
Assessment of hepatic steatosis in children with metabolic dysfunction-associated steatotic liver disease using ultrasound derived fat fraction: first large-scale clinical evaluation
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is an increasingly prevalent condition among children and adolescents (1,2). MASLD has become a major public health issue. From 2008 to 2018, the prevalence of MASLD among Chinese adults was 29.2%, with a yearly increase in incidence (3). Among Chinese children, the prevalence of MASLD is 6.3%, and it is as high as 40.4% among overweight and children with obesity (4). The early and accurate diagnosis of MASLD is crucial for preventing its progression to more severe liver diseases, such as steatohepatitis, fibrosis, and cirrhosis. MASLD often has an insidious onset, and without early intervention, it can progress to steatohepatitis, fibrosis, and even cirrhosis (5).
Traditionally, liver biopsy has been considered the gold standard for diagnosing MASLD (6); however, it is invasive and not suitable for routine screening, especially in pediatric populations. Other diagnostic methods include alanine aminotransferase (ALT) measurement, which has poor specificity; magnetic resonance imaging (MRI), which is costly and not easy to be available for patients; and traditional ultrasound, which has low sensitivity for fat content below 20% and is subject to operator variability (7). Therefore, there is a growing need for non-invasive, reliable, and cost-effective diagnostic tools. Among the emerging techniques, Ultrasound Derived Fat Fraction (uDFF) has shown promise in quantifying liver fat content (8-10). uDFF is a quantitative ultrasound technique based on quantifying the attenuation coefficient and the backscatter coefficient of acoustic waves in liver tissue and calculating the echo signal to obtain the attenuation coefficient of the liver, thus reflecting the changes in acoustic attenuation due to steatosis. It is a quantitative ultrasound technique based on quantifying the attenuation of sound waves in liver tissue. This method offers a multi-dimensional, non-invasive quantitative assessment of liver fat content and fibrosis, making it a valuable tool for the evaluation of diffuse liver diseases such as MASLD.
Despite the promise of uDFF, there is a notable gap in the literature regarding its application in the pediatric population for the assessment of MASLD. Although there are a small number of diagnostic evaluation studies of uDFF for MASLD in adults (10-12), there are very few studies in children. In a study involving 46 children, uDFF was highly concordant with pediatric MRI-derived proton density fat fraction (MRI-PDFF). However, the study was mainly of healthy children and only 10 children had MASLD (13). In another study using MRI-PDFF for the assessment of hepatic steatosis in children and adolescents, MASLD severity was found to correlate with several clinical indicators, but that study did not address uDFF (14). And it has been noted that younger age is associated with poorer outcomes in uDFF (12). The limited number of studies underscores the need for more extensive research to establish the diagnostic value of uDFF in pediatric MASLD. Future studies should aim to include larger sample sizes and diverse populations to validate the efficacy and reliability of uDFF in children. Additionally, longitudinal studies could provide insights into the utility of uDFF in monitoring disease progression and response to treatment in the pediatric population.
This study aims to evaluate the diagnostic value of uDFF in quantifying liver fat in children with MASLD and to explore the correlation between uDFF values and various anthropometric and biochemical parameters, including body weight, waist circumference, fasting blood glucose, lipid profile, and liver function tests. By assessing the effectiveness of uDFF, we seek to provide a non-invasive alternative for the early detection and management of MASLD in pediatric patients, potentially offering a more accessible and safer option for routine clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2393/rc).
Methods
Study population
This study was part of the 2022 “Childhood Obesity and Vision Health Management” project. This study used a cross-sectional research design, and the study subjects were children and adolescents from kindergarten to high school in 11 schools selected by randomized cluster sampling in a district of Beijing, China. A total of 230 children and adolescents diagnosed by ultrasonography (US) as having steatotic liver and 38 healthy children were finally enrolled in the study. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Capital Institute of Pediatrics (No. SHERLL2022043) and informed consent was obtained from all children’s legal guardians.
The inclusion criteria of MASLD children were: (I) children aged 6–12 years; (II) diagnosed with steatotic liver via ultrasound; (III) no history of alcohol consumption; and (IV) informed consent obtained. The exclusion criteria of MASLD children were: (I) presence of alcoholic liver disease, autoimmune liver disease, infectious diseases, etc.; (II) presence of endocrine disorders (such as thyroid disease), tumors, or psychiatric disorders. The healthy controls were carefully screened, and inclusion criteria included (I) no history of metabolic disorders; (II) normal abdominal ultrasound findings; and (III) no biochemical abnormalities.
Study measurements
Laboratory tests
Fasting venous blood samples (5 mL) were collected after 12 hours of fasting to conduct routine blood tests and biochemical analyses. Fasting blood glucose was measured using the hexokinase method. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured using direct methods. Total cholesterol (TC) was measured using an enzymatic method, and triglycerides (TG) were measured using the glycerol phosphate oxidase-peroxidase-phenol aminoantipyrine (GPO-PAP) method. All measurements were reported in mmol/L, and the testing equipment was provided by Siemens (Munich, Germany).
Abdominal ultrasound examination
Abdominal ultrasound was performed using two different instruments, a GE LOGIQ E20 (Waukesha, USA) and a Siemens Acuson Sequoia. The LOGIQ E20 was equipped with a convex array probe with a frequency of 1–5 MHz. Patients were examined on an empty stomach, in the supine or left lateral position, and in a calm state. High-quality, clear images of the liver parenchyma are obtained at the time of evaluation. Conventional two-dimensional (2D) ultrasound (conventional ultrasound) is used to visualize the size, shape, and parenchymal echotexture of the liver. Diagnosis of MASLD is made using 2D ultrasound.
Elastography
After the child was diagnosed with MASLD, further ultrasound examinations were performed by a pediatric sonographer with more than 10 years of experience and training in elastography. This physician determined liver fat content using the ultrasound-guided attenuation parameter (UGAP) technique on a GE machine and uDFF to reflect liver stiffness using a Siemens machine, respectively.
uDFF (Figure 1)
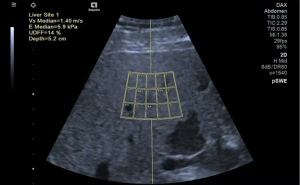
Acuson Sequoia ultrasonic diagnostic instrument manufactured by Siemens, Germany, with 5C1 probe frequency of 1.0–5.7 MHz was used. The patient was examined by a physician (with 10–20 years of experience in liver US). The patients were fasted for 6 h. They were placed in the left lateral position with the right arm raised and abducted. B-mode ultrasound (BMUS) was used to observe the morphology and echogenicity of the liver, after which the patient was instructed to breathe calmly and hold his/her breath for 10 s. uDFF Acquisition: The child was swept in the left lateral position with the right arm raised to the head. The probe was placed in the right intercostal space with the sound beam at 90° to the hepatic envelope, and the region of interest (ROI) frame was placed at a fixed depth of 1.5 cm below the hepatic envelope, avoiding the major blood vessels and rib shadows. A total of 5 sets of uDFF and Auto point shear wave elastography (pSWE) sequences were collected simultaneously during a brief apnea in free breathing. Median Auto pSWE-related parameters (velocity, elasticity) and uDFF values calculated by the system were recorded.
UGAP
The patient is placed in the supine position with the right upper extremity elevated above the head to optimize intercostal access, and to stretch the intercostal muscles and to obtain an appropriate scanning window during the examination. First, BMUS image was scanned to detect the presence of liver lesions. Second, the UGAP mode was activated and segment V of the liver (lower right anterior lobe) was examined through the intercostal space with the transducer perpendicular to the skin surface and the subject held his/her breath for 3–5 seconds. Third, because the depth of the ROI is fixed between 4 and 8 cm, the operator can only move the ROI in the left-right axis to avoid bile ducts, blood vessels, and shadow artifacts. We recorded 12 consecutive measurements on different frames and show the median and interquartile range (IQR)/median values. We defined a measurement with an IQR/median <15% of the 12 measurements as valid and successful.
Evaluation criteria
The diagnosis of steatotic liver was based on the criteria outlined in the “A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement” (15). The diagnosis of MASLD was based on the evidence of hepatic steatosis in combination with overweight/obesity, type 2 diabetes (T2D), or the presence of metabolic dysregulation. Grade 0/1/2/3 MASLD based on 2D ultrasound images (grade 0 is no steatotic liver, 1 mild, 2 moderate, 3 severe). Grade 1 MASLD was characterized by increased liver parenchymal echogenicity without posterior attenuation and clear vascular structures. Grade 2 MASLD was identified by increased echogenicity with slight posterior attenuation and less distinct vascular structures. Grade 3 MASLD was diagnosed when there was marked increase in echogenicity with significant posterior attenuation and unclear vascular structures.
Data processing and statistical analysis
Data entry was performed using Epidata 3.0, with double data entry by two independent personnel to ensure accuracy. Data managers checked the consistency, logical coherence, and boundary values of the two entries. Errors identified through these checks and survey questionnaires were corrected to ensure data accuracy. All survey data were merged and linked using a “unique identifier” code.
For quantitative variables, normally distributed data were described using mean ± standard deviation (). For skewed distributions (e.g., TG, insulin), median (IQR) were used to describe average levels. Categorical variables were described using counts and percentages [N (%)]. For comparisons between two samples, normally distributed data were analyzed using t-tests, while non-normally distributed data were analyzed using rank-sum tests. Chi-squared tests were used for comparisons of categorical data between groups. The t-tests were used to compare UGAP, and uDFF between study subjects. Multivariate logistic regression analyses were conducted to examine the relationships between UGAP, uDFF, and biochemical indicators in children with MASLD. Statistical analyses were performed using SPSS 22.0 software, with P<0.05 considered statistically significant.
Results
Demographic and clinical characteristics
The demographic and biochemical characteristics of the study participants are summarized in Tables 1,2. The study included a total of 230 steatotic liver children and 38 healthy controls. Children with steatotic liver exhibited significantly higher values than controls for height (150.5±10.0 vs. 138.4±13.4 cm, t=−6.581, P<0.001), weight (61.9±14.6 vs. 33.9±12.0 kg, t=−11.207, P<0.001), body mass index (BMI) (27.0±4.0 vs. 17.3±3.6 kg/m2, t=−14.110, P<0.001), and blood metabolic parameters (all P<0.05) (Table 1). Boys predominated in the steatotic liver group (73.0% vs. 44.7% in controls, χ2=12.223, P<0.001). No significant age difference was observed between groups (9.8±1.4 vs. 9.7±1.6 years, t=−0.678, P=0.498).
Table 1
| Characteristics | Steatotic liver group (N=230) | Control group (N=38) | t or χ2 | P |
|---|---|---|---|---|
| Age, years | 9.8±1.4 | 9.7±1.6 | −0.678 | 0.498 |
| Height, cm | 150.5±10.0 | 138.4±13.4 | −6.581 | <0.001 |
| Weight, kg | 61.9±14.6 | 33.9±12.0 | −11.207 | <0.001 |
| BMI, kg/m2 | 27.0±4.0 | 17.3±3.6 | −14.110 | <0.001 |
| Gender, boys | 168 (73.0) | 17 (44.7) | 12.223 | <0.001 |
| Blood glucose, mmol/L | 4.9±0.4 | 4.3±0.4 | −8.870 | <0.001 |
| Total cholesterol, mmol/L | 4.3±0.7 | 3.4±0.6 | −7.534 | <0.001 |
| Triglycerides, mmol/L | 1.3±0.6 | 0.9±0.3 | −4.345 | <0.001 |
| HDL-C, mmol/L | 1.3±0.3 | 1.2±0.3 | −2.556 | 0.011 |
| LDL-C, mmol/L | 2.9±0.7 | 2.1±0.7 | −5.635 | <0.001 |
| ALT, U/L | 37.4±32.7 | 23.1±9.9 | −2.672 | 0.008 |
| AST, U/L | 28.0±14.5 | 21.5±5.6 | −2.704 | 0.007 |
Continuous information is expressed as mean ± standard deviation and categorical information is expressed as n (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 2
| Characteristics | Total steatotic liver children (N=230) | Steatotic liver, boys (n=168) | Steatotic liver, girls (n=62) | t or χ2 | P |
|---|---|---|---|---|---|
| Age, years | 9.8±1.4 | 9.8±1.4 | 9.8±1.4 | −0.08 | 0.933 |
| Height, cm | 150.5±10.0 | 150.5±9.9 | 150.5±10.3 | −0.03 | 0.979 |
| Weight, kg | 61.9±14.6 | 61.7±14.0 | 62.4±16.2 | −0.33 | 0.745 |
| Waist circumference, cm | 88.3±9.3 | 89.6±9.6 | 84.6±7.5 | 4.10 | <0.001 |
| BMI, kg/m2 | 27.0±4.0 | 27.0±3.7 | 27.2±4.7 | −0.37 | 0.713 |
| Blood glucose, mmol/L | 4.9±0.4 | 5.0±0.4 | 4.9±0.3 | 1.52 | 0.130 |
| Total cholesterol, mmol/L | 4.3±0.7 | 4.3±0.7 | 4.2±0.6 | 1.06 | 0.289 |
| Triglycerides, mmol/L | 1.3±0.6 | 1.3±0.6 | 1.3±0.5 | −0.23 | 0.819 |
| HDL-C, mmol/L | 1.3±0.3 | 1.3±0.3 | 1.3±0.2 | 1.48 | 0.141 |
| LDL-C, mmol/L | 2.9±0.7 | 2.9±0.8 | 2.8±0.6 | 0.78 | 0.437 |
| ALT, U/L | 37.4±32.7 | 40.5±32.3 | 29.1±32.4 | 2.34 | 0.020 |
| AST, U/L | 28.0±14.5 | 29.2±14.5 | 24.6±14.1 | 2.13 | 0.034 |
| Steatotic liver | 3.19 | 0.074 | |||
| Grade 1 | 191 (83.0) | 135 (80.4) | 56 (90.3) | ||
| Grade 2 | 39 (17.0) | 33 (19.6) | 6 (9.7) |
Continuous information is expressed as mean ± standard deviation and categorical information is expressed as n (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Among children with steatotic liver (Table 2), boys and girls showed comparable age (9.8±1.4 vs. 9.8±1.4 years, t=−0.08, P=0.933), height (150.5±9.9 vs. 150.5±10.3 cm, t=−0.03, P=0.979), weight (61.7±14.0 vs. 62.4±16.2 kg, t=−0.33, P=0.745), and BMI (27.0±3.7 vs. 27.2±4.7 kg/m2, t=−0.37, P=0.713). Boys demonstrated larger waist circumferences than girls (89.6±9.6 vs. 84.6±7.5 cm, t=4.10, P<0.001). Liver enzymes differed significantly by sex: boys had higher ALT (40.5±32.3 vs. 29.1±32.4 U/L, t=2.34, P=0.020) and aspartate aminotransferase (AST) levels (29.2±14.5 vs. 24.6±14.1 U/L, t=2.13, P=0.034). No sex-based differences were observed in blood glucose, lipid profiles, or steatotic liver severity (Grade 1: 80.4% boys vs. 90.3% girls; Grade 2: 19.6% boys vs. 9.7% girls, χ2=3.19, P=0.074).
Comparison of quantitative liver assessment in control group, Grade 1 and Grade 2 steatotic liver disease
Table 3 presents the comparison of fat scores between Grade 1 and Grade 2 steatotic liver groups. Similarly, the UGAP was significantly higher in the Grade 2 group (0.83±0.06 dB/cm/MHz) compared to the Grade 1 group (0.70±0.07 dB/cm/MHz, P<0.001). The uDFF also showed a significant increase in the Grade 2 group (19.10%±6.45%) compared to the Grade 1 group (11.52%±5.44%, P<0.001). When stratified by gender, in boys, UGAP (0.83±0.05 vs. 0.70±0.07 dB/cm/MHz, P<0.001) and uDFF (18.58%±6.13% vs. 11.59%±5.41%, P<0.001) were significantly higher with Grade 2 steatotic liver compared to those with Grade 1 steatotic liver. The UGAP was also significantly higher in the Grade 2 group (0.80±0.11 dB/cm/MHz) compared to the Grade 1 group (0.69±0.07 dB/cm/MHz, P=0.001). Additionally, the uDFF was significantly higher in girls with Grade 2 steatotic liver (22.00%±7.95%) compared to those with Grade 1 steatotic liver (11.34%±5.56%, P<0.001). In both boys and girls, UGAP and uDFF were significantly higher in the steatotic liver group of Grade 1 than in the control group.
Table 3
| Assessment | Grade 1 | Grade 2 | t* | P* | Control | t# | P# |
|---|---|---|---|---|---|---|---|
| Boys and girls | |||||||
| UGAP (dB/cm/MHz) | 0.70±0.07 | 0.83±0.06 | −10.1 | <0.001 | 0.53±0.03 | 25.351 | <0.001 |
| uDFF (%) | 11.52±5.44 | 19.10±6.45 | −7.67 | <0.001 | 2.83±0.85 | 20.678 | <0.001 |
| Boys | |||||||
| UGAP (dB/cm/MHz) | 0.70±0.07 | 0.83±0.05 | −11.19 | <0.001 | 0.52±0.03 | 18.385 | <0.001 |
| uDFF (%) | 11.59±5.41 | 18.58±6.13 | −6.46 | <0.001 | 2.67±0.82 | 17.368 | <0.001 |
| Girls | |||||||
| UGAP (dB/cm/MHz) | 0.69±0.07 | 0.80±0.11 | −3.39 | 0.001 | 0.53±0.03 | 14.763 | <0.001 |
| uDFF (%) | 11.34±5.56 | 22.00±7.95 | −4.28 | <0.001 | 2.95±0.87 | 10.934 | <0.001 |
Continuous information is expressed as mean ± standard deviation. *, comparison between grade 1 and grade 2 steatotic liver; #, comparison between grade 1 steatotic liver and control group. uDFF, ultrasound derived fat fraction; UGAP, ultrasound-guided attenuation parameter.
To validate the reliability of the uDFF and UGAP data, a Pearson correlation consistency test was performed (Figure 2). The results demonstrated a significant correlation between the two measurements, particularly within the Grade 2 steatotic liver group.
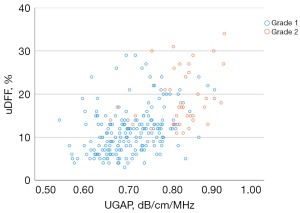
Multivariate linear regression analysis of UGAP, uDFF in children with Grade 1 to Grade 2 steatotic liver disease
Table 4 presents the results of the multiple linear regression analysis for the UGAP in children with Grade 1 to Grade 2 steatotic liver disease, conducted for the overall group as well as separately for boys and girls. In the overall group, TC [β=−0.19, 95% confidence interval (CI): −0.27 to −0.11, P<0.001], TG (β=0.06, 95% CI: 0.03 to 0.09, P<0.001), HDL-C (β=0.18, 95% CI: 0.09 to 0.26, P<0.001), LDL-C (β=0.16, 95% CI: 0.09 to 0.23, P<0.001), and ALT (β=0.00, 95% CI: 0.00 to 0.00, P=0.020) were found to be significant predictors of UGAP. Other factors such as weight, waist circumference, BMI, fasting blood glucose, and AST did not show significant associations with UGAP. When analyzed by gender, TC, TG, HDL-C, LDL-C and ALT were significant predictors in boys but not in girls.
Table 4
| Index | Total | Boys | Girls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |||
| Weight | 0.00 | 0.00 to 0.00 | 0.800 | 0.00 | 0.00 to 0.00 | 0.917 | 0.00 | 0.00 to 0.01 | 0.466 | ||
| Waist | 0.00 | 0.00 to 0.00 | 0.457 | 0.00 | 0.00 to 0.00 | 0.659 | 0.00 | 0.00 to 0.01 | 0.581 | ||
| BMI | 0.00 | −0.01 to 0.01 | 0.842 | 0.00 | 0.00 to 0.00 | 0.359 | 0.00 | −0.01 to 0.01 | 0.836 | ||
| Blood glucose | 0.00 | −0.02 to 0.03 | 0.784 | −0.01 | −0.05 to 0.02 | 0.442 | 0.06 | −0.01 to 0.12 | 0.070 | ||
| Total cholesterol | −0.19 | −0.27 to −0.11 | <0.001 | −0.19 | −0.28 to −0.10 | <0.001 | −0.10 | −0.28 to 0.08 | 0.289 | ||
| Triglycerides | 0.06 | 0.03 to 0.09 | <0.001 | 0.05 | 0.02 to 0.09 | 0.002 | 0.04 | −0.03 to 0.10 | 0.270 | ||
| HDL-C | 0.18 | 0.09 to 0.26 | <0.001 | 0.18 | 0.07 to 0.28 | 0.001 | 0.11 | −0.07 to 0.28 | 0.232 | ||
| LDL-C | 0.16 | 0.09 to 0.23 | <0.001 | 0.16 | 0.08 to 0.24 | <0.001 | 0.09 | −0.07 to 0.25 | 0.283 | ||
| ALT | 0.00 | 0.00 to 0.00 | 0.020 | 0.00 | 0.00 to 0.00 | 0.006 | 0.00 | 0.00 to 0.00 | 0.462 | ||
| AST | 0.00 | 0.00 to 0.00 | 0.542 | 0.00 | 0.00 to 0.00 | 0.252 | 0.00 | 0.00 to 0.00 | 0.166 | ||
Adjusts for gender, age, and all variables in the table, but does not adjust for gender when grouped by gender. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UGAP, ultrasound-guided attenuation parameter.
Table 5 presents the results of the multiple linear regression analysis for uDFF in children with Grade 1 to Grade 2 steatotic liver disease, conducted for the overall group as well as separately for boys and girls. In the overall group, waist circumference (β=0.17, 95% CI: 0.01 to 0.33, P=0.040), TC (β=−7.98, 95% CI: −13.74 to −2.22, P=0.007), TG (β=2.54, 95% CI: 0.48 to 4.61, P=0.016), HDL-C (β=6.89, 95% CI: 0.67 to 13.11, P=0.030), LDL-C (β=6.59, 95% CI: 1.53 to 11.65, P=0.011), ALT (β=0.14, 95% CI: 0.07 to 0.20, P<0.001), and AST (β=−0.18, 95% CI: −0.32 to −0.04, P=0.013) were found to be associated with uDFF. Other factors such as weight, BMI, and fasting blood glucose did not show significant associations with uDFF.
Table 5
| Index | Total | Boys | Girls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |||
| Weight | 0.06 | −0.08 to 0.20 | 0.414 | 0.08 | −0.09 to 0.26 | 0.351 | 0.04 | −0.29 to 0.38 | 0.801 | ||
| Waist | 0.17 | 0.01 to 0.33 | 0.040 | 0.24 | 0.01 to 0.48 | 0.049 | 0.18 | −0.17 to 0.53 | 0.332 | ||
| BMI | −0.26 | −0.67 to 0.15 | 0.214 | −0.55 | −1.09 to −0.01 | 0.049 | −0.12 | −1.06 to 0.80 | 0.809 | ||
| Blood glucose | 1.29 | −0.77 to 3.36 | 0.219 | −0.04 | −2.44 to 2.35 | 0.971 | 4.55 | −0.64 to 9.86 | 0.098 | ||
| Total cholesterol | −7.98 | −13.74 to −2.22 | 0.007 | −7.23 | −13.81 to −0.65 | 0.031 | −6.43 | −21.94 to 9.09 | 0.414 | ||
| Triglycerides | 2.54 | 0.48 to 4.61 | 0.016 | 2.22 | −0.15 to 4.59 | 0.066 | 2.10 | −3.32 to 7.51 | 0.445 | ||
| HDL-C | 6.89 | 0.67 to 13.11 | 0.030 | 6.79 | −0.44 to 14.03 | 0.066 | 3.72 | −11.18 to 18.50 | 0.622 | ||
| LDL-C | 6.59 | 1.53 to 11.65 | 0.011 | 6.12 | 0.36 to 11.88 | 0.037 | 5.88 | −7.82 to 19.61 | 0.398 | ||
| ALT | 0.14 | 0.07 to 0.20 | <0.001 | 0.15 | 0.08 to 0.22 | <0.001 | 0.06 | −0.10 to 0.23 | 0.443 | ||
| AST | −0.18 | −0.32 to −0.04 | 0.013 | −0.23 | −0.39 to −0.07 | 0.005 | 0.01 | −0.36 to 0.38 | 0.956 | ||
Adjust for gender, age and all variables in the table, not for gender when grouping by gender. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; uDFF, ultrasound derived fat fraction.
When analyzed by gender, waist circumference, BMI, TC, ALT and AST were significant in boys but not in girls. These findings indicate that waist circumference, TC, TG, HDL-C, LDL-C, ALT, and AST are significant predictors of uDFF in children with Grade 1 to Grade 2 steatotic liver disease, particularly in boys.
Relationship of uDFF and UGAP to other clinical measures
The relationship between uDFF, UGAP and various clinical measures was assessed using Pearson correlation coefficients (Table 6). The results are presented in the table below. Weight showed a significant positive correlation with uDFF (r=0.293, 95% CI: 0.160 to 0.417, P<0.001). Similarly, waist circumference was also significantly positively correlated with uDFF (r=0.331, 95% CI: 0.187 to 0.463, P<0.001). BMI exhibited a significant positive correlation with uDFF as well (r=0.228, 95% CI: 0.087 to 0.365, P=0.001). Fasting blood glucose had a Grade 2 positive correlation with uDFF (r=0.18, 95% CI: 0.031 to 0.322, P=0.008). TC, TG, HDL-C and LDL-C did not exhibit a significant correlation with uDFF. Liver enzymes showed significant correlations with uDFF: ALT had a strong positive correlation (r=0.357, 95% CI: 0.210 to 0.490, P<0.001) and AST also showed a significant positive correlation (r=0.247, 95% CI: 0.100 to 0.397, P<0.001). The details of UGAP related results were also shown in Table 6.
Table 6
| Index | uDFF | UGAP | |||
|---|---|---|---|---|---|
| Pearson’s correlation coefficient | P | Pearson’s correlation coefficient | P | ||
| Weight | 0.293 | <0.001 | 0.120 | 0.070 | |
| Waist | 0.331 | <0.001 | 0.130 | 0.050 | |
| BMI | 0.228 | 0.001 | 0.090 | 0.200 | |
| Blood glucose | 0.18 | 0.008 | 0.142 | 0.040 | |
| Total cholesterol | −0.017 | 0.804 | 0.030 | 0.070 | |
| Triglycerides | 0.142 | 0.036 | 0.090 | 0.170 | |
| HDL-C | −0.11 | 0.106 | 0.010 | 0.870 | |
| LDL-C | 0.036 | 0.593 | 0.010 | 0.840 | |
| ALT | 0.357 | <0.001 | 0.330 | <0.001 | |
| AST | 0.247 | <0.001 | 0.239 | <0.001 | |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; uDFF, ultrasound derived fat fraction; UGAP, ultrasound-guided attenuation parameter.
Relationship of steatotic liver to other clinical measures
Table 7 presents the biserial correlation analysis of steatotic liver with various clinical measures in children with Grade 1 to Grade 2 steatotic liver disease. Weight showed a significant positive correlation with steatotic liver (r=0.171, 95% CI: 0.050 to 0.298, P=0.011). Similarly, waist circumference was also significantly positively correlated with steatotic liver (r=0.22, 95% CI: 0.083 to 0.358, P=0.001). BMI exhibited a marginally significant positive correlation with steatotic liver (r=0.118, 95% CI: −0.008 to 0.242, P=0.082). Fasting blood glucose, TC, TG and HDL-C had no significant correlation with steatotic liver.
Table 7
| Index | Biserial correlation coefficient | 95% CI | P |
|---|---|---|---|
| Weight | 0.171 | 0.050 to 0.298 | 0.011 |
| Waist | 0.22 | 0.083 to 0.358 | 0.001 |
| BMI | 0.118 | −0.008 to 0.242 | 0.082 |
| Blood glucose | 0.028 | −0.113 to 1.63 | 0.677 |
| Total cholesterol | −0.062 | −1.94 to 0.060 | 0.360 |
| Triglycerides | 0.032 | −0.087 to 0.160 | 0.642 |
| HDL-C | −0.077 | −0.225 to 0.082 | 0.258 |
| LDL-C | −0.006 | −0.138 to 0.122 | 0.929 |
| ALT | 0.147 | 0.032 to 0.307 | 0.030 |
| AST | 0.117 | 0.008 to 0.280 | 0.084 |
| uDFF | 0.461 | 0.335 to 0.576 | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; uDFF, ultrasound derived fat fraction.
Liver enzymes showed significant correlations with steatotic liver: ALT had a significant positive correlation (r=0.147, 95% CI: 0.032 to 0.307, P=0.030), while AST exhibited a marginally significant positive correlation (r=0.117, 95% CI: 0.008 to 0.280, P=0.084). The uDFF showed the strongest positive correlation with steatotic liver (r=0.461, 95% CI: 0.335 to 0.576, P<0.001), indicating a robust association.
Discussion
This study represents the first evaluation of uDFF specifically in children with MASLD, highlighting its potential as a non-invasive diagnostic tool in pediatric populations. The results of this study indicate that as the severity of steatotic liver in children with MASLD increases, the measurements of uDFF and UGAP also rise. For UGAP and uDFF, lipid parameters and liver enzymes were significant predictors in MASLD boys but not in MASLD girls. This highlights potential metabolic differences in how hepatic fat accumulation affects liver attenuation properties between genders. The strong positive correlations between uDFF and clinical measures such as weight, waist circumference, BMI, fasting blood glucose, ALT, and AST suggest that uDFF is a reliable marker for assessing steatotic liver disease.
A previous study in adults has shown that uDFF has a higher diagnostic value than US in detecting steatosis (10). And this study further demonstrated that as the severity of steatotic liver increased, the measurements of UGAP and uDFF also rose. This observation was consistent across both boys and girls, suggesting that these quantitative ultrasound parameters are reliable indicators of hepatic steatosis severity. uDFF and UGAP leverage the attenuation and the backscatter characteristics of ultrasound waves as they propagate through the steatotic liver. Hepatocellular steatosis is often accompanied by cell swelling and ballooning degeneration, which increases the attenuation of ultrasound waves passing through the liver. Therefore, quantitative ultrasound techniques can effectively assess the degree of steatotic liver (10,16,17). Both UGAP and uDFF consistently showed significant increases with greater steatotic liver severity across all groups, reinforcing their robustness as diagnostic tools.
There are several limitations in this study. It is a single-center study and lacks comparative analysis with histopathology and MRI-PDFF. Future research should focus on multicenter, high-quality clinical studies to evaluate diagnostic efficacy, establish diagnostic thresholds, and examine the correlation or consistency with MRI-PDFF. In addition, given the wide variation between platforms of ultrasound fat score assessment, caution must be exercised when interpreting and comparing fat score values obtained from different ultrasound platforms in clinical practice (18).
Conclusions
In conclusion, this study underscores the reliability of uDFF, and UGAP as non-invasive quantitative tools for assessing hepatic steatosis in children with MASLD. The observed gender differences in the predictors of these measures highlight the need for tailored approaches in diagnosing and managing MASLD in boys and girls. Future research should aim to validate these findings in larger, multicenter studies and explore the underlying mechanisms driving these gender-specific differences.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2393/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2393/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Capital Institute of Pediatrics (No. SHERLL2022043) and informed consent was obtained from all children’s legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic Fatty Liver Disease in Children. Semin Liver Dis 2018;38:1-13. [Crossref] [PubMed]
- Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0140908. [Crossref] [PubMed]
- Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology 2019;70:1119-33. [Crossref] [PubMed]
- Dai W, Yao ZZ, Ou-Yang SS, Xu NA, Zhou HX, Li XW, Zhong Y, Luo JY. A cross-sectional study on the prevalence rate and influencing factors of non-alcoholic fatty liver disease in overweight/obese children. Zhongguo Dang Dai Er Ke Za Zhi 2023;25:448-56. [Crossref] [PubMed]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. [Crossref] [PubMed]
- Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, Xanthakos SA. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319-34. [Crossref] [PubMed]
- Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol 2019;25:1307-26. [Crossref] [PubMed]
- Dardanelli EP, Orozco ME, Oliva V, Lutereau JF, Ferrari FA, Bravo MG, Ruvinsky S, Roel M, Barvosa PC, Armeno M, Kaplan JS. Ultrasound attenuation imaging: a reproducible alternative for the noninvasive quantitative assessment of hepatic steatosis in children. Pediatr Radiol 2023;53:1618-28. [Crossref] [PubMed]
- Chen H, Shen H, Han J, Wang P, Song D, Shen H, Wei X, Yang B, Li J. Performance of ATT and UDFF in the diagnosis of non-alcoholic fatty liver: An animal experiment. Heliyon 2024;10:e27993. [Crossref] [PubMed]
- De Robertis R, Spoto F, Autelitano D, Guagenti D, Olivieri A, Zanutto P, Incarbone G, D'Onofrio M. Ultrasound-derived fat fraction for detection of hepatic steatosis and quantification of liver fat content. Radiol Med 2023;128:1174-80. [Crossref] [PubMed]
- Labyed Y, Milkowski A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J Ultrasound Med 2020;39:2427-38. [Crossref] [PubMed]
- Tavaglione F, Flagiello V, Terracciani F, Gallo P, Capparelli E, Spiezia C, De Vincentis A, Palermo A, Scriccia S, Galati G, Napoli N, Daniels SJ, Blau JE, Carlsson B, Khazrai YM, Incalzi RA, Picardi A, Vespasiani-Gentilucci U. Non-invasive assessment of hepatic steatosis by ultrasound-derived fat fraction in individuals at high-risk for metabolic dysfunction-associated steatotic liver disease. Diabetes Metab Res Rev 2024;40:e3787. [Crossref] [PubMed]
- Zalcman M, Barth RA, Rubesova E. Real-time ultrasound-derived fat fraction in pediatric population: feasibility validation with MR-PDFF. Pediatr Radiol 2023;53:2466-75. [Crossref] [PubMed]
- Ko HJ, Woo S, Han J, Kim YM, Lim HJ, Kim MJ, Park YS, Park KH. Which obesity index is the most useful marker for predicting hepatic steatosis in children and adolescents with obesity? A cross-sectional study using quantitative magnetic resonance imaging. Obes Res Clin Pract 2023;17:335-42. [Crossref] [PubMed]
- Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020;73:202-9. [Crossref] [PubMed]
- Ferraioli G, Berzigotti A, Barr RG, Choi BI, Cui XW, Dong Y, Gilja OH, Lee JY, Lee DH, Moriyasu F, Piscaglia F, Sugimoto K, Wong GL, Wong VW, Dietrich CF. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med Biol 2021;47:2803-20. [Crossref] [PubMed]
- Gatos I, Drazinos P, Yarmenitis S, Theotokas I, Koskinas J, Koullias E, Mitranou A, Manesis E, Zoumpoulis PS. Liver Ultrasound Attenuation: An Ultrasound Attenuation Index for Liver Steatosis Assessment. Ultrasound Q 2022;38:124-32. [Crossref] [PubMed]
- Jeon SK, Lee JM. Inter-platform reproducibility of ultrasound-based fat fraction for evaluating hepatic steatosis in nonalcoholic fatty liver disease. Insights Imaging 2024;15:46. [Crossref] [PubMed]


