
Endovascular coiling versus microsurgical clipping for extremely small intracranial aneurysms: a comparative analysis of treatment strategies, complications, and clinical outcomes
Introduction
An extremely small intracranial aneurysm (ESIA) has a maximum diameter of approximately 2 mm (1). The condition has traditionally been under-researched and had a relatively low detection rate, and has thus received minimal attention from the scientific community. However, the advent and subsequent widespread adoption of three-dimensional digital subtraction angiography (3D-DSA) technology has significantly improved detection rates. The latest research reveals that the average size of treated aneurysms has decreased significantly, with an average reduction of 0.71 mm every 5 years. Additionally, as operative safety has improved in recent years, more and more small aneurysms have been identified and treated (2). The International Subarachnoid Aneurysm Trial (ISAT) demonstrated that among patients with aneurysms treated with endovascular coiling (EC) and microsurgical clipping (MC), the outcomes associated with EC were superior to those of MC (3). However, this conclusion omitted ESIAs because they were not included in the ISAT. Thus, there remains ongoing debate over ESIAs treatment (4,5). The EC of ESIAs presents significant technical challenges owing to their constrained intraluminal dimensions. In addition, the MC of these lesions is complicated by their narrow neck morphology, which predisposes to compromised clip stability and potential iatrogenic rupture during the intervention. Furthermore, sometimes, when microsurgical (MC) operations are challenging, it may be necessary to sacrifice a portion of the parent artery to achieve complete clipping. This sacrifice can result in arterial stenosis or occlusion, which may subsequently lead to ischemic injury (6). Due to the unpredictable nature of ESIA rupture, it is generally acknowledged that individuals with significant risk factors, including younger age, presence of aneurysms in the anterior communicating artery (ACoA), and hypertension, should receive prompt treatment (7). Moreover, a study revealed that most ESIAs are wide-necked, making EC and MC treatment difficult (8). In this study, we conducted a comprehensive comparison of the aneurysmal embolization rates, associated complications, and both short- and long-term outcomes associated with 2 treatment methods: EC and MC. The primary objective of this comparison was to ascertain which of these treatment approaches provides greater benefits for patients with ESIAs. By evaluating these critical factors, we aimed to offer valuable insights into the efficacy and safety of each method, ultimately guiding clinical decisions in the management of ESIAs. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2848/rc).
Methods
Study design
This retrospective study analyzed clinical data spanning an 11-year period (February 2013 to December 2023) retrieved from the medical record management system at the Department of Neurosurgery, Renmin Hospital of Wuhan University. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was reviewed and approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2024-K083[X01]) and informed consent was provided by all individual participants.
All aneurysms were confirmed using digital subtraction angiography (DSA) and/or computed tomography angiography (CTA).
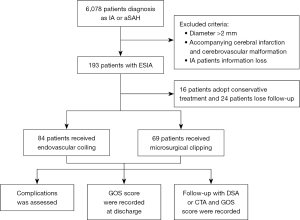
The inclusion criteria were as follows: (I) aneurysms were confirmed by DSA or CTA; (II) aneurysm diameter was less than 2 mm; (III) all patients underwent surgical treatment and signed informed consent. The exclusion criteria included the following: (I) aneurysm diameter was greater than 2 mm; (II) aneurysms were associated with other cerebrovascular diseases, such as cerebrovascular malformation, intracranial venous thrombosis, moyamoya disease or cerebral infarction; (III) the patient refused to sign the informed consent or loss of patient information (Figure 1). All patients underwent clinical follow-up at the hospital, through outpatient visits, or by telephone 6 months after discharge. All aneurysm data, including aneurysm size, neck width, location, and number, were diagnosed and measured using DSA or CTA. The degree of embolization was assessed and recorded by two experienced neurosurgeons and/or neuro-interventionists immediately following the surgery (Figure 2).
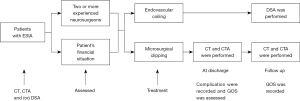
Treatment methods
Endovascular procedures
Patients with ruptured ESIAs who decided to receive endovascular therapy were orally administered 300 mg clopidogrel and 300 mg aspirin 2 hours before surgery or 5 mL tirofiban intravenously during stent release, with 5 mL/h continued for 24 hours if a stent was used. All patients underwent endotracheal intubation after general anesthesia. Following a successful puncture of the femoral artery, a standard DSA of the entire cerebral vasculature was conducted to rule out additional vascular issues and to identify the location, configuration, dimensions, and parent artery of the aneurysm. After cerebral angiography, systemic heparinization was administered with 50 IU/kg of heparin. Subsequent doses were reduced by half each hour, with a minimum dose of 1,000 IU maintained until the end of the operation. The 3D-DSA guided the selection of optimal surgical trajectories, angulation parameters, and stent configurations. A 6F (French, 6F ≈2 mm) multipurpose catheter was advanced into the distal segments of the internal carotid or vertebral artery under fluoroscopic guidance. For cases necessitating stent-assisted coil embolization, the stent microcatheter tip was navigated to the parent artery’s distal segment and precisely aligned using real-time roadmap imaging, with device deployment facilitated by micro-guidewire tracking. The microcatheter was carefully pushed into the aneurysm cavity using a micro-guidewire. Depending on the aneurysm’s dimensions and contour, suitable coils were chosen to effectively pack it while the stent was partially deployed. Coils measuring 1.5 or 1 mm in diameter were chosen due to the constrained space inside the aneurysm. Once coiling was completed, the stent was fully deployed. A follow-up procedure was conducted to assess the level of embolization and to check for any stenosis in the parent artery. Dyna computed tomography (CT) was performed to check whether the stent was fully open and to exclude intracranial hemorrhage. Patients undergoing stent-assisted coil embolization were routinely administered dual antiplatelet therapy, including 75 mg of clopidogrel daily for at least 6 months and 100 mg of aspirin daily for a longer period. Additionally, if the CYP2C19 genotype (clopidogrel-related gene) indicated slow metabolism, 90 mg of ticagrelor was administered orally every 12 hours for 3 months. Treatment strategies, including coil embolization alone or stent-assisted, stent type, initial angiographic results, and follow-up angiography, were documented.
Microsurgical procedures
The pterional or interhemispheric approach was adopted depending on the location and direction of the aneurysms. First, we dissected the internal carotid cistern to release cerebrospinal fluid and reduce intracranial pressure in ruptured ESIAs. Subsequently, the parent artery and adjacent nerves of ESIAs were exposed. The parent artery was blocked with a suitable temporary clip. Before the clipping procedure, a thorough examination of the blood vessels and nerves surrounding the aneurysm was conducted. This ensured that no critical perforating vessels or nerves were harmed during the operation and that normal neurological function was preserved. Following this careful assessment, a mini clip was precisely applied to the neck of the aneurysm. After clip placement, we verified that there was no significant narrowing of the parent artery and confirmed complete occlusion of the aneurysm. To assess the success of the intervention, a postoperative CTA was performed, allowing for a detailed evaluation of both the parent artery and the state of the aneurysm occlusion. Additionally, any intraoperative complications such as rupture or clip slippage, along with the details of preoperative ventriculo-peritoneal shunt (V-P shunt) insertion and the specific surgical approach taken, were meticulously documented to ensure comprehensive patient records and facilitate future medical evaluations.
Complications, clinical outcomes, and angiographic results at follow-up
Before discharge, complications including intraoperative rupture, ischemic stroke, intracranial infection, pleural effusion, hydrocephalus, pulmonary infection, postoperative rebleeding, hepatic or renal insufficiency, urinary tract infection, hypoproteinemia, anemia, seizures, electrolyte disturbance, and decompressive craniectomy were recorded. The degree of aneurysm occlusion was evaluated using the modified Raymond Scale (9): grade I, complete occlusion (no contrast agents observed in the aneurysm); grade II, neck remnant (contrast agents observed at the aneurysmal neck); and grade III, partial occlusion (contrast agents observed within the aneurysmal cavity). The scale was used immediately post-intervention and during follow-up. The Glasgow Outcome Scale (GOS) was used to assess outcomes, with scores as follows: 5 (return to everyday life, despite mild impairment); 4 (slightly disabled but able to live independently and work under protection); 3 (severe disability, sober, disabled, and needing care in daily life); 2 (plant survival with minimal response, for example, eyes open with sleep/wake cycles); and 1 (death) (10). We recorded the discharge and 6-month follow-up GOS scores for both the EC and MC groups.
Statistical analysis
The software GraphPad Prism (version 10.1, RRID: SCR_002798; GraphPad Software, San Diego, CA, USA) was utilized for the graphical representation of the data, whereas SPSS (version 27.0, RRID: SCR_016479; IBM Corp., Armonk, NY, USA) was employed for the statistical analysis. Data were expressed as mean ± standard deviation, median (interquartile spacing), and percentage as appropriate. The unpaired t-test was used to analyze the numerical variables, whereas the categorical variables were represented by n (%). To analyze the relative risk, the statistical test of Chi-squared was employed. Statistical significance was defined as P<0.05. Taxonomic variables were analyzed using the Chi-squared test, the continuity-corrected Chi-squared test, or Fisher’s exact test. The incidence and relative risk associated with dichotomous variables were evaluated and compared between the EC group and the MC group. This analysis was conducted with a confidence interval (CI) set at 95%, providing a statistical framework to ensure the reliability and validity of the findings.
Results
A total of 153 patients were included in the study; 84 underwent EC treatment and 69 MC treatment at the Neurosurgical Department of Renmin Hospital of Wuhan University from 2013 to 2023. A non-significant difference in baseline characteristics was identified between the two groups. In addition, the final intention-to-treat analysis revealed that all patients were included in the study. All data, including gender, age, aneurysm location, size, complications, discharge, and follow-up GOS, were documented.
Demographic, clinical, and aneurysm data
The mean age of the patients was 58.6±11.5 years (range, 19–81 years) in the EC group and 57.0±9.7 years (range, 28–79 years) in the MC group. In the EC group, there were 62 (73.8%) women and 22 (26.2%) men, whereas the MC group included 46 (66.7%) women and 23 (33.3%) men. The most common comorbidity was hypertension (63.1% vs. 58.0%), followed by smoking (25.0% vs. 24.6%) in EC and MC groups, respectively. Hunt-Hess grading revealed 14 cases of grade 0, 18 cases of grade 1, 23 cases of grade 2, 23 cases of grade 3, 5 cases of grade 4, and 1 case of grade 5 in the EC group and 10 cases of grade 0, 9 cases of grade 1, 25 cases of grade 2, 18 cases of grade 3, and 6 cases of grade 4, and 1 case of grade 5 in the MC group. Fisher grading identified 14 cases of grade 0, 21 cases of grade 1, 29 cases of grade 2, 12 cases of grade 3, and 8 cases of grade 4 in the EC group, and 10 cases of grade 0, 17 cases of grade 1, 14 cases of grade 2, 12 cases of grade 3, and 16 cases of grade 4 in the MC group. The baseline characteristics of the patients are summarized in Table 1. A total of 254 aneurysms were diagnosed in these 153 patients, with 166 being ESIAs and 88 being non-ESIAs. In total, 105 (68.6%) patients had ruptured ESIAs, and 48 (31.4%) had unruptured ones. In total, 153 cases of ESIAs included 7 anterior cerebral artery aneurysms, 4 anterior choroidal artery aneurysms, 42 ACoA aneurysms, 6 basilar artery aneurysms, 34 internal carotid artery aneurysms, 19 middle cerebral artery aneurysms, 1 posterior cerebral artery aneurysm, 33 posterior communicating artery aneurysms, 4 posterior inferior cerebellar artery aneurysms, 2 superior cerebellar artery aneurysms, and 1 vertebral artery aneurysm. The median aneurysm diameter was 2.0 mm (1.8, 2.0 mm). The width of the neck was approximately 1.8 mm (1.5, 2.0 mm). The aneurysm data are presented in Table 2.
Table 1
| Characteristics | All (n=153) | Endovascular group (n=84) | Clipping group (n=69) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 57.9±10.7 | 58.6±11.5 | 57.0±9.7 | 0.366 |
| Sex, n (%) | 0.335 | |||
| Male | 45 (29.4) | 22 (26.2) | 23 (33.3) | |
| Female | 108 (70.6) | 62 (73.8) | 46 (66.7) | |
| Past medical history, n (%) | ||||
| Smoking | 38 (24.8) | 21 (25.0) | 17 (24.6) | 0.959 |
| Hypertension | 93 (60.8) | 53 (63.1) | 40 (58.0) | 0.518 |
| Presentation (ESIA), n (%) | 0.564 | |||
| Unruptured | 48 (31.4) | 28 (33.3) | 20 (29.0) | |
| Rupture | 105 (68.6) | 56 (66.7) | 49 (71.0) | |
| H-H grade | 0.373 | |||
| 0 | 14 | 10 | ||
| 1 | 18 | 9 | ||
| 2 | 23 | 25 | ||
| 3 | 23 | 18 | ||
| 4 | 5 | 6 | ||
| 5 | 1 | 1 | ||
| Fisher grade | 0.094 | |||
| 0 | 14 | 10 | ||
| 1 | 21 | 17 | ||
| 2 | 29 | 14 | ||
| 3 | 12 | 12 | ||
| 4 | 8 | 16 |
ESIA, extremely small intracranial aneurysms; H-H grade, Hunt-Hess grade; SD, standard deviation.
Table 2
| Characteristics | Total | Endovascular group (n=84) | Clipping group (n=69) | P value |
|---|---|---|---|---|
| Location of ESIAs, n (%) | 0.827 | |||
| ACA | 2 (2.4) | 5 (7.2) | ||
| AChA | 3 (3.6) | 1 (1.4) | ||
| ACoA | 21 (25.0) | 21 (30.4) | ||
| BA | 6 (7.1) | 0 | ||
| ICA | 28 (33.3) | 6 (8.7) | ||
| MCA | 3 (3.6) | 16 (23.2) | ||
| PCA | 1 (1.2) | 0 | ||
| PCoA | 15 (17.9) | 18 (26.1) | ||
| PICA | 2 (2.4) | 2 (2.9) | ||
| SCA | 2 (2.4) | 0 | ||
| VA | 1 (1.2) | 0 | ||
| Size (mm), median (range) | 2.0 (1.8, 2.0) | 1.95 (1.8, 2.0) | 2.0 (1.8, 2.0) | 0.473 |
| Neck (mm), median (range) | 1.8 (1.5, 2.0) | 1.65 (1.4, 2.0) | 1.9 (1.6, 2.0) | 0.003* |
| Number of aneurysms | ||||
| 1 | 32 | 40 | ||
| 2 | 43 | 22 | ||
| 3 | 6 | 6 | ||
| 4 | 3 | 1 | ||
| Number of ESIAs | ||||
| 1 | 72 | 68 | ||
| 2 | 12 | 1 |
*, P<0.05 indicates a statistical difference between the endovascular group and the clipping group. ACA, anterior cerebral artery; AChA, anterior choroidal artery; ACoA, anterior communicating artery; BA, basilar artery; ESIAs, extremely small intracranial aneurysms; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; PCoA, posterior communicating artery; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery; VA, vertebral artery.
EC, MC procedure, and complications
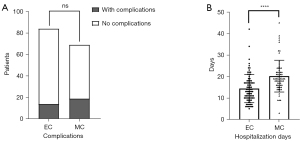
All patients in the study underwent successful treatment using either EC or MC techniques. Within the EC group specifically, the treatment approach involved various methods of embolization. A total of 20 cases were treated with a single microcatheter embolization, whereas 4 cases utilized double microcatheter embolization. Additionally, stent-assisted embolization was employed in 60 cases, highlighting the diverse techniques applied within the EC group to achieve effective treatment outcomes. Following the operation, angiography confirmed 71 cases of Raymond grade I and 13 cases of Raymond grade II. After 6 months of follow-up, there were 74 cases of Raymond grade I and 8 cases of Raymond grade II (2 patients died during follow-up). In the patients assigned to undergo MC, the procedure to clip the ESIAs was successful, but 3 cases of aneurysm rupture, 2 of slippage, and 7 of VP-shunt insertion prior to surgery were noted. Postoperative cerebral CTA showed that the aneurysm was completely occluded (Table 3). Hospitalization days in the EC group (14.40±6.57 days) were significantly lower than those in the MC group (20.17±7.38 days). A single patient could experience multiple complications. In the EC group, no complications such as decompressive craniectomy, postoperative rebleeding, intraoperative rupture, intracranial infection, pleural effusion, hepatic and/or renal insufficiency, urinary tract infection, or seizures occurred after careful operation and adequate anticoagulant treatment. However, there were 6 cases of developed hydrocephalus, 2 cases of ischemic stroke, 9 cases of pulmonary infection, 3 cases of electrolyte disturbance, 2 cases of hypoproteinemia, and 1 case of anemia. In the MC group, complications included 4 cases of hydrocephalus, 6 cases of decompressive craniectomy, 3 cases of ischemic stroke, 15 cases of pulmonary infection, 2 cases of pleural effusion, 3 cases of hepatic and/or renal insufficiency, 2 cases of urinary tract infection, 3 cases of electrolyte disturbance, 2 cases of anemia, and 1 case of seizures. No postoperative rebleeding, intracranial infection, or hypoproteinemia occurred in the MC group. Pulmonary infection, pleural effusion, hepatic or renal insufficiency, and urinary tract infections are general complications, whereas the remaining complications are specific to the surgical method, which we categorize as special complications. General complications are influenced by perioperative care; therefore, they cannot be included in the EC and MC groups when comparing the incidence of complications. As shown in Table 4 and Figure 3, there was no significant difference between the EC group and the MC group in terms of specific complications (16.67% vs. 27.54%). Figure 4 illustrates typical cases treated with EC, and Figure 5 illustrates typical cases treated with MC.
Table 3
| Characteristics | Values |
|---|---|
| Coiling group (n=84) | |
| Treatment methods | |
| Single microcatheter | 20 (23.8) |
| Double microcatheter | 4 (4.8) |
| Stenting with coiling | 60 (71.4) |
| Stent type | |
| Atlas | 21 (35.0) |
| LVIS | 20 (33.3) |
| LEO | 4 (6.7) |
| Pipeline | 4 (6.7) |
| Enterprise | 3 (5.0) |
| Others | 8 (13.3) |
| Raymond grade (at discharge/follow-up) | |
| 1 | 71/74 |
| 2 | 13/8 |
| Clipping group (n=69) | |
| Intraoperative variables | |
| Intraoperative rupture | 3 (4.3) |
| Clip slippage | 2 (2.9) |
| Preoperative V-P shunt | 7 (10.1) |
| Surgery approach | |
| Pterional | 66 (95.7) |
| Coronary | 2 (2.9) |
| Supraorbital lateral | 1 (1.4) |
Data are presented as n (%) or n. EC, endovascular coiling; LEO, trade name, a self-expanding intracranial stent; MC, microsurgical clipping; LVIS, low-profile visualized intraluminal support; V-P shunt, ventriculo-peritoneal shunt.
Table 4
| Characteristic | Endovascular coiling | Microsurgery clipping | P value |
|---|---|---|---|
| Complications | |||
| Hydrocephalus | 6 (7.1) | 4 (5.8) | |
| Decompressive craniectomy | 0 | 6 (8.7) | |
| Rebleeding postoperative | 0 | 0 | |
| Ischemic stroke | 2 (2.4) | 3 (4.3) | |
| Intracranial infection | 0 | 0 | |
| Electrolyte disturbance | 3 (3.6) | 3 (4.3) | |
| Hypoproteinemia | 2 (2.4) | 0 | |
| Anemia | 1 (1.2) | 2 (2.9) | |
| Seizures | 0 | 1 (1.4) | |
| Pulmonary infection | 9 (10.7) | 15 (21.7) | |
| Pleural effusion | 0 | 2 (2.9) | |
| Hepatic and (or) renal insufficiency | 0 | 3 (4.3) | |
| Urinary tract infection | 0 | 2 (2.9) | |
| General complications | 9 (10.7) | 22 (31.9) | 0.0012* |
| Special complications | 14 (16.7) | 19 (27.5) | 0.1038 |
| Total complications | 23 (27.4) | 41 (59.4) | <0.0001* |
| Hospitalization days | 14.40±6.57 | 20.17±7.38 | <0.0001* |
Data are presented as n (%) or mean ± standard deviation. *, P<0.05 indicates a statistical difference between the endovascular group and the clipping group.

Discharge and follow-up outcomes
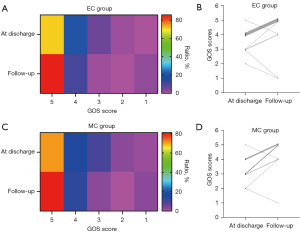
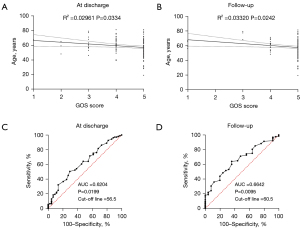
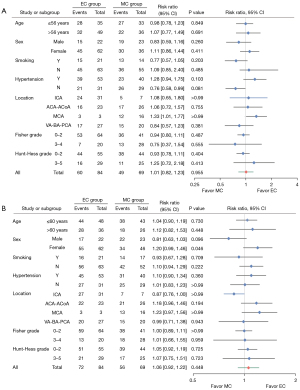
The DSA follow-up of 82 patients in the EC group demonstrated good recovery. In the EC group, angiography revealed modified Raymond scale grade I in 74 patients (88.10%) and grade II in 8 patients (9.52%). A total of 2 patients died during follow-up. In the MC group, cerebral CTA examination revealed recurrence in 7 patients (10.14%). The GOS evaluation was conducted for all participants at the point of hospital discharge and again after 6 months had elapsed. A comparison was made between patients in the EC group (71.43%, 60/84) and those in the MC group (71.01%, 49/69). It was found that a higher percentage of patients in the EC group demonstrated good recovery (GOS score 5) at discharge; however, this difference was not statistically significant (P=0.96, 95% CI: 0.82–1.23). After 6 months, the recovery rate increased to (85.71%, 72/84) in the EC group and (81.16%, 56/69) in the MC group; however, the GOS score 5 remained statistically non-significant (P=0.45, 95% CI: 0.92–1.22). In some cases, patients with initial GOS scores lower than 5 exhibited improvement, achieving a score of 5 at subsequent follow-up evaluations, with 13 cases observed in the EC group and 8 cases in the MC group. However, 2 patients died in the EC group, and the GOS 2 or 3 score changed to 1; 1 patient died in the MC group, and the GOS 2 score changed to 1 (Figure 6). Younger patients demonstrated better prognosis at discharge and during follow-up. Receiver operating characteristic (ROC) curve analysis identified 56 years as the optimal cutoff age at discharge and 60 years at follow-up (Figure 7). Subgroup analyses for a favorable outcome (GOS score 5) revealed no differences between the two groups in age (>56 or ≤56 years at discharge, >60 or ≤60 years at follow-up), hypertension, smoking, aneurysm location, excellent or poor clinical condition (Hunt-Hess and Fisher grades) at admission and 6 months follow-up. Subgroup analysis for a favorable outcome revealed no sex-related differences between EC and MC groups at discharge. However, a difference emerged during follow-up (Figure 8).
Discussion
ESIA differs from traditional very small intracranial aneurysms (VSIAs, with a diameter of less than 3 mm) and has a smaller diameter (1,11,12). It has been established through previous studies on VSIAs that EC and MC treatments can yield favorable outcomes (13,14). In comparison to VSIAs, ESIAs are characterized by narrower inner spaces and thinner wall structures. This design difference renders ESIAs more susceptible to damage when undergoing EC treatment, increasing the likelihood of rupture during the procedure. Conversely, when subjected to MC treatment, ESIAs demonstrate a higher tendency for clip slippage, highlighting the distinct vulnerabilities associated with their structural attributes. Both treatments are complicated and have high risks. In previous medical understanding, small aneurysms, specifically those with a diameter measuring less than 7 mm, were generally perceived to possess a low likelihood of rupture (15). However, when patients present with high-risk factors associated with rupture, including youth, aneurysms in the ACoA, hypertension, and smoking, surgical intervention may be a viable option (7,16). In our study, 24 patients had ruptured IAs on the ipsilateral side, whereas an additional 24 patients exhibited a high risk of rupture. Consequently, after discussions with the patients and their families, and upon obtaining informed consent, all 48 patients underwent surgical treatment. With the development of 3D-DSA technology, more and more ESIAs have been detected following subarachnoid hemorrhage (SAH). However, no prior research had reported whether EC or MC treatment is more effective in treating ESIAs. Therefore, to investigate the difference between the two treatment methods, we compared the efficiency, complications, and postoperative outcomes of 153 patients with ESIAs treated with EC and MC.
EC treatment of ESIAs
The rapid advances in endovascular manufacturing technologies, such as the development of newer, softer, smaller coils and more minor and denser stent mesh, have made coil embolization of very tiny lesions safer (17). The classification of endovascular treatment is twofold, with the two main types being pure coil embolization and stent-assisted coil embolization. Pure coil embolization is generally adequate for treating narrow-necked ESIAs, as it effectively facilitates the occlusion of the aneurysm without the need for further support. In contrast, wide-necked ESIAs present greater challenges, and therefore, stent-assisted embolization is necessary. This technique is crucial for preventing coil prolapse, which can compromise the effectiveness of the treatment. A variety of stents are available for this purpose, including the Neuroform, LEO® Stent, low-profile visualized intraluminal support (LVIS), Solitaire, and Enterprise stents, each offering distinct features and capabilities suited for different clinical scenarios. Selecting the appropriate stents for ESIAs is crucial. The braid stents have a mesh diameter of less than 1 mm, which can prevent the escape of the smallest coil from the aneurysm. For example, LVIS stent has a high metal coverage (up to 23%) and has been discovered to function as a flow diverter (1). LVIS Junior and LEO stents are ideal for wide-necked aneurysms with a parent artery diameter of less than 2 mm (7). In the course of our research, we primarily utilized LVIS, LVIS Junior, and LEO stents as essential tools for facilitating the embolization process in cases of wide-necked ESIAs.
In the past, intraoperative rupture of aneurysms was a frequent and serious complication associated with EC treatment. A study of 422 aneurysms revealed that intraoperative rupture occurred in 35 cases, constituting an 8.3% rupture rate (18), indicating that such ruptures are relatively common during the embolization process. The incidence of intraoperative rupture for ESIAs is more than double that observed for giant aneurysms, with a rupture rate of 7.7% (16). The findings of recent research, which include our previous study, demonstrate a reduced incidence of intraprocedural rupture when compared with results obtained in previous studies (1,12,19). In the present study, among the 84 patients who underwent EC treatment, only 3 patients (3.57%) experienced intraoperative rupture, which may be attributed to several factors. First, the excellent plasticity of the microcatheter helped to stabilize it in the aneurysm cavity, reducing the microcatheter stimulation to the vascular wall. Second, selecting the first coil with a diameter of less than or equal to that of the aneurysm reduced the pressure of the coil on the aneurysm wall. Furthermore, thrombosis is a prevalent complication associated with endovascular treatment, notably in cases where stent-assisted embolization is employed. Studies reported intra-procedure thrombosis rates of 8.1% (20) and 11.2% (21) in stent-assisted embolization for ruptured normal-size IA, which were lower than that of ESIAs (22). However, in our research, anticoagulant treatment was administered prior to, throughout, and following the surgery, whereas high-pressure saline was continuously infused to avert catheter thrombosis. Consequently, only 2 (2.38%) out of 84 patients experienced thrombosis. The recurrence rate of aneurysms treated by EC ranged from 4% to 20%, with recurrence highly dependent on aneurysm location, size, neck width, and incomplete embolization (13). ESIAs that were treated using a pure coil exhibited strong stability and compactness in embolic formation. Stent-assisted embolization is primarily employed for ESIAs with wide necks. This technique has been demonstrated to induce modifications in the hemodynamic characteristics within the parent vessel. It functions as an organizational framework for the proliferation of endothelial cells, thereby contributing to a reduction in the incidence of recurrence following endovascular treatment (23).
MC treatment of ESIAs
MC remains the primary treatment option for ESIA when EC fails or faces high rupture risk. This incorporates single-clip (24), double-clip (11,14), and cotton-assisted (6) surgical clipping techniques. The reduced dimensions of ESIAs render MC treatment susceptible to clip slippage. Recently, MC treatment of ESIAs has adopted double-clip technology or post-wrap clamping to reduce clip slippage. In our study, MC exhibited a total occlusion rate of 100%, without remnant aneurysms or rebleeding. However, clip slippages occurred in 2 (2.90%) of the 69 patients with ESIA in our study, a frequency that varies from other previous studies (14,24). Intraoperative rupture constitutes a lethal complication in the context of MC. A number of factors have been identified as contributing to the probability of rupture occurring intraoperatively. These include the location of the aneurysm, its morphology, fluctuations in intraoperative blood pressure, the presence of epilepsy, and the surgical experience of the operating physician (25,26).
In the present series of cases, complete exposure of the parent artery and aneurysm was achieved. In addition, the proximal and distal parent arteries were blocked by means of temporary clips. Mini-clipping was adjusted to clip the aneurysm, followed by a careful check to ensure the completeness of the clipping and that no perforating arteries were occluded. In the present study, intraoperative aneurysm rupture occurred in 3 patients; they were completely clipped after a temporary occlusion of the parent artery. However, with the development of surgical techniques, complications after MC are unavoidable. Bruneau et al. reported a 98.2% total occlusion rate of aneurysms, with only 2.7% of the patients experiencing persistent microsurgical complications and no mortality (24). According to Grasso, ischemia linked to surgery was observed in 15% of patients, whereas bleeding was noted in 13.2% (27). In our series study, 1 (1.45%) patient died from severe preoperative cerebral hemorrhage, 1 died from respiratory failure caused by postoperative lung infection, 3 (4.35%) developed postoperative ischemic stroke, and none exhibited postoperative rebleeding. Other complications, including electrolyte disturbances in 3 patients with ACoA aneurysms, occurred after surgery, which may be closely related to the damaged hypothalamic branch originating from the ACoA. Analysis shows that hypothalamic perforating arteries have 2–6 branches, supplying blood to different regions of the hypothalamus. The study also found that 10% of these arteries originate on one side of the ACoA and terminate on the opposite side of the hypothalamus. Damage to these arteries during surgical procedures can lead to symptoms such as electrolyte imbalances, disturbances in consciousness, cognitive impairments, and elevated body temperature (28).
EC versus MC
Due to no studies having compared the safety and efficacy between EC and MC treatment of ESIAs, we reported 84 patients with ESIAs treated with EC and 69 patients treated with MC. A retrospective study of 162 patients diagnosed with VSIAs who were treated with EC or MC was conducted to analyze the clinical outcomes. The analysis revealed a slight trend indicating that EC treatment yielded improved outcomes, although the results did not achieve statistical significance (13). An additional investigation encompassing 130 patients diagnosed with ruptured ACoA aneurysms also revealed no substantial variations in complication and reintervention rates at discharge or during 1- and 3-year post-intervention follow-ups (29). In China, patients choose MC treatment because EC treatment is more expensive. However, with the recent inclusion of interventional materials in medical insurance coverage, the costs of both surgical methods have become almost similar.
In this study, the mortality rates were 2.38% for EC and 2.90% for MC. Patients in the MC group had to recover from surgical incisions, had more extended hospital stays, and demonstrated an increased risk of infections. The occurrence of procedure-related complications was observed in 27.54% of the MC group and 16.67% of the EC group. The complications included hydrocephalus, decompressive craniectomy, postoperative rebleeding, cerebral infarction, intracranial infection, electrolyte disturbance, hypoproteinemia, anemia, and seizures. Subtotal embolism was evident in 13 patients of the EC group following the operation, whereas 7 of these patients achieved complete aneurysm occlusion upon subsequent angiographic follow-up. Conversely, 7 patients within the MC group exhibited aneurysm recurrence as indicated by follow-up angiograms, despite the presence of complete clipping prior to discharge. The EC group had a lower occlusion rate at discharge (84.52% vs. 100%), but this increased during follow-up (88.10% vs. 89.86%). This discrepancy is due to subsequent thrombosis following coil embolization in the EC group.
Further investigation revealed that blood pressure was controllable and the stent promoted aneurysm occlusion. There were two recurrent patients with uncontrolled hypertension.
Our subgroup analysis for a favorable outcome revealed a gender-related difference between the EC and MC groups at follow-up. The long-term prognosis of female patients treated with EC was better than that of those treated with MC. This may be attributed to several factors. First, female patients may prefer EC, as they are less likely to accept shaving their hair. Additionally, female patients are more sensitive. Patients who receive EC treatment are healthier physically and mentally and have better recovery outcomes. Second, 45 of 62 female patients underwent stent-assisted embolization and took aspirin orally every day in the EC. It was reported that inflammation plays a critical role in the development and rupture of IAs, thus, aspirin may reduce the recurrence and progress by inhibiting inflammatory mediators (30).
Limitations
There are several limitations to this study. First, our follow-up duration was limited to 6 months. Although there was no difference in complications between the EC and MC groups during the study period, probably because long-term complications associated with antiplatelet therapy were not thoroughly investigated, our assessment of both EC and MC was inadequate. We anticipate that future studies will extend the follow-up duration and retain the imaging data to better assess long-term prognostic differences. Second, it was a retrospective study without a fully randomized control group. This could introduce publication bias, as studies with positive results are more likely to be reported and published. Nonetheless, the two groups exhibited no significant discrepancies in terms of age, clinical grade, Fisher grade, or treatment timing. It should be noted that this study was conducted in a single institution; therefore, its findings are specific to our center and may not necessarily represent the results obtained at other facilities. However, our results are consistent with the prevailing trends observed in the outcomes of aneurysm treatments.
Conclusions
This is the first report to compare the safety and efficacy of EC and MC treatment for ESIAs. The results showed that no significant difference was observed in outcome at discharge and during follow-up. As a consequence, EC and MC treatments are both suitable for patients with ESIAs. However, the EC group exhibited fewer hospitalization days than the MC group, whereas the latter demonstrated a higher occlusion rate. Additionally, female patients demonstrated better long-term outcomes after EC treatment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2848/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2848/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was reviewed and approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2024-K083[X01]) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tian Q, Dong W, Zhang W, Xu Z, Wang J, Chen Q, Li M. Embolization with Stent-Assisted Technique for Wide-Necked Extremely Small Intracranial Aneurysm with Diameter no more than 2 mm. J Stroke Cerebrovasc Dis 2020;29:105388. [Crossref] [PubMed]
- Khorasanizadeh M, Pettersson SD, Maglinger B, Garcia A, Wang SJ, Ogilvy CS. Trends in the size of treated unruptured intracranial aneurysms over 35 years. J Neurosurg 2023;139:1328-38. [Crossref] [PubMed]
- Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock PInternational Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809-17. [Crossref] [PubMed]
- Suzuki S, Kurata A, Ohmomo T, Sagiuchi T, Niki J, Yamada M, Oka H, Fujii K, Kan S. Endovascular surgery for very small ruptured intracranial aneurysms. Technical note. J Neurosurg 2006;105:777-80. [Crossref] [PubMed]
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman RInternational Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267-74. [Crossref] [PubMed]
- Liu J, Gao G, Zhang S, Huang Y, Wu J, Hu X, Lu J, Zhang Q, Zhou L, Huang Y. Cotton-Assisted Surgical Clipping of Very Small Aneurysms: A Two-Center Study. World Neurosurg 2019;127:e242-50. [Crossref] [PubMed]
- Lee GJ, Eom KS, Lee C, Kim DW, Kang SD. Rupture of Very Small Intracranial Aneurysms: Incidence and Clinical Characteristics. J Cerebrovasc Endovasc Neurosurg 2015;17:217-22. [Crossref] [PubMed]
- AlMatter M, Bhogal P, Aguilar Pérez M, Schob S, Hellstern V, Bäzner H, Ganslandt O, Henkes H. The Size of Ruptured Intracranial Aneurysms : A 10-Year Series from a Single Center. Clin Neuroradiol 2019;29:125-33. [Crossref] [PubMed]
- Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398-403. [Crossref] [PubMed]
- Saul TG, Ducker TB, Salcman M, Carro E. Steroids in severe head injury: A prospective randomized clinical trial. J Neurosurg 1981;54:596-600. [Crossref] [PubMed]
- Rahmanian A, Ghaffarpasand F, Alibai E, Choque-Velasquez J, Jahromi BR, Hernesniemi J. Surgical Outcome of Very Small Intracranial Aneurysms Utilizing the Double Clip Technique. World Neurosurg 2018;110:e605-11. [Crossref] [PubMed]
- Qin F, Li Z, Fang X, Zhao X, Liu J, Wu D, Lai N. Therapeutic effect of enterprise stent-assisted embolization for very small ruptured intracranial aneurysms. Medicine (Baltimore) 2017;96:e7832. [Crossref] [PubMed]
- Li J, Su L, Ma J, Kang P, Ma L, Ma L. Endovascular Coiling Versus Microsurgical Clipping for Patients With Ruptured Very Small Intracranial Aneurysms: Management Strategies and Clinical Outcomes of 162 Cases. World Neurosurg 2017;99:763-9. [Crossref] [PubMed]
- Kiran NA, Jahromi BR, Velasquez JC, Hijazy F, Goehre F, Kivisaari R, Siangprasertkij C, Munoz Gallegos LF, Lehto H, Hernesniemi J. Double-clip technique for the microneurosurgical management of very small (< 3 mm) intracranial aneurysms. Neurosurgery 2015;11:3-7. [PubMed]
- Wu P, Ocak PE, Wang D, Ocak U, Xu S, Li Y, Zhang T, Shi H. Endovascular Treatment of Ruptured Tiny Intracranial Aneurysms with Low-Profile Visualized Intraluminal Support Device. J Stroke Cerebrovasc Dis 2019;28:330-7. [Crossref] [PubMed]
- van Rooij WJ, Keeren GJ, Peluso JP, Sluzewski M. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol 2009;30:835-9. [Crossref] [PubMed]
- Gupta V, Chugh M, Jha AN, Walia BS, Vaishya S. Coil embolization of very small (2 mm or smaller) berry aneurysms: feasibility and technical issues. AJNR Am J Neuroradiol 2009;30:308-14. [Crossref] [PubMed]
- Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: report of a consecutive series and a meta-analysis. Stroke 2010;41:116-21. [Crossref] [PubMed]
- Lu J, Liu JC, Wang LJ, Qi P, Wang DM. Tiny intracranial aneurysms: endovascular treatment by coil embolisation or sole stent deployment. Eur J Radiol 2012;81:1276-81. [Crossref] [PubMed]
- Yang P, Zhao K, Zhou Y, Zhao R, Zhang L, Zhao W, Hong B, Xu Y, Huang Q, Krings T, Liu J. Stent-assisted Coil Placement for the Treatment of 211 Acutely Ruptured Wide-necked Intracranial Aneurysms: A Single-Center 11-Year Experience. Radiology 2015;276:545-52. [Crossref] [PubMed]
- Ryu CW, Park S, Shin HS, Koh JS. Complications in Stent-Assisted Endovascular Therapy of Ruptured Intracranial Aneurysms and Relevance to Antiplatelet Administration: A Systematic Review. AJNR Am J Neuroradiol 2015;36:1682-8. [Crossref] [PubMed]
- Yamaki VN, Brinjikji W, Murad MH, Lanzino G. Endovascular Treatment of Very Small Intracranial Aneurysms: Meta-Analysis. AJNR Am J Neuroradiol 2016;37:862-7. [Crossref] [PubMed]
- Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery 2007;61:460-8; discussion 468-9. [Crossref] [PubMed]
- Bruneau M, Amin-Hanjani S, Koroknay-Pal P, Bijlenga P, Jahromi BR, Lehto H, Kivisaari R, Schaller K, Charbel F, Khan S, Mélot C, Niemela M, Hernesniemi J. Surgical Clipping of Very Small Unruptured Intracranial Aneurysms: A Multicenter International Study. Neurosurgery 2016;78:47-52. [Crossref] [PubMed]
- Chen SF, Kato Y, Kumar A, Tan GW, Oguri D, Oda J, Watabe T, Imizu S, Sano H, Wang ZX. Intraoperative rupture in the surgical treatment of patients with intracranial aneurysms. J Clin Neurosci 2016;34:63-9. [Crossref] [PubMed]
- Lakićević N, Vujotić L, Radulović D, Cvrkota I, Samardžić M. Factors Influencing Intraoperative Rupture of Intracranial Aneurysms. Turk Neurosurg 2015;25:858-85. [Crossref] [PubMed]
- Grasso G, Perra G. Surgical management of ruptured small cerebral aneurysm: Outcome and surgical notes. Surg Neurol Int 2015;6:185. [Crossref] [PubMed]
- Chen J, Li M, Zhu X, Chen Y, Zhang C, Shi W, Chen Q, Wang Y. Anterior Communicating Artery Aneurysms: Anatomical Considerations and Microsurgical Strategies. Front Neurol 2020;11:1020. [Crossref] [PubMed]
- Moon K, Levitt MR, Almefty RO, Nakaji P, Albuquerque FC, Zabramski JM, McDougall CG, Spetzler RF. Treatment of Ruptured Anterior Communicating Artery Aneurysms: Equipoise in the Endovascular Era? Neurosurgery 2015;77:566-71; discussion 571. [Crossref] [PubMed]
- Fuentes AM, Stone McGuire L, Amin-Hanjani S. Sex Differences in Cerebral Aneurysms and Subarachnoid Hemorrhage. Stroke 2022;53:624-33. [Crossref] [PubMed]


