0.18 | 1.30±0.22 | 1.37±0.17 | |||||
| Contralateral to glioma | 1.45±0.20 | 1.42±0.21 | 1.22±0.19 | |||||
| IDH mutation, wild type | 0.35 | 0.42 | 0.24 | |||||
| Ipsilateral to glioma | 1.20±0.10 | 1.30±0.23 | 1.32±0.21 | |||||
| Contralateral to glioma | 1.26±0.20 | 1.35±0.22 | 1.26±0.13 | |||||
| Tumor location, frontal | 0.02 | 0.08 | 0.04 | |||||
| Ipsilateral to glioma | 1.33±0.18 | 1.29±0.15 | 1.40±0.21 | |||||
| Contralateral to glioma | 1.45±0.24 | 1.39±0.18 | 1.25±0.13 | |||||
| Tumor location, temporal | 0.04 | 0.43 | 0.14 | |||||
| Ipsilateral to glioma | 1.30±0.20 | 1.37±0.18 | 1.31±0.18 | |||||
| Contralateral to glioma | 1.43±0.17 | 1.40±0.22 | 1.22±0.17 | |||||
| Tumor location, parietal | 0.39 | 0.01 | 0.97 | |||||
| Ipsilateral to glioma | 1.30±0.20 | 1.20±0.16 | 1.30±0.19 | |||||
| Contralateral to glioma | 1.38±0.30 | 1.38±0.15 | 1.30±0.17 | |||||
| Tumor location, occipital | – | – | 0.42 | |||||
| Ipsilateral to glioma | – | – | 1.34±0.27 | |||||
| Contralateral to glioma | – | – | 1.24±0.21 | |||||
| Tumor location, basal | 0.02 | 0.66 | 0.21 | |||||
| Ipsilateral to glioma | 1.30±0.17 | 1.32±0.35 | 1.35±0.08 | |||||
| Contralateral to glioma | 1.42±0.15 | 1.40±0.32 | 1.04±0.07 | |||||
| Survival, alive | 0.01 | 0.04 | 0.01 | |||||
| Ipsilateral to glioma | 1.32±0.18 | 1.30±0.23 | 1.37±0.17 | |||||
| Contralateral to glioma | 1.45±0.20 | 1.42±0.22 | 1.23±0.17 | |||||
| Survival, death | 0.83 | 0.59 | 0.33 | |||||
| Ipsilateral to glioma | 1.26±0.17 | 1.29±0.22 | 1.31±0.20 | |||||
| Contralateral to glioma | 1.23±0.25 | 1.34±0.20 | 1.24±0.15 | |||||
ALPS, analysis along the perivascular space; IDH, isocitrate dehydrogenase; SD, standard deviation.
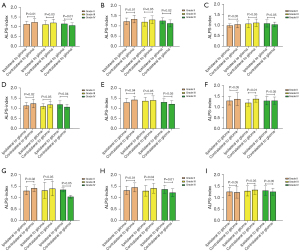
Group differences in the ALPS-index and other tensor metrics
Differences in patients and HC
The results showed a significant difference of ALPS-index in the ipsilateral gliomas between the patient group and the HC (1.31±0.20 vs. 1.56±0.22; P<0.01). Furthermore, the ALPS-index in the contralateral cerebral hemisphere of the glioma patients remained lower than that of the HC (1.35±0.21 vs. 1.58±0.22; P<0.01). There was no significant difference between right hemispheres and left hemispheres in the HC group (1.54±0.22 vs. 1.57±0.23; P=0.25) (Figure 4A and Table S2).
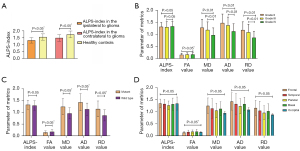
Differences in grade II, grade III, and grade IV
Our analysis found a difference in ALPS-index and other tensor metrics among the grade II, III, and IV patients. The results were as follows: ALPS-index (grade II 1.30±0.03 vs. grade III 1.31±0.03 vs. grade IV 1.35±0.03; P=0.43); FA values (grade II 0.16±0.06 vs. grade III 0.17±0.07 vs. grade IV 0.17±0.08; P=0.67); MD values (grade II 1.25±0.04 vs. grade III 1.20±0.05 vs. grade IV 1.01±0.04; P<0.01); AD values (grade II 1.45±0.05 vs. grade III 1.39±0.05 vs. grade IV 1.18±0.05; P<0.01); RD values (grade II 1.16±0.04 vs. grade III 1.10±0.05 vs. grade IV 0.93±0.04; P<0.01) (Figure 4B and Table S3).
Differences in IDH-mutant and wild type
The ALPS-index of the IDH-mutant was not significantly different from that of wild type (IDH-mutant 1.31±0.02 vs. wild type 1.33±0.03; P=0.60). Other tensor metrics were statistically significant; the results were as follows: FA values (IDH-mutant 0.15±0.06 vs. wild type 0.18±0.08; P<0.01); MD values (IDH-mutant 1.25±0.34 vs. wild type 0.96±0.24; P<0.01); AD values (IDH-mutant 1.43±0.36 vs. wild type 1.14±0.26; P<0.01); RD values (IDH-mutant 1.15±0.34 vs. wild type 0.88±0.24; P<0.01) (Figure 4C and Table S4).
Differences in tumor location
We did not find any statistical difference in tumor locations regarding ALPS-index and other tensor metrics. Table 3 and Figure 4D show the results: ALPS-index in frontal/temporal/parietal/occipital/basal of 1.33±0.03,1.33±0.03, 1.27±0.04, 1.32±0.04, and 1.37±0.11, respectively, P=0.72; FA values in frontal/temporal/parietal/occipital/basal of 0.14±0.05, 0.17±0.08, 0.18±0.07, 0.18±0.09, and 0.16±0.03, respectively, P=0.19; MD values in frontal/temporal/parietal/occipital/basal of 1.22±0.05, 1.15±0.05, 1.06±0.06, 1.14±0.07, and 1.01±0.19, respectively, P=0.37; AD values in frontal/temporal/parietal/occipital/basal of 1.39±0.05, 1.33±0.06, 1.25±0.07, 1.34±0.07, and 1.15±0.20, respectively, P=0.44; RD values in frontal/temporal/parietal/occipital/basal of 1.13±0.05, 1.06±0.05, 0.97±0.06, 1.04±0.06, and 0.93±0.19, respectively, P=0.34.
Table 3
| Characteristics | Frontal | Temporal | Parietal | Occipital | Basal | P value |
|---|---|---|---|---|---|---|
| ALPS-index | 1.33±0.03 | 1.33±0.03 | 1.27±0.04 | 1.32±0.04 | 1.37±0.11 | 0.72 |
| FA value | 0.14±0.05 | 0.17±0.08 | 0.18±0.07 | 0.18±0.09 | 0.16±0.03 | 0.19† |
| MD value | 1.22±0.05 | 1.15±0.05 | 1.06±0.06 | 1.14±0.07 | 1.01±0.19 | 0.37 |
| AD value | 1.39±0.05 | 1.33±0.06 | 1.25±0.07 | 1.34±0.07 | 1.15±0.20 | 0.44 |
| RD value | 1.13±0.05 | 1.06±0.05 | 0.97±0.06 | 1.04±0.06 | 0.93±0.19 | 0.34 |
Continuous variables are shown as mean ± standard deviation. †, Kruskal-Wallis H test was used for data that did not satisfy the association variance condition. AD, axial diffusivity; ALPS, analysis along the perivascular space; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity.
The ROC curves for metrics in predicting IDH1 mutation
The diagnostic efficacy of IDH mutation by different metrics of grade II
The ROC curve showed that MD had the highest diagnostic efficiency, with an AUC of 0.81. The AUC value of RD and AD was 0.80. The ALPS-index and FA diagnostic efficiency was relatively low, with AUCs of 0.72 and 0.76, respectively. In the combined diagnosis of ALPS value and other tensor metrics, AUC improved significantly. Table 4 shows the AUC results: ALPS-index + FA, 0.82; ALPS-index + MD, 0.88; ALPS-index + AD, 0.88; ALPS-index + RD, 0.89.
Table 4
| Grades | Parameter metric | AUC | Cutoff value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Grade II | ALPS-index | 0.72 | 1.35 | 56.50 | 100 |
| FA value | 0.76 | 0.16 | 85.70 | 69.60 | |
| MD value | 0.81 | 1.25 | 63.00 | 100 | |
| AD value | 0.80 | 1.26 | 76.10 | 85.70 | |
| RD value | 0.80 | 1.15 | 63.00 | 100 | |
| ALPS-index + FA | 0.82 | 0.15 | 69.60 | 85.70 | |
| ALPS-index + MD | 0.88 | 0.09 | 71.70 | 100 | |
| ALPS-index + AD | 0.88 | 0.10 | 76.10 | 100 | |
| ALPS-index + RD | 0.89 | 0.10 | 71.70 | 100 | |
| Grade III | ALPS-index | 0.49 | 0.22 | 72.40 | 50 |
| FA value | 0.37 | 0.06 | 20.70 | 85.70 | |
| MD value | 0.64 | 0.28 | 27.60 | 100 | |
| AD value | 0.65 | 0.27 | 41.40 | 85.70 | |
| RD value | 0.64 | 0.28 | 27.60 | 100 | |
| ALPS-index + FA | 0.61 | 0.27 | 7.10 | 92.90 | |
| ALPS-index + MD | 0.64 | 0.29 | 93.10 | 35.70 | |
| ALPS-index + AD | 0.65 | 0.29 | 79.30 | 50 | |
| ALPS-index + RD | 0.65 | 0.29 | 93.10 | 35.70 | |
| Grade IV | ALPS-index | 0.60 | 0.27 | 90.90 | 35.70 |
| FA value | 0.36 | 0.05 | 22.70 | 82.10 | |
| MD value | 0.70 | 0.43 | 62.80 | 75 | |
| AD value | 0.68 | 0.41 | 59.10 | 82.10 | |
| RD value | 0.71 | 0.40 | 68.20 | 71.40 | |
| ALPS-index + FA | 0.63 | 0.37 | 72.70 | 64.30 | |
| ALPS-index + MD | 0.70 | 0.40 | 54.50 | 85.70 | |
| ALPS-index + AD | 0.68 | 0.41 | 59.10 | 82.10 | |
| ALPS-index + RD | 0.70 | 0.40 | 54.50 | 85.70 |
AD, axial diffusivity; ALPS, analysis along the perivascular space; AUC, area under the curve; FA, fractional anisotropy; IDH, isocitrate dehydrogenase; MD, mean diffusivity; RD, radial diffusivity.
The diagnostic efficacy of IDH mutation by different metrics of grade III and grade IV
The results demonstrated that the AUCs were relatively lower than 0.80 in both univariate analysis and combined multi-metrics of grade III and IV, as shown in Table 4.
Cox analyses associated with OS
The results of Cox analyses about the factors associated with OS are summarized in Table 5. Univariate Cox model analysis indicated that clinical factors (grade, sex, age, IDH) and DTI metrics (MD/AD/RD) were significantly associated with OS (P<0.01). On stepwise multivariable Cox analyses, grade, IDH mutation, and sex were the independent predictive factors of OS (P<0.02).
Table 5
| Parameter metric | Univariate Cox | Multivariate Cox | |||||
|---|---|---|---|---|---|---|---|
| HR | P value | 95% CI | HR | P value | 95% CI | ||
| Grade (LGG vs. HGG) | 0.28 | <0.01 | 0.15, 0.53 | 0.31 | 0.02 | 0.12, 0.83 | |
| Sex (female vs. male) | 0.37 | <0.01 | 0.18, 0.78 | 0.41 | 0.02 | 0.19, 0.86 | |
| Age (<44 vs. ≥44 years) | 0.27 | <0.01 | 0.14, 0.55 | 0.67 | 0.47 | 0.23, 1.95 | |
| IDH (wide type vs. mutant) | 8.54 | <0.01 | 4.18, 17.44 | 10.96 | <0.01 | 3.60, 33.42 | |
| ALPS-index (<1.18 vs. ≥1.18) | 1.55 | 0.18 | 0.82, 2.91 | – | – | – | |
| FA value (<0.19 vs. ≥0.19) | 1.05 | 0.91 | 0.48, 2.27 | – | – | – | |
| MD value (<0.99 vs. ≥0.99) | 2.48 | <0.01 | 1.35, 4.58 | 4.38 | 0.09 | 0.81, 23.60 | |
| AD value (<1.19 vs. ≥1.19) | 2.73 | <0.01 | 1.46, 5.09 | 0.18 | 0.13 | 0.02, 1.62 | |
| RD value (<0.98 vs. ≥0.98) | 2.49 | <0.01 | 1.33, 4.65 | 1.61 | 0.66 | 0.20, 13.25 | |
Age cutoff represents median age in the cohort. Cutoff values for ALPS-index, FA, MD, AD, and RD were determined by ROC curve analysis. AD, axial diffusivity; ALPS, analysis along the perivascular space; CI, confidence interval; DTI, diffusion tensor image; FA, fractional anisotropy; HGG, high-grade glioma; HR, hazard ratio; IDH, isocitrate dehydrogenase; LGG, low-grade glioma; MD, mean diffusivity; RD, radial diffusivity; ROC, receiver operating characteristic.
Discussion
In this study, we introduced the ALPS-index to represent the alterations in GS function in different grades of gliomas. We also combined ALPS-index with DTI-derived tensor metrics and glioma clinical indicators. We found that the ALPS-index of the hemisphere contralateral to glioma was higher than that of ipsilateral hemisphere in LGG patients, but the conclusion was reversed in HGG patients, which has never been reported in previous studies. The ALPS-index of glioma patients was always significantly lower than that of HC. Furthermore, this is the first research reporting that different tumor locations may have different effects on GS. Finally, we found some predictors of OS in glioma patients.
Maintaining an efficient lymphatic drainage system and brain waste elimination throughout one’s life could be crucial. The ALPS-index is thought to serve as an estimate of human GS function. Compared with healthy volunteers, the ALPS-index of glioma patients was reduced, indicating that the brain waste elimination mechanism was impaired, which is consistent with several studies (25,26,31). The researchers discovered that AQP4 knockout rats had significantly longer interstitial fluid (ISF) clearance in the thalamus, significantly greater water/waste retention, and increased intracranial pressure in the brain compared to normal rats, suggesting that AQP4 dysfunction may be a major contributing factor to the GS imbalance (32,33). In addition, research has revealed that the decline in GS function may be attributed to the increased physical compression of brain tissue by tumor volume (31). Whole-brain pathways associated with GS include glymphatic CSF-ISF circulation in the brain as well as CSF drainage channels (34). The second finding was that the decrease of the ALPS-index in the contralateral cerebral hemisphere, suggesting that the GS as a whole was affected by the tumor.
In LGG, the ALPS-index in the glioma ipsilateral hemisphere was significantly lower than that in the contralateral hemisphere, which may be due to direct invasion and destruction of the tumor, altered ISF dynamics caused by compression, and infiltration of inflammatory cells (35). However, in HGG, it is intriguing and surprising to learn that the ALPS-index in the glioma ipsilateral hemisphere was paradoxically higher than that in the contralateral hemisphere, which was contrary to our hypothesis. We think it is still related to the expression of AQP4. It has been reported that all CNS tumors with edema produce high levels of vascular endothelial growth factor (VEGF), which induces capillary permeability, endothelial proliferation, and AQP4 is positively regulated by VEGF (36). In this context, the upregulation of AQP4 should represent a protective response aimed at avoiding secondary cytotoxic brain edema by promoting the re-absorption of excess fluid (37). Therefore, it can be explained that the ALPS-index in the ipsilateral hemisphere was higher than that in the contralateral hemisphere in the HGG group of our study. This finding can also be applied to explain the results of the comparison of other different categories (e.g., IDH mutation, tumor location, survival). Our study has potential clinical implications. Since cerebral edema can impair drug delivery efficacy (38), future therapies targeting AQP4 modulation to enhance GS function may offer significant therapeutic benefits.
The inter-group comparison showed that the ALPS-index of HGG was higher than that of LGG, although there was no statistical significance. Suzuki et al. demonstrated a positive correlation between the expression level of aquaporins and histological tumor grade by using [11 C] TTN-020 positron emission tomography (PET) imaging of aquaporins (39), which supported our findings. However, our result differs from the findings of Zhu et al. (25), who reported that the ALPS-index in HGG was significantly lower than it was in LGG. We guess there is a combination of factors beyond mere aquaporins expression that eventually complicate the glymphatic efficiency; these factors incorporate tumor physical compression, disruption of the blood-brain barrier-altered ISF dynamics (31), and the remodeling of glymphatic pathways (10). Our findings emphasize the importance of considering diverse physiological and pathological mechanisms in assessing glymphatic function in gliomas. Although aquaporin expression contributes, the combined impact of TME changes and glymphatic pathway alterations likely significantly influences the ALPS-index. To develop a comprehensive evaluation model, future studies should integrate the ALPS-index with multimodal MRI techniques such as arterial spin labeling (ASL) for cerebral blood flow assessment and magnetic resonance spectroscopy (MRS) for metabolic imaging.
The DTI-derived metrics (MD/AD/RD) of the inter-group comparison in this study were statistically significant. DTI provides a macroscopic view of microstructures of white matter in the CNS (40). FA reflects the integrity of nerve fibers and the degree of cellular structure arrangement. MD is sensitive to initial cell swelling (cytotoxic edema) which restricts diffusion (41). AD tends to be strongly affected by axon damage, whereas RD is sensitive to white matter damage due to demyelination (42). In this study, the FA value in HGG was slightly higher than that in LGG, although there was no significant difference in FA values among all levels of gliomas. In HGG, the tumor cells were arranged in a pseudopalisading structure, and in LGG, the cells were arranged loosely (43). These results suggest that although white matter fiber bundle destruction is more severe in HGG than LGG, the increased cell density and vascular distribution in HGG compensate for the decreased FA. We found that MD/AD/RD values were significantly reduced in HGG, which was consistent with the findings of Zhao et al. (44) and Jiang et al. (45). Zhao et al. found that the MD value was significantly lower in HGG, whereas Jiang observed significant differences in AD and RD values between HGG and LGG in a cohort of 53 patients. These studies showed that MD/AD/RD values were negatively correlated with histological grade. This may reflect increased cellular proliferation, severe axonal damage, and a higher degree of demyelination in HGG. However, our findings extend beyond these previous studies by providing a more comprehensive assessment of tumor microstructure, which may have significant implications for non-invasive diagnosis and treatment planning.
Additionally, we investigated, for the first time, whether the ALPS-index and DTI-derived parameters varied across different tumor locations, and the results were negative. This implies that the GS function is impaired and white matter fiber bundle destruction occurs at different locations in the brain. Recent evidence (46) shows that the pathophysiological process of the tumor is not restricted locally but also spreads globally through the white matter pathways to other brain regions. Meanwhile, we observed that tumor location did not consistently correlate with ALPS-index differences, likely due to several factors. First, the small sample size in certain regions (e.g., occipital and basal areas) limited statistical power. Second, IDH-mutant gliomas predominantly occur in the frontal lobe near the rostral extension of the lateral ventricle (47). Given the higher prevalence of IDH-mutant cases in our cohort, significant ALPS-index differences were more readily detected in the frontal regions. Therefore, we believe that a larger sample size is needed in our future studies.
In this study, there was no difference in ALPS-index between the IDH-mutant group and IDH wild type group, which was different from previous reports (25,26,48); the FA value of the IDH wild type group was significantly higher than that of IDH-mutant group. We believe that data bias was responsible for this discrepancy. For the reasons described above, the IDH-wild type in this study was mainly in HGG, tending to behave more aggressively than IDH-mutant gliomas. The compensatory up-regulation of AQP4 due to poor fluid circulation may offset the reduction in ALPS-index, whereas the increase of dense cell density and vascular distribution reversed the FA value significantly. The reduction of MD, AD, and RD values of wild type is usually related to more severe white matter destruction and demyelination, which is similar to previous reports (49).
Our ROC curve analysis for IDH mutation revealed that the combined model significantly enhances diagnostic efficiency for LGG, with the ALPS-index playing a key role. This aligns with the findings of Zhu et al. (25), who also reported improved diagnostic performance when combining DTI metrics with the ALPS-index compared to using the ALPS-index alone. However, unlike Zhu et al., we stratified our analysis by tumor grade, revealing that the ALPS-index and DTI metrics are particularly critical in LGG diagnosis. We believe that LGG may be less invasive (50), so it is reasonable to assume that the microstructure of the tumor parenchyma is relatively normal, and the GS of LGG may withstand less severe damage. Therefore, the DTI parameter, which represents the change of microstructure, and the ALPS-index, which can reflect the function of the GS, and their combined model can more comprehensively reflect the pathophysiological information of LGG. In HGG, the increased heterogeneity of tumor microstructure and multifactorial impacts on GS efficiency (10,31) highlight the need for additional biomarkers, such as mean kurtosis (51), to better capture microstructural complexity in non-Gaussian distributed tissues. Future research should clarify the relative contributions of these factors, advancing non-invasive diagnostics and personalized treatments. Future clinical research should aim to stratify management, identify various contributing factors based on tumor grade, and direct preoperative planning and postoperative radiation.
Regarding the parameters predicting OS, the univariate Cox analysis results indicated that DTI was more predictive than the ALPS-index. We contend that the ALPS-index only indirectly reflects the characteristics of the tumor, and is far less convincing than the DTI parameter which directly represents the tumor itself. Multivariate Cox analysis showed that general clinical data, especially IDH wild type, were the most important independent factor for poor prognosis, which was similar to the results of Zeng et al. (48). This may be attributed to the substantial overlap between IDH status and tumor grade.
Our study possesses certain limitations. Firstly, the sample size of this study was relatively small, especially in certain regions (e.g., occipital and basal areas), which may have resulted in the impacts of tumors in diverse locations on the GS have not been identified. Secondly, we lacked histopathological evidence representing AQP4. A multi-center study with a bigger sample size should be carried out in the future to gather information on glioma patients and examine the relationship between the histopathological findings of ALPS-index and AQP4. Thirdly, the study’s findings rely on specific pre-processing steps, and variations among different techniques may exist. Future research could compare the impact of various pre-processing methods on the ALPS-index. Additionally, the subjectivity of ROI mapping remains an inherent limitation.
It is important to note that the ALPS-index is an incomplete representation of GS function. Since the ALPS-index was originally used to describe the subcortical GS, human studies of a CSF tracer (gadobitol) have shown only a sparse tracer enhancement in the white matter region (52), suggesting that CSF-ISF exchange and thus glymphatic clearance appear to play a secondary role in brain clearance of deep white matter. Meanwhile the principle of ALPS-index was based on the expectation that the perivascular spaces is continuous between the white matter and the cortex, but in fact, most cortical vessels do not directly connected with white matter vessels (53). Finally, the ALPS-index merely reflects the free diffusion parallel to the x-axis, rather than along the glymphatic flow along the y-or-z axis (54). Therefore, based on the above, we must be cautious when using ALPS-index to represent GS functions. It is still necessary to expand the sample size, collect images under higher field intensity to obtain better ROI placement area, and use a multiple methods (such as the combination of imaging and histopathology) to conduct a comprehensive evaluation of GS.
Conclusions
The ALPS-index can indirectly reflect the impairment of the GS in gliomas. The GS on the ipsilateral side of glioma may increase compensatorily in HGG patients. The combined model of the ALPS-index and DTI parameter is significant for predicting IDH mutation in LGG. The ALPS-index and other non-invasive imaging biomarkers can reflect the characteristics of glioma more comprehensively. However, the GS impairment of gliomas in different locations still requires further study.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2710/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2710/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R, Reifenberger G. Glioma. Nat Rev Dis Primers 2015;1:15017. [Crossref] [PubMed]
- Du N, Zhou X, Mao R, Shu W, Xiao L, Ye Y, Xu X, Shen Y, Lin G, Fang X, Li S. Preoperative and Noninvasive Prediction of Gliomas Histopathological Grades and IDH Molecular Types Using Multiple MRI Characteristics. Front Oncol 2022;12:873839. [Crossref] [PubMed]
- Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, Bondy ML, Berger M, Jenkins R, Wrensch M. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus 2015;38:E6. [Crossref] [PubMed]
- Rimmer B, Bolnykh I, Dutton L, Lewis J, Burns R, Gallagher P, Williams S, Araújo-Soares V, Menger F, Sharp L. Health-related quality of life in adults with low-grade gliomas: a systematic review. Qual Life Res 2023;32:625-51. [Crossref] [PubMed]
- Kannan S, Murugan AK, Balasubramanian S, Munirajan AK, Alzahrani AS. Gliomas: Genetic alterations, mechanisms of metastasis, recurrence, drug resistance, and recent trends in molecular therapeutic options. Biochem Pharmacol 2022;201:115090. [Crossref] [PubMed]
- Sharma P, Aaroe A, Liang J, Puduvalli VK. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol Adv 2023;5:vdad009. [Crossref] [PubMed]
- Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 2018;15:422-42. [Crossref] [PubMed]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337-41. [Crossref] [PubMed]
- Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020;577:689-94. [Crossref] [PubMed]
- Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 2020;30:229-43. [Crossref] [PubMed]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111. [Crossref] [PubMed]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015;212:991-9. [Crossref] [PubMed]
- Zhang L, Min Z, Tang M, Chen S, Lei X, Zhang X. The utility of diffusion MRI with quantitative ADC measurements for differentiating high-grade from low-grade cerebral gliomas: Evidence from a meta-analysis. J Neurol Sci 2017;373:9-15. [Crossref] [PubMed]
- Sun Y, Su C, Deng K, Hu X, Xue Y, Jiang R. Mean apparent propagator-MRI in evaluation of glioma grade, cellular proliferation, and IDH-1 gene mutation status. Eur Radiol 2022;32:3744-54. [Crossref] [PubMed]
- Huang Z, Lu C, Li G, Li Z, Sun S, Zhang Y, Hou Z, Xie J. Prediction of Lower Grade Insular Glioma Molecular Pathology Using Diffusion Tensor Imaging Metric-Based Histogram Parameters. Front Oncol 2021;11:627202. [Crossref] [PubMed]
- Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, Kishimoto T, Naganawa S. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol 2017;35:172-8. [Crossref] [PubMed]
- Cai X, Chen Z, He C, Zhang P, Nie K, Qiu Y, Wang L, Wang L, Jing P, Zhang Y. Diffusion along perivascular spaces provides evidence interlinking compromised glymphatic function with aging in Parkinson's disease. CNS Neurosci Ther 2023;29:111-21. [Crossref] [PubMed]
- Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, Cai J, Chen S, Fang L, Sun J, Lou M. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage 2021;238:118257. [Crossref] [PubMed]
- Shen T, Yue Y, Ba F, He T, Tang X, Hu X, Pu J, Huang C, Lv W, Zhang B, Lai HY. Diffusion along perivascular spaces as marker for impairment of glymphatic system in Parkinson's disease. NPJ Parkinsons Dis 2022;8:174. [Crossref] [PubMed]
- Hsu JL, Wei YC, Toh CH, Hsiao IT, Lin KJ, Yen TC, Liao MF, Ro LS. Magnetic Resonance Images Implicate That Glymphatic Alterations Mediate Cognitive Dysfunction in Alzheimer Disease. Ann Neurol 2023;93:164-74. [Crossref] [PubMed]
- Vittorini MG, Sahin A, Trojan A, Yusifli S, Alashvili T, Bonifácio GV, Paposhvili K, Tischler V, Lampl C, Sacco SSchool of Advanced Studies of the European Headache Federation (EHF-SAS). The glymphatic system in migraine and other headaches. J Headache Pain 2024;25:34. [Crossref] [PubMed]
- Lee DA, Park BS, Ko J, Park SH, Lee YJ, Kim IH, Park JH, Park KM. Glymphatic system dysfunction in temporal lobe epilepsy patients with hippocampal sclerosis. Epilepsia Open 2022;7:306-14. [Crossref] [PubMed]
- Kaur J, Ding G, Zhang L, Lu Y, Luo H, Li L, Boyd E, Li Q, Wei M, Zhang Z, Chopp M, Jiang Q. Imaging glymphatic response to glioblastoma. Cancer Imaging 2023;23:107. [Crossref] [PubMed]
- Xu D, Zhou J, Mei H, Li H, Sun W, Xu H. Impediment of Cerebrospinal Fluid Drainage Through Glymphatic System in Glioma. Front Oncol 2021;11:790821. [Crossref] [PubMed]
- Zhu H, Xie Y, Li L, Liu Y, Li S, Shen N, Zhang J, Yan S, Liu D, Li Y, Zhu W. Diffusion along the perivascular space as a potential biomarker for glioma grading and isocitrate dehydrogenase 1 mutation status prediction. Quant Imaging Med Surg 2023;13:8259-73. [Crossref] [PubMed]
- Toh CH, Siow TY. Factors Associated With Dysfunction of Glymphatic System in Patients With Glioma. Front Oncol 2021;11:744318. [Crossref] [PubMed]
- Calabrese E, Villanueva-Meyer JE, Rudie JD, Rauschecker AM, Baid U, Bakas S, Cha S, Mongan JT, Hess CP. The University of California San Francisco Preoperative Diffuse Glioma MRI Dataset. Radiol Artif Intell 2022;4:e220058. [Crossref] [PubMed]
- Zuo XN, Anderson JS, Bellec P, Birn RM, Biswal BB, Blautzik J, et al. Milham MP. An open science resource for establishing reliability and reproducibility in functional connectomics. Sci Data 2014;1:140049. [Crossref] [PubMed]
- Qin Y, Li X, Qiao Y, Zou H, Qian Y, Li X, Zhu Y, Huo W, Wang L, Zhang M. DTI-ALPS: An MR biomarker for motor dysfunction in patients with subacute ischemic stroke. Front Neurosci 2023;17:1132393. [Crossref] [PubMed]
- Taoka T, Ito R, Nakamichi R, Kamagata K, Sakai M, Kawai H, Nakane T, Abe T, Ichikawa K, Kikuta J, Aoki S, Naganawa S. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol 2022;40:147-58. [Crossref] [PubMed]
- Gao M, Liu Z, Zang H, Wu X, Yan Y, Lin H, Yuan J, Liu T, Zhou Y, Liu J. A Histopathologic Correlation Study Evaluating Glymphatic Function in Brain Tumors by Multiparametric MRI. Clin Cancer Res 2024;30:4876-86. [Crossref] [PubMed]
- Teng Z, Wang A, Wang P, Wang R, Wang W, Han H. The Effect of Aquaporin-4 Knockout on Interstitial Fluid Flow and the Structure of the Extracellular Space in the Deep Brain. Aging Dis 2018;9:808-16. [Crossref] [PubMed]
- Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol 2007;22:778-84. [Crossref] [PubMed]
- Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019;65:106-19. [Crossref] [PubMed]
- Preusser M, Haberler C, Hainfellner JA. Malignant glioma: neuropathology and neurobiology. Wien Med Wochenschr 2006;156:332-7. [Crossref] [PubMed]
- Maugeri R, Schiera G, Di Liegro CM, Fricano A, Iacopino DG, Di Liegro I. Aquaporins and Brain Tumors. Int J Mol Sci 2016;17:1029. [Crossref] [PubMed]
- Yang L, Wang X, Zhen S, Zhang S, Kang D, Lin Z. Aquaporin-4 upregulated expression in glioma tissue is a reaction to glioma-associated edema induced by vascular endothelial growth factor. Oncol Rep 2012;28:1633-8. [Crossref] [PubMed]
- Ma Q, Schlegel F, Bachmann SB, Schneider H, Decker Y, Rudin M, Weller M, Proulx ST, Detmar M. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci Rep 2019;9:14815. [Crossref] [PubMed]
- Suzuki Y, Nakamura Y, Yamada K, Kurabe S, Okamoto K, Aoki H, Kitaura H, Kakita A, Fujii Y, Huber VJ, Igarashi H, Kwee IL, Nakada T. Aquaporin Positron Emission Tomography Differentiates Between Grade III and IV Human Astrocytoma. Neurosurgery 2018;82:842-6. [Crossref] [PubMed]
- Concha L. A macroscopic view of microstructure: using diffusion-weighted images to infer damage, repair, and plasticity of white matter. Neuroscience 2014;276:14-28. [Crossref] [PubMed]
- Wang Z, Guan F, Duan W, Guo Y, Pei D, Qiu Y, Wang M, Xing A, Liu Z, Yu B, Zheng H, Liu X, Yan D, Ji Y, Cheng J, Yan J, Zhang Z. Diffusion tensor imaging-based machine learning for IDH wild type glioblastoma stratification to reveal the biological underpinning of radiomic features. CNS Neurosci Ther 2023;29:3339-50. [Crossref] [PubMed]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front Neurol 2018;9:92. [Crossref] [PubMed]
- Ahn SJ, Shin HJ, Chang JH, Lee SK. Differentiation between primary cerebral lymphoma and glioblastoma using the apparent diffusion coefficient: comparison of three different ROI methods. PLoS One 2014;9:e112948. [Crossref] [PubMed]
- Zhao J, Wang YL, Li XB, Hu MS, Li ZH, Song YK, Wang JY, Tian YS, Liu DW, Yan X, Jiang L, Yang ZY, Chu JP. Comparative analysis of the diffusion kurtosis imaging and diffusion tensor imaging in grading gliomas, predicting tumour cell proliferation and IDH-1 gene mutation status. J Neurooncol 2019;141:195-203. [Crossref] [PubMed]
- Jiang L, Xiao CY, Xu Q, Sun J, Chen H, Chen YC, Yin X. Analysis of DTI-Derived Tensor Metrics in Differential Diagnosis between Low-grade and High-grade Gliomas. Front Aging Neurosci 2017;9:271. [Crossref] [PubMed]
- Lv K, Cao X, Wang R, Du P, Fu J, Geng D, Zhang J. Neuroplasticity of Glioma Patients: Brain Structure and Topological Network. Front Neurol 2022;13:871613. [Crossref] [PubMed]
- Tejada Neyra MA, Neuberger U, Reinhardt A, Brugnara G, Bonekamp D, Sill M, Wick A, Jones DTW, Radbruch A, Unterberg A, Debus J, Heiland S, Schlemmer HP, Herold-Mende C, Pfister S, von Deimling A, Wick W, Capper D, Bendszus M, Kickingereder P. Voxel-wise radiogenomic mapping of tumor location with key molecular alterations in patients with glioma. Neuro Oncol 2018;20:1517-24. [Crossref] [PubMed]
- Zeng S, Huang Z, Zhou W, Ma H, Wu J, Zhao C, Yang Z, Qiu H, Chu J. Noninvasive evaluation of the glymphatic system in diffuse gliomas using diffusion tensor image analysis along the perivascular space. J Neurosurg 2025;142:187-96. [PubMed]
- Mader MM, Deuter D, Sauvigny T, Borchert P, Faizy TD, Bester M, Westphal M, Rosengarth K, Schmidt NO, Sedlacik J, Dührsen L. Diffusion tensor imaging changes in patients with glioma-associated seizures. J Neurooncol 2022;160:311-20. [Crossref] [PubMed]
- Forst DA, Nahed BV, Loeffler JS, Batchelor TT. Low-grade gliomas. Oncologist 2014;19:403-13. [Crossref] [PubMed]
- Xie Y, Li S, Shen N, Gan T, Zhang S, Liu WV, Zhu W. Assessment of Isocitrate Dehydrogenase 1 Genotype and Cell Proliferation in Gliomas Using Multiple Diffusion Magnetic Resonance Imaging. Front Neurosci 2021;15:783361. [Crossref] [PubMed]
- Ringstad G. Glymphatic imaging: a critical look at the DTI-ALPS index. Neuroradiology 2024;66:157-60. [Crossref] [PubMed]
- Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull 1981;7:519-79. [Crossref] [PubMed]
- Bae YJ, Kim JM, Choi BS, Ryoo N, Song YS, Nam Y, Yoon IY, Cho SJ, Kim JH. Altered Brain Glymphatic Flow at Diffusion-Tensor MRI in Rapid Eye Movement Sleep Behavior Disorder. Radiology 2023;307:e221848. [Crossref] [PubMed]


