
Incidental Kommerell diverticulum with aberrant left subclavian artery and right aortic arch: a single-center observational study
Introduction
Kommerell diverticulum (KD) is a rare congenital abnormality of the aortic arch that arises as a result of failed regression of the primitive fourth dorsal arch during embryological development, presenting as an aneurysmal enlargement at the orifice of the aberrant subclavian artery (1,2). It was first reported by Burckhard Friedrich Kommerell in 1936 when a patient presented with an aortic diverticulum in the left aortic arch originating from the aberrant right subclavian artery (ARSA), causing dysphagia via compression of the esophagus (3).
Dilation of the KD can compress adjacent structures, resulting in clinical manifestations such as dysphagia, dyspnea, wheezing, recurrent pneumonia, obstructive emphysema, or chest pain (4-6). However, the majority of patients with KD are diagnosed incidentally on computed tomography (CT) or magnetic resonance imaging (MRI) for other clinical conditions, and most do not have any symptoms. Only 5% of adult patients with aberrant subclavian arteries develop symptoms (7). The clear recommendation for intervention is the presence of clinical symptoms attributed to KD with aberrant subclavian artery, but the indications for surgery in asymptomatic patients have been controversial. Given the scarcity of literature on natural history, there is a lack of agreement regarding the management of asymptomatic KD.
Salomonowitz et al. (8) divided aortic diverticulum into three types according to pathogenesis: KD in ARSA with left aortic arch (LAA-ARSA), KD in aberrant left subclavian artery with right aortic arch (RAA-ALSA), and aortic diverticulum at the aortoductal junction (also classified as the pulmonary ductus diverticulum). KD is always associated with the occurrence of anomalies in LAA-ARSA or RAA-ALSA. Edward’s hypothetical double aortic arch model enables the elucidation of various aortic arch anomalies by interrupting the arch system at specific locations (1,3) (Figure 1A). LAA-ARSA occurs when the dorsal segment of the right aortic arch is severed between the right carotid artery (RCA) and the right subclavian artery (RSA), and the right ductus arteriosus regresses during the development of the double aortic arch (Figure 1B,1C). The emergence of RAA-ALSA is attributed to the severance of the dorsal segment of the left aortic arch between the left common carotid artery and the LSA, along with the regression of the right ductus arteriosus during the development of the double aortic arch (Figure 1D,1E).

As reported in the literature (3), KD is common in RAA-ALSA, yet not common in LAA-ARSA. KD in RAA-ALSA is commonly larger in size compared to other types of aortic diverticulum, such as KD in LAA-ARSA (9,10). Sex and age have been associated with atherosclerosis and cardiovascular risk factors, and are recognized as risk factors for the rupture of aortic aneurysms (11-14). KD in RAA-ALSA is considered a unique form of aneurysm, with a higher incidence in males (3), and its diameter tends to increase with age (15). Due to the peculiarities of the anomalies of RAA-ALSA concomitant with KD and the lack of knowledge about its natural history, we conducted a single-center study to explore the association of KD measurements in RAA-ALSA patients with demographic features such as sex and age, and to present follow-up results in patients who were incidentally diagnosed in our center. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2547/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (No. 2018065). As this was a retrospective study, the requirement for informed consent was waived by the Ethics Committee.
Patient cohort
From January 2013 to April 2023, patients diagnosed with KD through CT scans at our institution were included retrospectively. The CT imaging data included non-enhanced or enhanced chest CT, as well as cervical CT angiography (CTA). The hospital’s CT database was searched using specific keywords, including KD, ALSA, or RAA. Patients were also identified using the International Classification of Diseases, Ninth Revision codes. Two expert radiologists evaluated the image equality and excluded images with excessive artifacts. All patient clinical data were extracted from the institutional medical records system.
Imaging data and measurements
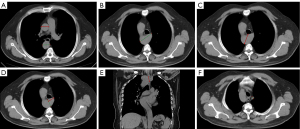
Based on the dataset in the radiology image system, axial CT images at 2–5 mm slices and coronal reformatted images at a thickness of 5 mm from non-enhanced or enhanced chest CT scans, as well as cervical CTA, were used for measurements. All the image analysis and parameter measurements were performed on the Picture Archiving and Communication System (PACS) workstation. KD is defined as the widening of the base of the subclavian artery by more than 1.5 times the diameter of the distal left subclavian artery (dLSA) (16). Ascending aorta diameter (AAo) and descending aorta diameter (DAo) were measured from the outer wall to the outer wall at the level of the pulmonary bifurcation on axial CT images (Figure 2A) (17-19). According to past literature reports on KD measurements (9,15,20,21), our research involved analyzing all axial CT scans to determine KD parameters, including the distance from the opposite aortic wall to the top of KD (DAW) (Figure 2B), the maximum distance from the KD wall adjacent to the trachea to the opposite descending aorta wall (DTAW) (Figure 2C), anteroposterior diameter of the KD orifice (KDA), the depth of KD (KDD) (Figure 2D), and the longitudinal diameter of the KD orifice (KDL) (Figure 2E). The dLSA was defined as 1 cm from the top of the KD (Figure 2F). KD parameters were indexed to AAo (KD measurements/AAo), DAo (KD measurements/DAo), and dLSA (KD measurements/dLSA) for internal normalization (9,22). It was recommended that surgery should be performed if the KDA or KDL is greater than 30 mm or if the DAW is greater than 50 mm (3). Based on that, we divided the patients into two groups: non-potential surgical candidates and potential surgical candidates.
Follow-up
Telephone follow-up was conducted on all included patients by a radiologist who was blinded to the baseline data. The endpoint events included newly developed symptoms related to KD, KD rupture, and death from any cause.
Statistical analysis
Data are shown as frequencies for categorical variables and median with interquartile range (IQR) or mean ± standard deviation (SD) for continuous variables. We used the Shapiro-Wilk test to assess the normality of data distribution. Intra- and inter-observer reliability were assessed in 30 randomly selected patients using Bland-Altman analysis. Two experienced radiologists who were blinded to the data measured the relevant parameters to evaluate interobserver reliability. One of the radiologists was randomly selected to repeat these measurements independently at a later time. Either the Chi-squared or Fisher’s exact test was used to compare categorical samples. Differences in measurements regarding KD between male and female patients and a comparison of the demographic features between potential surgical candidates and non-potential surgical candidates were assessed by the Mann-Whitney test or Student’s t-test. We tested the correlations of various measures of KD and aortic data with age using the Pearson correlation method or the Spearman correlation method, as appropriate. Kaplan-Meier methods were used to estimate cumulative event proportions. Statistical analyses were performed using SPSS software version 25.0 (IBM Corp., Armonk, NY, USA). A P value less than 0.05 was considered statistically significant.
Results
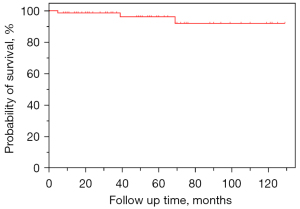
From January 2013 to April 2023, approximately 600,000 chest CT scans and 30,000 head and neck CTA were conducted in our institution. Among these, 77 KD patients with RAA-ALSA were identified and 10 were lost to follow-up due to incorrect contact information, resulting in 67 enrolled patients. Among the 10 patients lost to follow-up, 7 were male and 3 were female, and the average age was 48.9±12.7 years. The measurements (mean ± SD) for AAo, DAo, KDA, KDL, KDD, DAW, DTAW, and dLSA were 32.6±4.7, 30.8±7.4, 26.7±6.9, 26.1±6.6, and 19.8±4.3, 52.6±13.1, 36.6±12.6, and 7.9±2.8 mm, respectively. There were no statistically significant differences between lost patients and enrolled patients in sex, age, AAo, DAo, KDA, KDL, KDD, dLSA, DAW, and DTAW (all P values >0.05). Baseline clinical and imaging characteristics and follow-up results are presented in Table 1, according to gender. The age range of enrolled patients was from 16 to 84 years, with a mean age of 53.8±14.5 years at imaging. There were 42 males (62.7%) and 25 females (37.3%). All patients had a retroesophageal route of the ALSA, without clinical symptoms such as dysphagia or dyspnea caused by KD. The reasons why patients underwent imaging modality examinations were not related to KD and showed no significant differences between male and female patients. Non-enhanced chest CT was performed in 64.2% of patients, whereas other patients had available enhanced CT imaging with iodine contrast, including contrast-enhanced chest CT (4.5%), and head and neck CTA (31.3%). Excellent intraobserver reliability was present and intraclass correlation coefficients (ICCs) of all measurements ranged from 0.80 to 0.92. There was excellent interobserver reliability for all measurements between the two radiologists (ICCs were >0.79, ranging from 0.80 to 0.92). Patients who were male in our study were more likely to have a larger diameter in AAo (P=0.012), DAo (P=0.003), dLSA (P<0.001), KDA (P=0.029), and DTAW (P=0.012), and were significantly lower in some KD measurements than female patients, such as KDL/dLSA (P=0.021). There were no significant differences in other measurements and parameters between male and female patients (all P>0.05). None of the patients (100.0%) underwent surgical repair of KD. All patients (100.0%) had follow-up results and the median follow-up period was 50 months (ranging from 5 to 129 months). There was no significant difference in follow-up time between genders. Three male patients died during our follow-up period due to pulmonary infection or heart failure. The cumulative survival of KD is shown in Figure 3.
Table 1
| Variables | Total patients (N=67) | Male (N=42) | Female (N=25) | P value |
|---|---|---|---|---|
| Age (years) | 53.8 (14.5) | 55.2 (12.1) | 51.3 (17.8) | 0.367 |
| Symptoms for CT scanning | 0.209 | |||
| Neurologic issue | 28 (41.8) | 20 (47.6) | 8 (32.0) | |
| Cough | 2 (3.0) | 2 (4.8) | 0 (0.0) | |
| Pulmonary infection | 4 (6.0) | 4 (9.5) | 0 (0.0) | |
| Evaluate lungs (screening for COVID-19) | 7 (10.4) | 4 (9.5) | 3 (12.0) | |
| Respiratory tract infection | 2 (3.0) | 1 (2.4) | 1 (4.0) | |
| Malaise | 4 (6.0) | 3 (7.1) | 1 (4.0) | |
| Chest pain | 2 (3.0) | 1 (2.4) | 1 (4.0) | |
| Wheeze | 1 (1.5) | 1 (2.4) | 0 (0.0) | |
| Check-up examinations | 2 (3.0) | 1 (2.4) | 1 (4.0) | |
| Lung cancer screening/monitor pulmonary nodules | 4 (6.0) | 1 (2.4) | 3 (12) | |
| Asthma | 3 (4.5) | 1 (2.4) | 2 (8.0) | |
| Chest tightness | 1 (1.5) | 1 (2.4) | 0 (0.0) | |
| Numbness and weakness of the extremities | 1 (1.5) | 1 (2.4) | 0 (0.0) | |
| COPD | 1 (1.5) | 1 (2.4) | 0 (0.0) | |
| Induced abortion | 1 (1.5) | 0 (0.0) | 1 (4.0) | |
| Breast cancer | 1 (1.5) | 0 (0.0) | 1 (4.0) | |
| Hemoptysis | 1 (1.5) | 0 (0.0) | 1 (4.0) | |
| Diabetes mellitus | 2 (3.0) | 0 (0.0) | 2 (8.0) | |
| Measurements | ||||
| AAo (mm) | 31.5 (4.4) | 32.6 (4.1) | 29.7 (4.2) | 0.012 |
| DAo (mm) | 29.3 (5.1) | 30.7 (4.4) | 26.9 (5.4) | 0.003 |
| KDA (mm) | 25.0 (5.9) | 26.2 (6.4) | 23.0 (4.3) | 0.029 |
| KDL (mm) | 25.2 (5.6) | 26.0 (6.1) | 24.0 (4.6) | 0.125 |
| KDD (mm) | 19.3 (4.7) | 20.1 (5.0) | 17.0 (15.0, 21.0) | 0.068 |
| dLSA (mm) | 8.3 (1.9) | 9.0 (1.9) | 7.2 (1.5) | <0.001 |
| DAW (mm) | 49.1 (9.1) | 50.8 (8.8) | 46.4 (9.2) | 0.057 |
| DTAW (mm) | 34.9 (8.0) | 36.8 (7.9) | 31.7 (7.2) | 0.012 |
| KDA/AAo | 0.80 (0.16) | 0.81 (0.18) | 0.75 (0.68, 0.93) | 0.199 |
| KDL/AAo | 0.81 (0.17) | 0.80 (0.19) | 0.82 (0.15) | 0.943 |
| KDD/AAo | 0.62 (0.15) | 0.62 (0.15) | 0.62 (0.14) | 0.669 |
| DAW/AAo | 1.57 (0.24) | 1.57 (0.25) | 1.57 (0.25) | 0.938 |
| DTAW/AAo | 1.11 (0.20) | 1.13 (0.22) | 1.06 (0.17) | 0.228 |
| KDA/DAo | 0.86 (0.18) | 0.86 (0.20) | 0.87 (0.14) | 0.855 |
| KDL/DAo | 0.87 (0.17) | 0.85 (0.19) | 0.91 (0.15) | 0.205 |
| KDD/DAo | 0.67 (0.18) | 0.66 (0.18) | 0.69 (0.18) | 0.539 |
| DAW/DAo | 1.70 (0.28) | 1.67 (0.31) | 1.76 (1.56, 1.84) | 0.379 |
| DTAW/DAo | 1.20 (0.22) | 1.21 (0.23) | 1.18 (0.19) | 0.671 |
| KDA/dLSA | 3.10 (0.78) | 2.97 (0.72) | 3.30 (0.85) | 0.206 |
| KDL/dLSA | 3.15 (0.83) | 2.97 (0.78) | 3.45 (0.83) | 0.021 |
| KDD/dLSA | 2.43 (0.72) | 2.32 (0.71) | 2.61 (0.72) | 0.113 |
| DAW/dLSA | 6.15 (1.53) | 5.84 (1.27) | 6.69 (1.78) | 0.052 |
| DTAW/dLSA | 4.32 (1.06) | 4.19 (0.83) | 4.55 (1.27) | 0.344 |
| Symptoms related to KD | N/A | |||
| Asymptomatic | 67 (100.0) | 42 (100.0) | 25 (100.0) | |
| Cross sectional imaging modality | 0.270 | |||
| Chest CT | 43 (64.2) | 24 (57.1) | 19 (76.0) | |
| Contrast-enhanced chest CT | 3 (4.5) | 2 (4.8) | 1 (4.0) | |
| Head and neck CTA | 21 (31.3) | 16 (38.1) | 5 (20.0) | |
| Treatment | N/A | |||
| Conservative | 67 (100.0) | 42 (100.0) | 25 (100.0) | |
| Follow-up results | ||||
| Mortality number | 3 (4.5) | 3 (7.1) | 0 (0.0) | 0.449 |
| Mean follow-up time/survival (months) | 52.1 (33.5) | 54.1 (32.2) | 48.6 (36.1) | 0.371 |
| Mean time point for mortality (months) | 37.7 | 37.7 | N/A | N/A |
Data are presented as mean with standard deviation for normally distributed continuous variables, count (%) for ordinal categorical variables, and median with interquartile range for abnormally distributed continuous variables. Differences were assessed by the Mann-Whitney test or Student’s t-test or Chi-squared or Fisher’s exact test. AAo, ascending aorta diameter; CT, computed tomography; CTA, computed tomography angiography; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DAo, descending aorta diameter; DAW, the distance from the opposite aortic wall to the top of KD; dLSA, the diameter of the distal left subclavian artery; DTAW, the maximum distance from the diverticulum wall adjacent to the trachea to the opposite descending aorta wall; KD, Kommerell diverticulum; KDA, anteroposterior diameter of KD orifice; KDD, the depth of KD; KDL, longitudinal diameter of KD orifice; N/A, not available.
There were relatively high positive correlations between age and AAo (r=0.728, P<0.001), DAo (r=0.667, P<0.001), and KD measurements measured through CT imaging in female patients, including KDA (r=0.571, P=0.003), KDL (r=0.436, P=0.030), DAW (r=0.530, P<0.001), and DTAW (r=0.630, P<0.001) (Figure 4). We found that AAo was positively correlated with age in male patients (r=0.383, P=0.012). There were no correlations between age and DAo, KDA, KDL, DAW, or DTAW in male patients (all P>0.05) (Figure 4). Age did not correlate with KDD, dLSA, KDA/AAo, KDL/AAo, KDD/AAo, DAW/AAo, DTAW/AAo, KDA/DAo, KDL/DAo, KDD/DAo, DAW/DAo, DTAW/DAo, KDA/dLSA, KDL/dLSA, KDD/dLSA, DAW/dLSA, or DTAW/dLSA in both male and female patients (all P>0.05) (Figures 5-7). Potential surgical candidates were statistically significantly older than non-potential surgical candidates (P=0.004) (Table 2). There were no significant differences in sex, clinical information, or follow-up data between potential surgical candidates and non-candidates (all P>0.05) (Table 2).


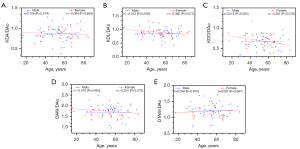
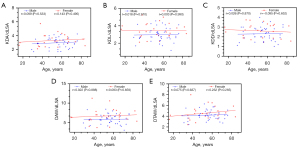
Table 2
| Variables | Total patients (N=67) |
Non-potential candidates (N=31) | Potential candidates (N=36) | P value |
|---|---|---|---|---|
| Age (years) | 53.8 (14.5) | 48.5 (15.7) | 58.3 (17.0) | 0.004 |
| Male | 42 (62.7) | 17 (54.8) | 25 (69.4) | 0.218 |
| Symptoms for CT scanning | 0.080 | |||
| Neurologic issue | 28 (41.8) | 15 (48.4) | 13 (36.1) | |
| Cough | 2 (3.0) | 2 (6.5) | 0 (0.0) | |
| Pulmonary infection | 4 (6.0) | 1 (3.2) | 3 (8.3) | |
| Evaluate lungs (screening for COVID-19) | 7 (10.4) | 0 (0.0) | 7 (19.4) | |
| Respiratory tract infection | 2 (3.0) | 0 (0.0) | 2 (5.6) | |
| Malaise | 4 (6.0) | 3 (9.7) | 1 (2.8) | |
| Chest pain | 2 (3.0) | 2 (6.5) | 0 (0.0) | |
| Wheeze | 1 (1.5) | 0 (0.0) | 1 (2.8) | |
| Check-up examinations | 2 (3.0) | 1 (3.2) | 1 (2.8) | |
| Lung cancer screening/monitor pulmonary nodules | 4 (6.0) | 2 (6.5) | 2 (5.6) | |
| Asthma | 3 (4.5) | 2 (6.5) | 1 (2.8) | |
| Chest tightness | 1 (1.5) | 1 (3.2) | 0 (0.0) | |
| Numbness and weakness of the extremities | 1 (1.5) | 0 (0.0) | 1 (2.8) | |
| COPD | 1 (1.5) | 0 (0.0) | 1 (2.8) | |
| Induced abortion | 1 (1.5) | 1 (3.2) | 0 (0.0) | |
| Breast cancer | 1 (1.5) | 0 (0.0) | 1 (2.8) | |
| Hemoptysis | 1 (1.5) | 0 (0.0) | 1 (2.8) | |
| Diabetes mellitus | 2 (3.0) | 1 (3.2) | 1 (2.8) | |
| Symptoms related to KD | N/A | |||
| Asymptomatic | 67 (100.0) | 31 (100.0) | 36 (100.0) | |
| Cross sectional imaging modality | 0.216 | |||
| Chest CT | 43 (64.2) | 17 (54.8) | 26 (72.2) | |
| Contrast-enhanced chest CT | 3 (4.5) | 1 (3.2) | 2 (5.6) | |
| Head and neck CTA | 21 (31.3) | 13 (41.9) | 8 (22.2) | |
| Treatment | N/A | |||
| Conservative | 67 (100.0) | 31 (100.0) | 36 (100.0) | |
| Follow-up results | ||||
| Mortality number | 3 (4.5) | 1 (3.2) | 2 (5.6) | >0.99 |
| Mean follow-up time/survival (months) | 52.1 (33.5) | 54.7 (31.6) | 49.8 (35.4) | 0.414 |
| Mean time point for mortality (months) | 37.7 | 39 | 37 | N/A |
Data are presented as mean with standard deviation for normally distributed continuous variables, count (%) for ordinal categorical variables. Differences were assessed by the Mann-Whitney test or Student’s t-test or Chi-squared or Fisher’s exact test. CT, computed tomography; CTA, computed tomography angiography; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; KD, Kommerell diverticulum; N/A, not available.
Discussion
RAA with isolation of the ALSA is the most common form of a RAA and is also highly associated with KD (23). A case considered to represent the better development of KD at the origin of the ALSA with RAA was thought to be attributed to the large amount of blood flow through the fetal ductus arteriosus during embryonic development (24). The retroesophageal route is the most common form of aberrant subclavian arteries (80%), with a lesser percentage between the esophagus and the trachea (15%), or anterior to the trachea (5%) (25,26). All the ALSA were retroesophageal in our study. This discrepancy may be due to the small sample size in the present study. Molz et al. (7) summarized a male predominance in KD with RAA-ALSA (2 to 1). In our cohort, 62.7% of the patients with KD and ALSA were male and 37.3% were female, showing a male predominance in CT scans. During the development of KD with ALSA, a patent left ductus arteriosus or ligamentum arteriosum often forms a vascular ring, and it is often loose, so in most cases, patients are asymptomatic (27,28). KD was incidentally diagnosed during a CT examination for other indications, and no apparent related symptoms were reported in our cohort.
As an aneurysmal dilation of a major cervicobrachial branch of the thoracic aorta, surgical or intra-arterial intervention would always be considered to prevent possible rupture of the lesion or other complications. However, the indication based on KD measurements for surgical intervention in asymptomatic patients remains controversial. Idrees et al. (21) proposed that the threshold for the surgery of KD should be if the diameter of the orifice is greater than 30 mm or the distance from the tip of KD to the opposite aortic wall (DAW) is more than 50 mm. Besides, the European Association for Cardio-Thoracic Surgery and the European Society for Vascular Surgery guidelines state that repairs are performed when the diameter of the subclavian aneurysm is at least 3 cm or the diameter of the KD orifice is at least 5.5 cm, and also point out that there is great controversy in the actual measurement of KD’s size without a clear consensus (29). Some groups may agree with aggressive surgical intervention for KD patients in their studies because the patients were symptomatic (30,31). Nevertheless, some studies have also indicated that the expansion of KD is a natural and slow trend. Hale et al. discovered that indexing maximal short-axis diameter of KD to DAo demonstrates no correlation with age and inferred that progressive dilation of KD out of proportion to somatic growth did not occur (9). With the increase of age, the size of aorta and related structures such as the KD can expand. The benefit of intervention for KD may be doubted if the enlargement is just a consequence of aging and mild atherosclerotic processes. In our study, we included more KD data in dimensions and KD-related data indexing to AAo, DAo, and dLSA, which can be used to comprehensively explore the relationship between age and KD measurements in an Asian population. ICCs of intra- and inter-observer reliability regarding KD measurements, AAo, DAo, and dLSA exceeded 0.75, indicating high reliability and reproducibility of the measurements. A previous study had shown that males had larger KD size compared with females (10); we can draw similar conclusions regarding aortic diameter and some KD measurements in our study. Hence, we deduce a correlation between gender and the sizes of the aorta and KD. Regarding the size of KD, our findings also indicated a correlation between certain KD measurements and the age of female patients. We can infer that the absolute dilation of KD would exist in female patients.
Furthermore, many reports have confirmed that the growth of thoracic aorta may continue throughout the lifetime and is not limited to the developmental stage (17-19). In adulthood, the diameter of the aortic root may increase by 0.8 mm in females and 0.9 mm in males for every decade (32). It was considered that the aortic size is a function of both body surface area and age in adults and might only be related to body size in childhood (33). To discriminate whether pathological expansion existed in KD, the measurements of KD were indexed to the diameter of aorta and dLSA. We found that KD measurements indexed to aortic diameter or dLSA were independent of age in both male and female patients. It is suggested that the slow growth of KD during life does not exceed the increase of the aorta diameter or dLSA, indicating a benign history of KD. Erben et al. (15) found that the growth rate in 45 asymptomatic patients with aberrant subclavian artery was 1.45±0.39 mm per year for KD and 2.29±0.47 mm per year for DAW and the majority of KD grew very slowly and showed a benign natural course. Hale et al. (9) followed up 21 KD patients (4 RAA-ALSA patients and 17 LAA-ARSA patients) and measured the KD over time, and no trend of enlargement was observed. In our study, none of the patients exhibited KD-related symptoms or experienced KD-related complications or fatal vascular incidents during the follow-up period. Therefore, it can be deduced that KD patients, who received an incidental diagnosis and showed no symptoms, generally exhibit a non-malignant progression and a favorable survival rate in the short term. Compared to previous studies reporting KD with rupture or dissection (31,34-37), we found that the diameter of KD in those cases tended to be larger, and the patients were generally older. Additionally, those patients were symptomatic.
Based on the surgical standard recommendations (3), we found that age was higher in potential surgical candidates compared to non-potential surgical candidates. With the unnoticed surgical indication of size, these patients were not treated for years, without related major adverse events. As it has been suggested that KD measurements may increase slowly with age (15), we also infer that the enlargement of incidental KDs may not be predominately pathological, indicating that surgery would not be urgent if no other indications were found. However, neglection of these patients should not be permitted. As a major risk factor for aortic aneurysm (38), atherosclerosis may also promote the enlargement of KDs. We would consider follow-up imaging and clinical visit more appropriate in cases with large KD and severe atherosclerosis, for early detection of disproportionate development of KDs requiring surgical intervention to prevent lethal events.
Study limitations
As a single-center observational study, the importance of our research is constrained due to the limited size of our sample. However, the study did present valuable information on incidentally diagnosed KDs. Further study with a larger sample size and prospective design across multiple centers is advised to facilitate appropriate management for the benefit of the patients. Another limitation is that most of the measurements were performed on non-enhanced chest CT scans, making it difficult to delineate the margins and leading to minor errors. However, the imperfections of measurements do not have a significant impact on the discovered tendency, especially on the indexed parameters. Finally, as incidental findings, additional CT scans were generally not conducted in the majority patients in the follow-up period, which makes it impossible for longitudinal comparison.
Conclusions
Our study demonstrated that incidentally diagnosed asymptomatic KD with RAA-ALSA patients tend to have a benign natural history and rarely experience deadly vascular events in the short term. However, in cases with large KD and severe atherosclerosis, regular imaging and clinical follow-up are needed to prevent major adverse events. Limited by sample size, we were unable to make definitive recommendations for the treatment of asymptomatic KD patients with RAA-ALSA. A larger sample size and prospective design across multiple centers are needed to detail management strategies for asymptomatic patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2547/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2547/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University (No. 2018065). As this was a retrospective study, the requirement for informed consent was waived by the Ethics Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Edwards JE. Anomalies of the derivatives of the aortic arch system. Med Clin North Am 1948;32:925-49. [PubMed]
- Tsukube T, Ataka K, Sakata M, Wakita N, Okita Y. Surgical treatment of an aneurysm in the right aortic arch with aberrant left subclavian artery. Ann Thorac Surg 2001;71:1710-1. [PubMed]
- Tanaka A, Milner R, Ota T. Kommerell's diverticulum in the current era: a comprehensive review. Gen Thorac Cardiovasc Surg 2015;63:245-59. [PubMed]
- Drucker MH, Symbas PN. Right aortic arch with aberrant left subclavian artery: symptomatic in adulthood. Am J Surg 1980;139:432-5. [PubMed]
- Mossad E, Farid I, Youssef G, Ando M. Diverticulum of Kommerell: a review of a series and a report of a case with tracheal deviation compromising single lung ventilation. Anesth Analg 2002;94:1462-4. table of contents. [PubMed]
- Patiniotis TC, Mohajeri M, Hill DG. Right aortic arch with aberrant left subclavian artery: aneurysmal dilatation causing symptomatic compression of the right main bronchus in an adult. Aust N Z J Surg 1995;65:690-2. [PubMed]
- Molz G, Burri B. Aberrant subclavian artery (arteria lusoria): sex differences in the prevalence of various forms of the malformation. Evaluation of 1378 observations. Virchows Arch A Pathol Anat Histol 1978;380:303-15. [PubMed]
- Salomonowitz E, Edwards JE, Hunter DW, Castaneda-Zuniga WR, Lund G, Cragg AH, Amplatz K. The three types of aortic diverticula. AJR Am J Roentgenol 1984;142:673-9. [PubMed]
- Hale BW, Lu JC, Romano JC, Lowery R, Yu S, Norris MD. Kommerell Diverticulum: Distinctions Between Arch Side and Evaluation of Morphology, Size, And Risk. Ann Thorac Surg 2022;114:848-56. [PubMed]
- Plotkin A, Ng B, Han SM, Weaver FA, Ham SW, Bowdish ME, Wilcox AG, Magee GA. Association of aberrant subclavian arteries with aortic pathology and proposed classification system. J Vasc Surg 2020;72:1534-43. [PubMed]
- Cui X, Wang L, Zhao Y, Wang B, Wu Z, Zhao Z, Zhang H, Chen L, Yang X. Risk Factors and Location of Intracranial Aneurysm Rupture in a Consecutive Chinese Han Population. World Neurosurg 2024;181:e214-21. [PubMed]
- Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994-2001. Circulation 2009;119:2202-8. [PubMed]
- Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011;8:92-102. [PubMed]
- Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998;29:251-6. [PubMed]
- Erben Y, Brownstein AJ, Velasquez CA, Li Y, Rizzo JA, Mojibian H, Tanweer M, Zafar MA, Ziganshin BA, Elefteriades JA. Natural history and management of Kommerell's diverticulum in a single tertiary referral center. J Vasc Surg 2020;71:2004-11. [PubMed]
- Backer CL, Russell HM, Wurlitzer KC, Rastatter JC, Rigsby CK. Primary resection of Kommerell diverticulum and left subclavian artery transfer. Ann Thorac Surg 2012;94:1612-7. [PubMed]
- Itani Y, Watanabe S, Masuda Y, Hanamura K, Asakura K, Sone S, Sunami Y, Miyamoto T. Measurement of aortic diameters and detection of asymptomatic aortic aneurysms in a mass screening program using a mobile helical computed tomography unit. Heart Vessels 2002;16:42-5. [PubMed]
- Mensel B, Heßelbarth L, Wenzel M, Kühn JP, Dörr M, Völzke H, Lieb W, Hegenscheid K, Lorbeer R. Thoracic and abdominal aortic diameters in a general population: MRI-based reference values and association with age and cardiovascular risk factors. Eur Radiol 2016;26:969-78. [PubMed]
- Mohiaddin RH, Schoser K, Amanuma M, Burman ED, Longmore DB. MR imaging of age-related dimensional changes of thoracic aorta. J Comput Assist Tomogr 1990;14:748-52. [PubMed]
- Griffeth EM, Stephens EH, Dearani JA, Francois C, Todd A, Miranda WR, Connolly HM, Bonnichsen CR, Pochettino A. Outcomes of Surgical Repair of Aberrant Subclavian Arteries in Adults. Ann Thorac Surg 2024;117:396-402. [PubMed]
- Idrees J, Keshavamurthy S, Subramanian S, Clair DG, Svensson LG, Roselli EE. Hybrid repair of Kommerell diverticulum. J Thorac Cardiovasc Surg 2014;147:973-6. [PubMed]
- Shehab M, Kosykh S, Wolf A, Haddad M, Fajer S, Hoffman RS, Bachar AR. Aberrant Right Subclavian Artery: Demographics, Morphological Features and Follow up CT Scans Dynamics. Vasc Endovascular Surg 2024;58:172-7. [PubMed]
- Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol 2009;10:176-84. [PubMed]
- van Son JA, Konstantinov IE, Burckhard F. Kommerell and Kommerell's diverticulum. Tex Heart Inst J 2002;29:109-12. [PubMed]
- Gomes MM, Bernatz PE, Forth RJ. Arteriosclerotic aneurysm of an aberrant right subclavian artery. Dis Chest 1968;54:549-52. [PubMed]
- Myers PO, Fasel JH, Kalangos A, Gailloud P. Arteria lusoria: developmental anatomy, clinical, radiological and surgical aspects. Ann Cardiol Angeiol (Paris) 2010;59:147-54. [PubMed]
- D'Souza VJ, Velasquez G, Glass TA, Formanek AG. Mirror-image right aortic arch: a proposed mechanism in symptomatic vascular ring. Cardiovasc Intervent Radiol 1985;8:134-6. [PubMed]
- Hanneman K, Newman B, Chan F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics 2017;37:32-51. [PubMed]
- Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg 2019;55:133-62. [PubMed]
- Austin EH, Wolfe WG. Aneurysm of aberrant subclavian artery with a review of the literature. J Vasc Surg 1985;2:571-7. [PubMed]
- Cinà CS, Althani H, Pasenau J, Abouzahr L. Kommerell's diverticulum and right-sided aortic arch: a cohort study and review of the literature. J Vasc Surg 2004;39:131-9. [PubMed]
- Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation 1995;91:734-40. [PubMed]
- Kaiser T, Kellenberger CJ, Albisetti M, Bergsträsser E, Valsangiacomo Buechel ER. Normal values for aortic diameters in children and adolescents--assessment in vivo by contrast-enhanced CMR-angiography. J Cardiovasc Magn Reson 2008;10:56. [PubMed]
- Bath J, D'Oria M, Rogers RT, Colglazier JJ, Braet DJ, Coleman DM, et al. Contemporary outcomes after treatment of aberrant subclavian artery and Kommerell's diverticulum. J Vasc Surg 2023;77:1339-1348.e6. [PubMed]
- Hosoba S, Suzuki T, Asai T, Nota H, Kuroyanagi S, Kinoshita T, Takashima N, Hayakawa M. Surgical repair of Kommerell's diverticulum and an aberrant subclavian artery. Surg Today 2014;44:247-51. [PubMed]
- Kwon YK, Park SJ, Choo SJ, Yun TJ, Lee JW, Kim JB. Surgical Outcomes of Kommerell Diverticulum. Korean J Thorac Cardiovasc Surg 2020;53:346-52. [PubMed]
- Wang Y, Li S, Jin M, Xue Y, Wang D, Zhou Q. Surgical treatment for right-side aortic arch concomitant with Kommerell's diverticulum: techniques selection and follow-up results. Eur J Med Res 2024;29:10. [PubMed]
- Yao L, Folsom AR, Alonso A, Lutsey PL, Pankow JS, Guan W, Cheng S, Lederle FA, Tang W. Association of carotid atherosclerosis and stiffness with abdominal aortic aneurysm: The atherosclerosis risk in communities (ARIC) study. Atherosclerosis 2018;270:110-6. [PubMed]


