
Evaluation of the ultrasonic arterial measurement and analysis system for predicting 10-year atherosclerotic cardiovascular disease risk in patients with type 2 diabetes
Introduction
Diabetes, particularly type 2 diabetes mellitus (T2DM), has emerged as a significant global public health concern, with implications for human health (1). In patients with T2DM, cardiovascular and cerebrovascular diseases are the leading causes of mortality, with atherosclerotic cardiovascular disease (ASCVD) posing the greatest burden (2). ASCVD typically manifests as complex and extensive lesions in this patient population, often with poor prognosis. Consequently, it is crucial to assess the cardiovascular risk profile in patients with T2DM.
Hyperglycemia is closely linked to atherosclerosis (AS), which forms the pathological basis for the development of ASCVD in diabetes. Vascular function is compromised even in the early stages of diabetes, often preceding any structural changes in the vasculature. Consequently, early detection of vascular damage in diabetic patients and intervention in the atherosclerotic process have become key areas of clinical research. Aortic stiffness is an independent risk factor for the onset and mortality from ASCVD and a key indicator for predicting future cardiovascular event risks (3). Non-invasive and invasive cardiovascular function tests are essential for assessing aortic stiffness, with carotid-femoral pulse wave velocity (cfPWV) being a well-established parameter for quantifying aortic stiffness (4).
Ultrasound Doppler imaging technology is preferred for its practicality, cost-effectiveness, safety, and non-invasiveness, enabling the precise measurement of cfPWV. The artery stiffness automated measurement system (AMAS) based on ultrasound Doppler imaging represents a novel advancement in ultrasound technology. It offers an automated, efficient, and accurate method for determining cfPWV in a clinical setting (5), thereby facilitating the standardization and automation of aortic stiffness assessment. Ultrasound evaluation of vascular structural parameters, such as carotid intima-media thickness (cIMT), has been extensively utilized in the risk stratification of patients with coronary heart disease (CHD) and in the prediction of adverse cardiovascular events, with a substantial body of related research (6-8). Nevertheless, studies employing the AMAS system for the measurement of cfPWV are relatively scarce.
In this study, we employed the prediction for ASCVD risk in China (China-PAR) (9-11) to estimate the 10-year risk of ASCVD as a surrogate endpoint. We aimed to investigate the utility of the ultrasound AMAS system-measured cfPWV in the cardiovascular disease (CVD) risk assessment of patients with T2DM and evaluate its superiority over cIMT in assessing the vascular health status of this population. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1620/rc).
Methods
Study population
This was an observational cross-sectional clinical study. The participants for this study were recruited at the Affiliated Hospital of Guangdong Medical University between February 2023 and October 2023. The criteria for enrollment for participants with T2DM at baseline were as follows: (I) meeting the 1999 World Health Organization (WHO) criteria for diabetes (12) and aged 20–80 years; (II) complete medical records; and (III) patients who have provided informed consent and are able to cooperate in completing the related examinations. The exclusion criteria included type 1 diabetes, recent diabetic ketoacidosis or hyperglycemic hyperosmolar state, CVDs, recent cardiovascular surgery, congenital or severe valvular heart diseases, malignant tumors, severe liver, kidney, or lung diseases, connective tissue diseases, and incomplete medical records. A total of 126 participants (64 males, 62 females) with an age range of 31–67 (52.13±7.93) years were enrolled. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study received approval from the Ethics Committee of Affiliated Hospital of Guangdong Medical University (No. PJKT2024-087) and informed consent was provided by all individual participants.
Data collection
Clinical data, including demographics, anthropometry, smoking/hypertension/diabetes history, family CVD history, and blood pressure, were collected. Height and weight were measured with 1 cm and 1 kg accuracy, respectively. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Waist circumference was measured 1 cm above the navel with 0.1 cm accuracy. Smoking and CVD history were self-reported. Hypertension was defined by systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg on 3 separate days, or self-reported/medication use. Family CVD history included at least one immediate family member with heart disease, stroke, or transient ischemic attack. Fasting blood tests included lipids, glucose, hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR), serum creatinine (SCR), and serum uric acid (SUA). Medication history included hypoglycemics, antihypertensives, and other cardiovascular drugs. Data were collected by trained operators.
Measurement of cfPWV and cIMT
cfPWV was measured using a VINNO color Doppler ultrasound device (VINNO G86E, Suzhou, China) with the AMAS system (Figure 1). Patients were positioned supine with their necks exposed and electrocardiograms attached. Following heart rate stabilization, the X4-12L probe captured Doppler flow spectra at the right common carotid artery (RCCA) and right common femoral artery (RCFA) bifurcations (5,13,14). A minimum of 15 stable cardiac cycles (heart rate variation ≤ ±3 beats/min) were preserved as dynamic spectral images. The probe position and spectral sampling site were marked on the body’s surface. The linear distance L between the RCCA and RCFA marks was recorded. This distance was measured three times with a soft tape measure positioned above the body surface. After retrieving RCCA dynamic spectral images, the AMAS feature was selected, setting a preset of 10 cardiac cycles. The carotid-R time feature was engaged to analyze 10 cardiac cycles. Similarly, the femoral-R time function was activated to derive the femoral-R time. The cfPWV was calculated by inputting the three L measurements in sequence.
The cIMT was assessed using the Aplio i900 Diagnostic Ultrasound System (Canon Medical Systems Europe, Tochigi, Japan) (Figure 2). Participants were positioned supine with exposed necks and electrocardiograms attached. A high-frequency probe (model PLI-2004BX, 24 MHz) was employed for sequential scans of the bilateral carotid arteries, ensuring clear visualization of the carotid bifurcation and proximal 3 cm. Intima-media thickness was measured at 1.5, 2.0, and 2.5 cm from the carotid bifurcation, coinciding with the R wave peak on the electrocardiogram. The average intima-media thickness of both carotid arteries constituted the cIMT.
The China-PAR model and 10-year ASCVD risk assessment
The 10-year ASCVD risk was evaluated using the China-PAR model (https://www.cvdrisk.com.cn), which is defined as the risk of developing the first ASCVD event over a 10-year period in a population without ASCVD at baseline. To more accurately predict the risk of CVD in the Chinese population, particularly in light of the unique risk factors and epidemiological characteristics prevalent in China, researchers have developed the China-PAR model based on the follow-up cohort of the China-PAR study. This model uses acute myocardial infarction, other CHD deaths, as well as fatal and non-fatal strokes as endpoints. The China-PAR model has demonstrated excellent predictive capabilities in both internal and external validations and has become an effective tool for CVD risk assessment within the country (9,10). Furthermore, Chinese guidelines recommend the use of the China-PAR model for the personalized overall risk assessment and stratification of CVDs, which has been recommended for both the general population and specific patient groups in China, providing a basis for individualized intervention strategies in clinical decision-making (11). Therefore, this study employed the China-PAR model to assess the 10-year risk of ASCVD as a surrogate endpoint. The model incorporated sex, age, urban-rural classification, geographical residence (northern or southern China, delineated by the Yangtze River), waist circumference, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), current blood pressure, antihypertensive treatment, diabetes mellitus, current smoking status, and family history of CVD. Participants were categorized into low- (<5.0%), moderate- (5.0–9.9%), and high- (≥10.0%) risk groups based on their 10-year ASCVD risk assessment.
Repeatability assessment
A total of 30 participants were randomly chosen to undergo a repeatability assessment for their cfPWV and cIMT measurements. The images were independently evaluated by two seasoned sonographers, isolated from the clinical data, and one of these sonographers re-evaluated the images after a two-week interval to assess the repeatability.
Statistical analysis
Continuous variables with normal distribution were presented as mean ± standard deviation (SD) and compared using analysis of variance (ANOVA) or Welch’s test. Non-normally distributed variables were reported as median (interquartile range: 25th–75th percentile) with the Kruskal-Wallis test for intergroup comparisons and Bonferroni correction for multiple comparisons. Count variables were presented as numbers (n) and percentages (%) and compared with chi-square tests. Linear regression was used to assess cfPWV influencing factors and their correlation with 10-year ASCVD risk, whereas logistic regression evaluated the association between cfPWV and high 10-year ASCVD risk. In the multivariate model, statistically significant univariate variables not accounted for in the China-PAR model were adjusted to examine the association between cfPWV and 10-year ASCVD risk. Receiver operating characteristic (ROC) curves were utilized to assess the ability of cfPWV and cIMT to differentiate patients with T2DM at high risk of 10-year ASCVD. The software SPSS 27.0 (IBM Corp., Armonk, NY, USA) was applied for data analysis, whereas GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was used to generate violin plots, scatter plots, and stacked bar charts. A two-tailed P value of <0.05 was considered statistically significant. Intraclass correlation coefficients (ICCs) were utilized to conduct both intra- and inter-operator repeatability assessments. Agreement levels were categorized as follows: poor (ICC ≤0.50), moderate (ICC >0.50 to 0.75), good (ICC >0.75 to 0.90), and very good (ICC >0.90).
Results
Basic characteristics of 10-year ASCVD risk groups
A graphical overview of the participant selection is presented in Figure 3. In this study, a total of 142 patients with T2DM were recruited at the Affiliated Hospital of Guangdong Medical University. Among 142 participants, 126 met the inclusion criteria for our study; 2 were excluded due to inability to obtain cfPWV or poor quality of ultrasound images, and a further 4 patients with type 1 diabetes were excluded. Additionally, 10 participants were excluded due to cardiovascular and cerebrovascular diseases such as CHD and cerebral infarction. Therefore, a total of 126 participants, with a mean age of 52.13±7.93 years, were included in the subsequent analyses. Of the enrolled participants, 64 (50.8%) were male and 38 (30.2%) had hypertension. Participants were stratified into low, moderate, and high-risk categories based on the China-PAR model. The 10-year ASCVD risk within the study population was 6.35% [interquartile range (IQR): 3.75–10.30%]. The distribution of patients across risk groups was as follows: low risk, 40 (31.746%); moderate risk, 46 (36.508%); and high risk, 40 (31.746%). Significant differences were observed in age, waist circumference, SBP, DBP, eGFR, SUA, and SCR level across the three risk groups (all P<0.05). Additionally, significant variations were noted in the prevalence of current smoking, hypertension, and the utilization of antihypertensive medications across the groups (all P<0.05) (Table 1).
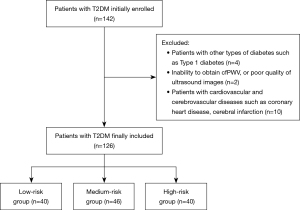
Table 1
| Variables | Total (n=126) | Low-risk (n=40) | Moderate-risk (n=46) | High-risk (n=40) | P value |
|---|---|---|---|---|---|
| Age (years) | 52.13±7.93 | 45.28±7.84 | 53.78±4.64 | 57.10±6.20 | <0.001 |
| Male | 64 (50.8) | 17 (42.5) | 23 (50.0) | 24 (60.0) | 0.219 |
| Urban | 49 (38.9) | 13 (32.5) | 19 (41.3) | 17 (42.5) | 0.601 |
| Height (cm) | 162.44±8.97 | 162.55±9.43 | 162.74±8.69 | 162.00±9.02 | 0.927 |
| Weight (kg) | 62.28±10.73 | 60.34±12.53 | 62.16±9.14 | 64.35±10.38 | 0.248 |
| BMI (kg/m2) | 23.09 (21.23–25.71) | 22.06 (20.64–24.46) | 23.55 (21.65–25.71) | 24.51 (21.68–26.58) | 0.075 |
| Waist (cm) | 85.83±8.49 | 81.99±9.24 | 85.76±6.62 | 89.76±8.02 | 0.001 |
| SBP (mmHg) | 135.43±12.88 | 125.03±9.67 | 135.28±10.50 | 146.00±9.22 | <0.001 |
| DBP (mmHg) | 81.00 (75.00–90.00) | 78.00 (72.25–81.75) | 81.50 (75.75–87.75) | 90.00 (80.00–98.00) | <0.001 |
| FPG (mmol/L) | 7.30 (5.50–10.71) | 7.18 (5.40–11.57) | 7.20 (5.31–9.69) | 7.77 (6.34–13.06) | 0.365 |
| HbA1c (%) | 10.28±2.89 | 10.87±2.79 | 9.82±2.65 | 10.23±3.20 | 0.243 |
| TG (mmol/L) | 1.59 (1.16–2.32) | 1.52 (0.99–2.29) | 1.65 (1.26–2.29) | 1.57 (1.21–2.35) | 0.437 |
| TC (mmol/L) | 5.41±1.19 | 5.35±1.18 | 5.40±1.26 | 5.48±1.13 | 0.878 |
| HDL-C (mmol/L) | 1.07 (0.90–1.25) | 1.14 (0.88–1.36) | 1.05 (0.89–1.25) | 1.07 (0.92–1.17) | 0.671 |
| LDL-C (mmol/L) | 3.35±0.97 | 3.30±0.84 | 3.30±1.12 | 3.46±0.93 | 0.688 |
| eGFR (mL/min) | 102.72 (86.17–110.24) | 110.83 (102.18–116.24) | 101.31 (89.53–107.59) | 91.39 (77.34–103.42) | <0.001 |
| SUA (μmol/L) | 304.15±93.65 | 278.60±74.20 | 301.97±89.04 | 332.23±109.43 | 0.036 |
| SCR (μmol/L) | 63.00 (51.75–78.25) | 57.00 (45.25–70.00) | 64.50 (51.50–77.00) | 66.00 (56.25–88.25) | 0.021 |
| Current smoking | 28 (22.2) | 5 (12.5) | 9 (19.6) | 14 (35.0) | 0.046 |
| Diabetic duration (years) | 4.00 (0.94–10.00) | 2.00 (0.43–5.00) | 5.00 (1.00–10.00) | 5.50 (0.63–10.00) | 0.194 |
| Lipid-lowering agents | 76 (60.3) | 25 (62.5) | 27 (58.7) | 24 (60.0) | 0.936 |
| Hypertension | 38 (30.2) | 1 (2.5) | 10 (21.7) | 27 (67.5) | <0.001 |
| Antihypertensive agents | 31 (24.6) | 1 (2.5) | 9 (19.6) | 21 (52.5) | <0.001 |
| Family history of cardiovascular disease | 23 (18.3) | 6 (15.0) | 11 (23.9) | 6 (15.0) | 0.460 |
| China-PAR risk (%) | 6.35 (3.75–10.30) | 2.70 (1.40–3.58) | 6.35 (5.60–7.33) | 11.00 (10.30–13.00) | <0.001 |
| cfPWV (m/s) | 8.31 (7.13–9.87) | 7.02 (6.38–8.01) | 8.27 (7.47–9.71) | 10.40 (8.72–12.04) | <0.001 |
| cIMT (×10−2 mm) | 70.84 (61.67–81.84) | 62.25 (53.80–70.17) | 72.84 (63.80–81.83) | 79.84 (69.63–88.25) | <0.001 |
Data are expressed as mean ± SD or median (interquartile range) for continuous variables, and as number (%) for categorical variables. China-PAR risk, 10-year ASCVD risk, as assessed by the China-PAR model. ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; cfPWV, carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness; China-PAR, prediction for ASCVD risk in China; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SCR, serum creatinine; SD, standard deviation; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides.
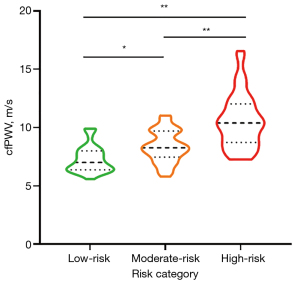
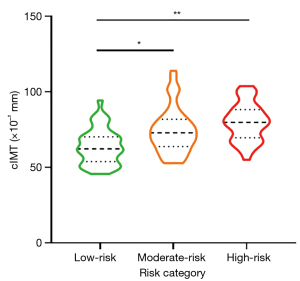
Statistical significance was achieved in the differentiation of cfPWV and cIMT among the low-, moderate-, and high-risk categories [cfPWV: low-risk: 7.02 (IQR: 6.38–8.01) m/s, moderate-risk: 8.27 (IQR: 7.47–9.71) m/s, and high-risk: 10.40 (IQR: 8.72–12.04) m/s, P<0.001; cIMT: low-risk: 62.25 (IQR: 53.80–70.17) ×10−2 mm, moderate-risk: 72.84 (IQR: 63.80–81.83) ×10−2 mm, and high-risk: 79.84 (IQR: 69.63–88.25) ×10−2 mm, P<0.001] (Table 1). Violin plots effectively depicted the distribution of cfPWV and cIMT across the respective risk categories (Figures 4,5). The moderate- and high-risk groups demonstrated significantly higher cfPWV and cIMT values when compared to the low-risk group (all P<0.05). Notably, the high-risk group presented with a significantly elevated cfPWV compared to the moderate-risk group (P<0.001). Although an increasing trend in cIMT was observed in the high-risk group relative to the moderate-risk group, this difference did not reach statistical significance (P>0.05).
Analysis of factors influencing cfPWV
The influencing factors of cfPWV in patients with T2DM are outlined in Table 2. Univariate analysis demonstrated a positive correlation between cfPWV and age (P<0.001), waist circumference (P=0.031), duration of diabetes mellitus (P=0.006), SBP (P<0.001), DBP (P<0.001), TC (P=0.008), low-density lipoprotein cholesterol (LDL-C; P=0.010), SUA (P=0.023), combined hypertension (P<0.001), and cIMT (P=0.014). cfPWV was inversely associated with eGFR (P<0.001). After controlling for confounders, SBP and combined hypertension continued to be significant risk factors for increased cfPWV among patients with T2DM.
Table 2
| Variables | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | t | P value | B | β | t | P value | ||
| Age (years) | 0.093 | 0.347 | 4.121 | <0.001 | 0.041 | 0.152 | 1.814 | 0.072 | |
| Gender | −0.002 | −0.0004 | −0.005 | 0.996 | |||||
| BMI (kg/m2) | 0.058 | 0.092 | 1.028 | 0.306 | |||||
| Waist (cm) | 0.048 | 0.193 | 2.188 | 0.031 | −0.009 | −0.035 | −0.471 | 0.639 | |
| SBP (mmHg) | 0.097 | 0.585 | 8.029 | <0.001 | 0.058 | 0.351 | 3.733 | <0.001 | |
| DBP (mmHg) | 0.099 | 0.478 | 6.058 | <0.001 | 0.025 | 0.119 | 1.308 | 0.194 | |
| TC (mmol/L) | 0.420 | 0.235 | 2.687 | 0.008 | 0.286 | 0.159 | 1.523 | 0.130 | |
| TG (mmol/L) | 0.030 | 0.028 | 0.317 | 0.752 | |||||
| HDL-C (mmol/L) | −0.143 | −0.019 | −0.210 | 0.834 | |||||
| LDL-C (mmol/L) | 0.503 | 0.229 | 2.623 | 0.010 | 0.246 | 0.112 | 1.077 | 0.284 | |
| eGFR (mL/min) | −0.035 | −0.332 | −3.913 | <0.001 | −0.005 | −0.051 | −0.611 | 0.543 | |
| SUA (μmol/L) | 0.005 | 0.203 | 2.305 | 0.023 | 0.001 | 0.026 | 0.330 | 0.742 | |
| cIMT (×10−2 mm) | 0.032 | 0.219 | 2.504 | 0.014 | −0.002 | −0.012 | −0.160 | 0.873 | |
| Diabetic duration (years) | 0.111 | 0.241 | 2.769 | 0.006 | 0.046 | 0.099 | 1.377 | 0.171 | |
| FpG (mmol/L) | 0.047 | 0.085 | 0.946 | 0.346 | |||||
| HbA1c (%) | −0.006 | −0.008 | −0.084 | 0.933 | |||||
| Smoking | 0.527 | 0.103 | 1.158 | 0.249 | |||||
| Hypertension | 2.360 | 0.511 | 6.620 | <0.001 | 0.992 | 0.215 | 2.632 | 0.010 | |
| Family history of cardiovascular disease | 0.641 | 0.117 | 1.311 | 0.192 | |||||
Variables that were significant statistically in univariate analysis (P<0.05) were included in multivariate linear regression analysis. BMI, body mass index; cfPWV, carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides; T2DM, type 2 diabetes mellitus.
Analysis of the relationship between cfPWV and the 10-year risk of ASCVD
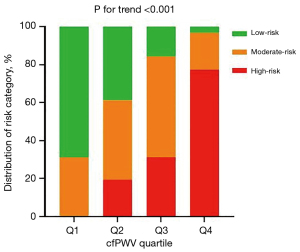
The 10-year ASCVD risk incrementally increased in correlation with elevated cfPWV levels (P<0.001) (Figure 6). The highest quartile of cfPWV exhibited the greatest proportion of individuals at high risk, in contrast to the lowest quartile, which comprised exclusively individuals at low to moderate risk, with a complete absence of high-risk individuals. Trend analysis indicated that there was a tendency for the proportion of high-risk individuals to escalate with increasing cfPWV, and this trend was statistically significant (P<0.001) (Figure 7).
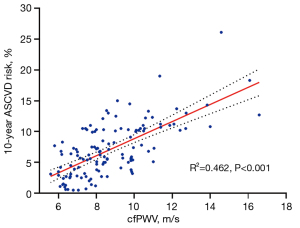
In univariate analyses, when the 10-year risk of ASCVD was treated as a continuous variable, an association was observed between cfPWV and the 10-year ASCVD risk. This association retained its significance following adjustment for covariates such as eGFR, SUA, SCR levels, and combined hypertension. For each 1 m/s increment in cfPWV, the 10-year ASCVD risk is augmented by 0.875% [B =0.875; 95% confidence interval (CI): 0.596–1.155, P<0.001]. After categorizing cfPWV into three grades according to its tertiles, the 10-year ASCVD risk in the middle tertile group rose by 2.216% compared to the lowest tertile (B =2.216, 95% CI: 0.923–3.509, P=0.001), and by 3.248% in the highest tertile group (B =3.248, 95% CI: 1.840–4.657, P<0.001). The findings are tabulated in Table 3.
Table 3
| 10-year ASCVD risk (%) | B | 95% CI | β | t | P value |
|---|---|---|---|---|---|
| Model 1 | |||||
| cfPWV (m/s) | 1.386 | 1.121–1.652 | 0.680 | 10.321 | <0.001 |
| cfPWV tertiles (m/s, median [range]) | |||||
| Q1 [6.66 (≤7.60)] | Reference | ||||
| Q2 [8.31 (7.61–9.37)] | 2.843 | 1.261–4.424 | 0.310 | 3.558 | 0.001 |
| Q3 [10.52 (≥9.38)] | 5.798 | 4.216–7.379 | 0.632 | 7.256 | <0.001 |
| P for trend† | <0.001 | ||||
| Model 2 | |||||
| cfPWV (m/s) | 0.875 | 0.596–1.155 | 0.429 | 6.210 | <0.001 |
| cfPWV tertiles (m/s), median (range) | |||||
| Q1 [6.66 (≤7.60)] | Reference | ||||
| Q2 [8.31 (7.61–9.37)] | 2.216 | 0.923–3.509 | 0.242 | 3.394 | 0.001 |
| Q3 [10.52 (≥9.38)] | 3.248 | 1.840–4.657 | 0.354 | 4.566 | <0.001 |
| P for trend † | <0.001 | ||||
Taking the 10-year ASCVD risk (%) assessed by the China-PAR model as the dependent variables. Model 1 was unadjusted for any variable, and model 2 was adjusted for eGFR, SUA, SCR, and combined hypertension. †, test for trend based on variable containing median value for each tertile. ASCVD, atherosclerotic cardiovascular disease; cfPWV, carotid-femoral pulse wave velocity; China-PAR, prediction for ASCVD risk in China; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio; SCR, serum creatinine; SUA, serum uric acid.
With the endpoint defined as a high 10-year ASCVD risk, an elevation in cfPWV was significantly correlated with an augmented risk of a high 10-year ASCVD risk [odds ratio (OR) =2.451, 95% CI: 1.758–3.417, P<0.001]. Following adjustment for potential confounders, including eGFR, SUA, SCR levels, and combined hypertension, the findings revealed that cfPWV was an independent risk factor (OR =2.015, 95% CI: 1.399–2.902, P<0.001). Upon categorization of cfPWV into three grades according to tertiles, the middle and highest tertile groups demonstrated a significantly higher risk of a high 10-year ASCVD risk, with OR of approximately 4.886 (OR =4.886, 95% CI: 1.028–23.218, P=0.046) and 11.59 (OR =11.594, 95% CI: 2.597–51.758, P=0.001), respectively, compared to the lowest tertile group. The data are tabulated in Table 4.
Table 4
| High 10-year ASCVD risk | B | Wald χ2 | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Model 1 | |||||
| cfPWV (m/s) | 0.897 | 27.953 | 2.451 | 1.758–3.417 | <0.001 |
| cfPWV tertiles (m/s), median (range) | |||||
| Q1 [6.66 (≤7.60)] | Reference | ||||
| Q2 [8.31 (7.61–9.37)] | 1.529 | 4.848 | 4.613 | 1.183–17.989 | 0.028 |
| Q3 [10.52 (≥9.38)] | 3.050 | 20.232 | 21.125 | 5.591–79.812 | <0.001 |
| P for trend† | <0.001 | ||||
| Model 2 | |||||
| cfPWV (m/s) | 0.701 | 14.170 | 2.015 | 1.399–2.902 | <0.001 |
| cfPWV tertiles (m/s), median (range) | |||||
| Q1 [6.66 (≤7.60)] | Reference | ||||
| Q2 [8.31 (7.61–9.37)] | 1.586 | 3.980 | 4.886 | 1.028–23.218 | 0.046 |
| Q3 [10.52 (≥9.38)] | 2.450 | 10.305 | 11.594 | 2.597–51.758 | 0.001 |
| P for trend† | <0.001 |
Taking the 10-year ASCVD risk (low-moderate-risk =0, high-risk =1) assessed by the China-PAR model as the dependent variables, Model 1 was unadjusted for any variable, and model 2 was adjusted for eGFR, SUA, SCR, and combined hypertension. Low-moderate-risk and high-risk were defined as 10-year ASCVD risk of less than 10% and 10% or more, respectively, as calculated by the China-PAR model. †, test for trend based on variable containing median value for each tertile. High 10-year ASCVD risk was defined as the China-PAR model calculated 10-year ASCVD risk ≥10%. ASCVD, atherosclerotic cardiovascular disease; cfPWV, carotid-femoral pulse wave velocity; China-PAR, prediction for ASCVD risk in China; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio; SCR, serum creatinine; SUA, serum uric acid.
ROC curve analyses for cfPWV and cIMT
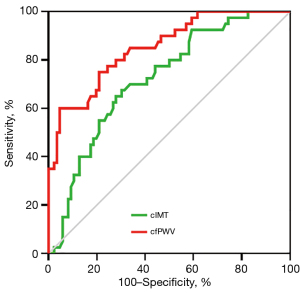
Utilizing ROC curve analyses, we evaluated and contrasted the efficacy of cfPWV and cIMT in identifying patients with T2DM who are at elevated risk for 10-year ASCVD (Figure 8). The results indicated that the area under the curves (AUCs) (95% CI) for cfPWV and cIMT were 0.852 (0.782–0.922) and 0.722 (0.630–0.814), respectively, with both demonstrating statistical significance (all P<0.001). The optimal cutoff values for cfPWV and cIMT were established as 10.02 m/s (sensitivity: 60.0%, specificity: 95.3%) and 74.50×10−2 mm (sensitivity: 67.5%, specificity: 69.8%), respectively. On the basis of AUC values, cfPWV exhibited enhanced predictive capability in comparison to cIMT for identifying patients with T2DM at elevated 10-year ASCVD risk (P=0.038).
Evaluation of repeatability in cfPWV and cIMT measurements
High levels of intra- and inter-observer concordance were observed for cfPWV and cIMT measurements, with statistical significance (all P<0.001; see Table 5).
Table 5
| Ultrasound parameters | Intra-observer | Inter-observer | |||||
|---|---|---|---|---|---|---|---|
| ICC | 95% CI | P value | ICC | 95% CI | P value | ||
| cfPWV | 0.992 | 0.984−0.996 | <0.001 | 0.982 | 0.962−0.992 | <0.001 | |
| cIMT 1.5 | 0.856 | 0.689−0.932 | <0.001 | 0.914 | 0.752−0.964 | <0.001 | |
| cIMT 2.0 | 0.899 | 0.764−0.954 | <0.001 | 0.939 | 0.868−0.971 | <0.001 | |
| cIMT 2.5 | 0.911 | 0.794−0.959 | <0.001 | 0.933 | 0.860−0.968 | <0.001 | |
| Mean cIMT | 0.918 | 0.784−0.965 | <0.001 | 0.957 | 0.910−0.980 | <0.001 | |
cIMT 1.5, the average intima-media thickness at 1.5 cm proximal to the bifurcation of both carotid arteries; cIMT 2.0, the average intima-media thickness at 2.0 cm proximal to the bifurcation of both carotid arteries; cIMT 2.5, the average intima-media thickness at 2.5 cm proximal to the bifurcation of both carotid arteries; Mean cIMT, the average intima-media thickness at the above 6 sites. cfPWV, carotid-femoral pulse wave velocity; CI, confidence interval; cIMT, carotid intima-media thickness; ICC, intraclass correlation coefficients.
Discussion
This study explored the application of cfPWV measured by the AMAS in cardiovascular risk assessment of patients with T2DM, and determined whether cfPWV outperforms cIMT in assessing vascular health in this patient population. It was found that cfPWV exhibited efficacy in screening for patients with T2DM at elevated 10-year ASCVD risk, with its performance surpassing that of cIMT. This method holds potential in aiding clinicians to identify high-risk patients more promptly, thus enabling early intervention and enhancing personalized treatment strategies for patients.
Diabetes is an independent risk factor for ASCVD. According to the China Chronic Disease Prevalence and Risk Factors Study, patients with diabetes face an approximately 1.5–2.5 times higher risk of concurrent CHD and stroke than the non-diabetic group. Additionally, the risk of cardiovascular death is approximately two times higher in patients with diabetes, constituting 43.2% of the total causes of death in this population (15,16). CVDs have become the leading cause of death in patients with diabetes, emphasizing the critical importance of early cardiovascular health assessment to prevent the onset and progression of ASCVD. However, the early assessment of future ASCVD risk is challenging. The China-PAR model is widely utilized for identifying adults who are at an elevated risk of ASCVD in China (9-11), and it has been demonstrated to effectively predict ASCVD risk among Chinese individuals in prior research (17). It is advisable as a first-line tool to predict the 10-year and lifetime ASCVD risk in the Chinese population, as indicated in native Chinese guidelines (11). Accordingly, we used the China-PAR model to assess the 10-year risk of ASCVD as a surrogate endpoint.
Arterial stiffness is closely associated with arteriosclerosis and can serve as an independent predictor of future cardiovascular events and prognosis, and PWV is used to quantitatively assess arterial stiffness and identify early vascular dysfunction (3,4,18). Specifically, an elevated PWV serves as an indicator of early AS. Moreover, cfPWV is regarded as “gold standard” for measuring arterial stiffness, serving as an indicator of aortic arterial stiffness and being associated with cardiovascular events (3,4). However, the measurement of cfPWV using pressure sensors is a prevalent method due to its simplicity. Nevertheless, this method is less sensitive and susceptible to various influences, including patient movement, ambient temperature fluctuations, sensor calibration issues, and individual anatomical differences (19-21). Conversely, the use of ultrasound imaging technology for cfPWV measurement offers higher precision and provides more detailed vascular information. This method effectively accomplishes the objective of evaluating both the vascular function and structure comprehensively. Moreover, in recent years, the advent of the Automated Measurement of AMAS system, which utilizes ultrasonic Doppler imaging technology, has brought about an automated approach to measuring cfPWV. When contrasted with the traditional standard manual method, the AMAS system has been shown to be highly feasible and time-efficient. Consequently, it provides a convenient, rapid, and reliable technique for assessing aortic stiffness in clinical practice (5).
To our knowledge, there is a paucity of literature, with only one report available, on the use of Doppler ultrasound imaging for acquiring cfPWV measurements. Thus, we employed the China-PAR model to assess the 10-year risk of ASCVD as a surrogate endpoint. We conducted a preliminary exploration of the correlation between cfPWV measured using the ultrasonic AMAS system, and the 10-year risk of ASCVD in patients with T2DM. The findings revealed a positive correlation between cfPWV and the 10-year risk of ASCVD in patients with T2DM (B =0.875, 95% CI: 0.596–1.155, P<0.001), highlighting cfPWV as an independent risk factor for a high 10-year ASCVD risk (OR =2.015, 95% CI: 1.399–2.902, P<0.001). This is consistent with a cross-sectional study conducted in a community-based population of 5,282 individuals in Beijing, China, which found that cfPWV correlates with the China-PAR assessment model, with higher cfPWV values associated with an increased 10-year risk of ASCVD (22). Moreover, this study’s ROC curve suggested that a cfPWV threshold exceeding 10.02 m/s necessitates closer monitoring, as it signifies an increased 10-year risk of ASCVD. In alignment with this finding, the cut-off point closely corresponds to the consensus threshold of 10 m/s (23). These results underscore the potential of cfPWV to streamline ASCVD risk assessment and stratification.
The ultrasonic evaluation of cIMT serves as a proxy for structural changes in the arterial wall, which are notably associated with atherosclerosis. Our study demonstrated that cIMT was significantly elevated in both the high- and intermediate-risk groups relative to the low-risk group (P<0.05 for both). Although there was a tendency for cIMT to increase in the high-risk group compared to the intermediate-risk group, this difference did not achieve statistical significance (P>0.05). Despite the utility of cIMT in identifying T2DM individuals at high risk for 10-year ASCVD incidence (AUC =0.722, P<0.001), its predictive performance is less robust than that of cfPWV (AUC =0.852 vs. 0.722; P=0.038). Roumeliotis et al. (24) identified cIMT as an independent predictor of cardiovascular event incidence and mortality risk in diabetic patients with chronic kidney disease, implying its potential for cardiovascular risk stratification. Conversely, Winckler et al. (25) did not endorse the use of cIMT as a predictive marker for cardiovascular events in patients with T2DM. Consequently, we advocate for the integration of cfPWV and cIMT in the ultrasonic assessment of vascular health in patients with T2DM to furnish a more comprehensive and precise clinical evaluation and to inform personalized treatment strategies.
Our study established a correlation between cfPWV, measured using the novel automated methodology AMAS system, and the 10-year risk of ASCVD in patients with T2DM. Furthermore, cfPWV, as a functional marker, outperformed cIMT, a traditional indicator of vascular structure, in identifying patients with T2DM at high 10-year ASCVD risk. However, it is important to acknowledge the limitations of this research. First, our study was cross-sectional and did not incorporate long-term clinical follow-up. Instead, we employed the China-PAR model to estimate the 10-year ASCVD risk as a surrogate endpoint. Nevertheless, the predictive accuracy of the China-PAR model for the Chinese population has been well-established in numerous prior studies (26-28). Second, the single-center recruitment strategy and the modest sample size of our study may hinder its applicability to broader geographic and demographic populations. Third, our study did not include a control group of non-diabetic individuals, which limits our ability to directly attribute the observed ASCVD risk to T2DM alone, as comparisons with a healthy population are not possible. Fourth, the study’s reliance on a single ultrasound examination precluded the exploration of the correlation between the trend of cfPWV changes and the risk of ASCVD. Consequently, to corroborate and enhance the comprehension of these findings, it is imperative to conduct high-quality, large-scale, multicenter cohort studies with extended follow-up periods. Ultimately, the China-PAR model is designed with a specific focus on the Chinese demographic, which inherently restricts its global generalizability. To address this limitation, future research endeavors should assess the integration of our model with established international risk assessment tools through a multicenter, large-scale study. This is essential for broadening the model’s applicability to a wider population. Additionally, prospective cohort studies could offer critical data to refine and validate the combined approach. The goal is to extend the model’s utility across diverse demographics and to ensure that the findings have enhanced universal applicability and clinical relevance.
Conclusions
The cfPWV, as measured by the ultrasonic AMAS system, exhibits a significant positive correlation with the 10-year ASCVD risk as evaluated by the China-PAR model in patients with T2DM. The utility of cfPWV in identifying individuals with T2DM at elevated 10-year ASCVD risk is demonstrated, and it surpasses cIMT in efficacy as a conventional indicator of vascular structure. The ultrasonic AMAS system emerges as an innovative and promising technology for the assessment of ASCVD risk. This technology holds potential in aiding clinicians to identify high-risk patients more promptly, thus enabling early intervention and enhancing personalized treatment strategies for patients.
Acknowledgments
The authors thank all the participants of the present study and the workers in The First Affiliated Hospital of Jinan University and the Affiliated Hospital of Guangdong Medical University.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1620/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1620/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study received approval from the Ethics Committee of Affiliated Hospital of Guangdong Medical University (No. PJKT2024-087) and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023;402:203-34. [Crossref] [PubMed]
- Hong T, Yan Z, Li L, Tang W, Qi L, Ye J, Ren J, Wan Q, Xiao W, Zhao D. The Prevalence of Cardiovascular Disease in Adults with Type 2 Diabetes in China: Results from the Cross-Sectional CAPTURE Study. Diabetes Ther 2022;13:969-81. [Crossref] [PubMed]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318-27. [Crossref] [PubMed]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, Struijker-Boudier H. followed by the addition of the ‘European Network for Non-invasive Investigation of Large Arteries’. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588-605. [Crossref] [PubMed]
- Wang Z, Wang D, Han M, Ai Y, Zhang X, Yuan L, Duan Y, Gao F, Yang Y. A Novel Methodology for Semi-automatic Measurement of Arterial Stiffness by Doppler Ultrasound: Clinical Feasibility and Reproducibility. Ultrasound Med Biol 2021;47:1725-36. [Crossref] [PubMed]
- Hensley B, Huang C, Cruz Martinez CV, Shokoohi H, Liteplo A. Ultrasound Measurement of Carotid Intima-Media Thickness and Plaques in Predicting Coronary Artery Disease. Ultrasound Med Biol 2020;46:1608-13. [Crossref] [PubMed]
- Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014;7:1025-38. [Crossref] [PubMed]
- Kabłak-Ziembicka A, Przewłocki T. Clinical Significance of Carotid Intima-Media Complex and Carotid Plaque Assessment by Ultrasound for the Prediction of Adverse Cardiovascular Events in Primary and Secondary Care Patients. J Clin Med 2021;10:4628. [Crossref] [PubMed]
- Yang X, Li J, Hu D, Chen J, Li Y, Huang J, Liu X, Liu F, Cao J, Shen C, Yu L, Lu F, Wu X, Zhao L, Wu X, Gu D. Predicting the 10-year risks of atheroscleroticcardiovascular disease in Chinese population: The China-PAR project (prediction for ASCVD risk in China). Circulation 2016;134:1430-40. [Crossref] [PubMed]
- Yang XL, Chen JC, Li JX, Cao J, Lu XF, Liu FC, Hu DS, Liu XQ, Shen C, Yu L, Lu FH, Wu XP, Zhao LC, Huang JF, Li Y, Wu XG, Gu DF. Risk stratification of atherosclerotic cardiovascular disease in Chinese adults. Chronic Dis Transl Med 2016;2:102-9. [Crossref] [PubMed]
- Guideline on the assessment and management of cardiovascular risk in China. Zhonghua Yu Fang Yi Xue Za Zhi 2019;53:13-35. [Crossref] [PubMed]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. [Crossref] [PubMed]
- Kaufmann CC, Breyer MK, Hartl S, Gross C, Schiffers C, Wouters EFM, Breyer-Kohansal R, Weber T, Huber K, Agusti A, Burghuber OC. Association of Preserved Ratio Impaired Spirometry with Arterial Stiffness. Ann Am Thorac Soc 2024;21:1289-98. [Crossref] [PubMed]
- Revzin MV, Imanzadeh A, Menias C, Pourjabbar S, Mustafa A, Nezami N, Spektor M, Pellerito JS. Optimizing Image Quality When Evaluating Blood Flow at Doppler US: A Tutorial. Radiographics 2019;39:1501-23. [Crossref] [PubMed]
- Bragg F, Holmes MV, Iona A, Guo Y, Du H, Chen Y, Bian Z, Yang L, Herrington W, Bennett D, Turnbull I, Liu Y, Feng S, Chen J, Clarke R, Collins R, Peto R, Li L, Chen ZChina Kadoorie Biobank Collaborative Group. Association Between Diabetes and Cause-Specific Mortality in Rural and Urban Areas of China. JAMA 2017;317:280-9. [Crossref] [PubMed]
- Bragg F, Li L, Yang L, Guo Y, Chen Y, Bian Z, Chen J, Collins R, Peto R, Wang C, Dong C, Pan R, Zhou J, Xu X, Chen ZChina Kadoorie Biobank (CKB) collaborative group. Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults. PLoS Med 2016;13:e1002026. [Crossref] [PubMed]
- Tang X, Zhang D, He L, Wu N, Si Y, Cao Y, Huang S, Li N, Li J, Dou H, Gao P, Hu Y. Performance of atherosclerotic cardiovascular risk prediction models in a rural Northern Chinese population: Results from the Fangshan Cohort Study. Am Heart J 2019;211:34-44. [Crossref] [PubMed]
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63:636-46. [Crossref] [PubMed]
- Aizawa K, Gates PE, Mawson DM, Elyas S, Casanova F, Gooding KM, Adingupu DD, Strain WD, Shore AC. Carotid-femoral pulse wave velocity acquisition methods and their associations with cardiovascular risk factors and subclinical biomarkers of vascular health. J Hypertens 2022;40:658-65. [Crossref] [PubMed]
- Meng K, Xiao X, Wei W, Chen G, Nashalian A, Shen S, Xiao X, Chen J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv Mater 2022;34:e2109357. [Crossref] [PubMed]
- Xu L, Zhou S, Wang L, Yao Y, Hao L, Qi L, Yao Y, Han H, Mukkamala R, Greenwald SE. Improving the accuracy and robustness of carotid-femoral pulse wave velocity measurement using a simplified tube-load model. Sci Rep 2022;12:5147. [Crossref] [PubMed]
- Yi T, Gao L, Fan F, Jiang Y, Jia J, Zhang Y, Li J, Huo Y. Association between pulse wave velocity and the 10-year risk of atherosclerotic cardiovascular disease in the Chinese population: A community-based study. J Clin Hypertens (Greenwich) 2023;25:278-85. [Crossref] [PubMed]
- Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T. followed by the addition of the ‘Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function,European Network for Noninvasive Investigation of Large Arteries’. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445-8. [Crossref] [PubMed]
- Roumeliotis A, Roumeliotis S, Panagoutsos S, Theodoridis M, Argyriou C, Tavridou A, Georgiadis GS. Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren Fail 2019;41:131-8. [Crossref] [PubMed]
- Winckler K, Thorsteinsson B, Wiinberg N, Jensen AK, Lundby-Christensen L, Heitmann BL, Lund SS, Krarup T, Jensen T, Vestergaard H, Breum L, Sneppen S, Boesgaard T, Madsbad S, Gluud C, Vaag A, Almdal TP, Tarnow L, Tarnow L. followed by the addition of the ‘CIMT trial group’. Prediction of carotid intima-media thickness and its relation to cardiovascular events in persons with type 2 diabetes. J Diabetes Complications 2020;34:107681. [Crossref] [PubMed]
- Cao Q, Li H, Pan X, Wang Y, Zhang P, He L, Wang J, Huang M, Xu F. A Systematic Review and Meta-Analysis of the Predictive Power of China-PAR Against Cardiovascular Disease. Clin Med Res 2024;22:28-36. [Crossref] [PubMed]
- Chen X, Tu Q, Wang D, Liu J, Qin Y, Zhang Y, Xiang Q. Effectiveness of China-PAR and Framingham risk score in assessment of 10-year cardiovascular disease risk in Chinese hypertensive patients. Public Health 2023;220:127-34. [Crossref] [PubMed]
- Zhiting G, Jiaying T, Haiying H, Yuping Z, Qunfei Y, Jingfen J. Cardiovascular disease risk prediction models in the Chinese population- a systematic review and meta-analysis. BMC Public Health 2022;22:1608. [Crossref] [PubMed]


