
Differentiating pulmonary embolism from contrast accumulation-induced beam hardening artifacts using dual-energy CT quantitative parameters
Introduction
Pulmonary embolism (PE) has a high fatality rate, with a 7-day all-cause mortality rate ranging from 1.9% to 2.9%, and a 30-day all-cause mortality rate ranging from 4.9% to 6.6%. The in-hospital mortality rate for acute PE in China has been gradually decreasing, from 25.1% in 1997 to 8.7% in 2008, and it is showing a further downward trend (1). Due to its high sensitivity and specificity, computed tomography pulmonary angiography (CTPA) is recommended as a preferred tool in the evaluation and management of patients with PE (2). However, when CTPA is conducted, even if there is enough saline flushing after the injection of contrast agent, there remains high concentration of contrast agent in the superior vena cava. Due to the absorption of low-energy X-rays, strip artifacts around high-concentrated contrast, namely beam-hardening artifacts (BHAs), can be generated (2,3). BHA can cause low-density areas at local pulmonary arteries on computed tomography (CT) images, obscuring pulmonary vessels and finally rendering an indeterminate diagnosis (4). The artifacts frequently interfere with the observations of the right upper pulmonary artery and the right pulmonary artery wall-attached thrombus. Young and inexperienced radiologists may be confused about whether a low-density area is caused by embolism or BHA, which impedes quick and accurate diagnosis (4,5).
The decrease of BHA has always been a focus of CTPA (5,6). Many efforts have been devoted to innovating image reconstruction algorithms, developing scanning technologies, and modifying contrast injection administration (4,6-8). In particular, fast-kVp switching dual-energy CT (DECT) could generate virtual monochromatic images (VMIs) with energies ranging from 40 to 140 keV, differing from conventional images generated at a polychromatic beam. Reports have stated that BHA could be the most decreased on 80–90 keV VMIs for contrast-enhanced CT imaging (6-8). These studies focused on the improvement of image quality and resolved BHA concerns to some extent; however, the artifact was not eliminated completely (6-8).
DECT also provides various quantitative tools, including the slope of Hounsfield unit (HU) curves (Slope), material decomposition-based material density, and effective atomic number (EffZ) for biochemical assessment, which are useful for vascularization and hemodynamics (9-12). The quantitative parameters derived from DECT have been widely applied in differentiating embolism from normal pulmonary artery with high efficacy, regardless of whether the PE was apparent or occult (9,13-15). Material identification and quantification are based on distinct X-ray attenuation curves for different chemical materials. As X-ray attenuation is modified by beam hardening, the BHA-induced low-CT-density area on pulmonary artery would not represent the true low-density tissue (6). Thus, we assumed quantitative parameters might recognize the beam hardening-decorated low CT density area from the low contrast density embolus and contrast-filled normal arteries. However, to our best knowledge, the above assumption was not verified.
Collectively, this study was conducted to explore the feasibility of quantitative parameters measured on DECT in the differentiation of artifacts on the superior vena cava caused by contrast injection, PE, and normal artery in CTPA for PE patients. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1385/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Zhongshan Hospital (Xiamen), Fudan University (No. B2023-109R) and the requirement for individual consent for this retrospective analysis was waived.
Retrospective and continuous collection was conducted of patients suspected of PE who underwent dual-energy-CTPA (DE-CTPA) at Zhongshan Hospital (Xiamen), Fudan University from March to October 2022. These patients had main complaints of chest tightness, suffocation, or high D-dimer levels.
The inclusion criteria were as follows: (I) patients with PE; (II) patients with contrast injection caused BHA on the right upper pulmonary artery; (III) age >18 years; (IV) no cardiac and renal insufficiency; (V) no iodine contrast contraindication; and (VI) stable vital signs. The exclusion criteria were as follows: (I) patients diagnosed as not having PE; (II) patients without artifacts of the right upper pulmonary artery; (III) those who had contrast allergy; (IV) those who could not cooperate well during the examination such as poor breath holding or continuous motion; (V) those with poor image quality caused by motion artifact and respiratory artifacts; and (VI) thrombus was too small to measure (<2 mm).
CT examination
CTPA was performed using a 256-row Revolution CT (GE HealthCare, Waukesha, WI, USA). All patients underwent imaging craniocaudally in the supine position. Patients were injected with a total of 30 mL iodine contrast medium (370 mg iodine/mL, Ultravist, Bayer, Berlin, Germany) and 30 mL normal saline via antecubital venous access at a rate of 3.5–4.0 mL/s. Patients were scanned during breath holding. The scanning began when the triggering threshold of 100 HU was reached at the level of the pulmonary trunk. The contrast-enhanced scanning was then performed in the gem spectral imaging mode with fast kVp switching between 80 and 140 kVp and smart tube current (range: 250–500 mA), helical pitch: 0.992:1, rotation time: 0.5 s, collimation thickness 0.625 mm, and image reconstruction: Adaptive Statistical Iterative Reconstruction-V (ASIR-V) 50%.
Image processing
The scanning data was transferred to Advanced Workstation 4.7 (GE HealthCare, USA) for image processing and evaluation. Three types of images were generated at 0.625 mm slice thickness: VMIs obtained at energies ranging from 40 to 140 keV, iodine-based material-decomposition images, and effective atom number images.
Measurements and calculation
Regions of interest (ROIs) were placed on the low-density area of the right upper pulmonary artery (artifact), the corresponding normal area of the left upper pulmonary artery (normal, if embolism happens here, change to the nearby normal right middle pulmonary artery), embolism, and pulmonary trunk. CT values of VMIs ranging from 40 to 100 keV (HU40–100 keV), EffZ, and iodine concentration (IC) of ROIs were measured. The minimum area of ROI was approximately 4 mm2, whereas the maximum area did not exceed that of the embolus or the distribution of the contrast agent. Measurements were repeated three times on adjacent slices, and the measured values were averaged for the following calculation. Slope of spectral curve ranging from 40 to 90 keV was calculated according to the following equation: Slope = (HU40 keV − HU90 keV)/(90 keV − 40 keV). The IC and EffZ of artifact, normal artery, and embolism were normalized by IC of pulmonary trunk, according to the following calculations: normalized IC (NIC) =IC(artifact, normal, or embolism)/IC of pulmonary artery trunk, normalized EffZ (NEffZ) =EffZ(artifact, normal, or embolism)/EffZ of pulmonary trunk.
Statistical analysis
Statistical analysis was performed via the software SPSS 26.0 (IBM Corp., Armonk, NY, USA). Quantitative data with normal distribution were expressed as mean ± standard derivation. The comparison among groups was conducted using Friedman method; the multiple comparison was performed utilizing Bonferroni test. Multivariable logistic regression was used to screen variables and to construct multivariable models for differentiation. Receiver operating characteristic (ROC) curve analysis was used for evaluating differentiation efficacy, model quality, area under the curve (AUC), Youden index, best cut-off, and corresponding sensitivity and specificity were obtained. A two-tailed P<0.05 was considered indicative of statistical significance.
Results
Patient characteristics
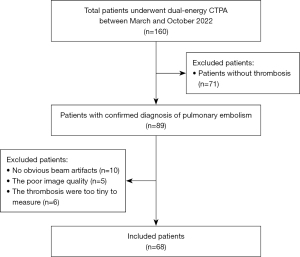
From March to October 2022, 160 patients underwent DE-CTPA scanning at Zhongshan Hospital (Xiamen), Fudan University. Of these, 71 patients without thrombosis were excluded (verified by two radiologists with 8–10-years of experience in chest CT diagnosis). In order to ensure the accuracy of the results, 5 patients were excluded due to poor image quality, 10 patients were excluded because there were no obvious BHAs, and 6 cases were excluded because the quantitative parameters could not be measured due to the too-tiny thrombosis. Finally, A total of 68 patients were included in our study (Figure 1).
There were 35 (51.5%) males and 33 (48.5%) females; their average age was 62.23±13.37 years. Among these 68 patients, 33 had a history of malignant tumors. A total of 59 patients had undergone testing of D-dimer levels, and almost all of them (57/59) showed abnormal results; 23 patients had undergone blood gas analysis testing, and 14 patients had hypoxemia. The embolism was most frequently observed on the segmental artery (34/68), followed by sub-segmental artery (21/68), and 13 cases had embolisms on both lobar and segmental arteries (Table 1).
Table 1
| Characteristics | Value or cases |
|---|---|
| Age (years) | 62.23±13.37 |
| Sex (male/female) | 35/33 |
| Elevated D-dimer level >0.8 mg/L (n=59) | 57 |
| Hypoxemia (n=23) | 14 |
| Location of embolism | |
| Main and lobar artery* | 13 |
| Segmental artery | 34 |
| Sub-segmental artery | 21 |
| History of malignant tumors (n=33) | |
| Liver cancer | 10 |
| Cervical carcinoma | 6 |
| Colorectal cancer | 6 |
| Lung cancer | 4 |
| Other cancer | 7 |
Data are presented as N or mean ± standard deviation. *, if there were multiple embolisms in both the lobar artery and segmental artery, the case was divided into the group of main and lobar artery.
Comparison of DECT quantitative parameters among groups
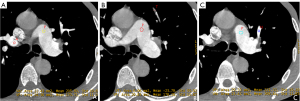
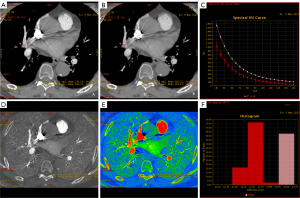
Artifact, embolism, and normal locations had different HU90 keV, HU100 keV, NIC, NEffZ, and Slope, with statistical significance among the three groups (all P<0.05). As shown in Table 2, normal arteries had the highest HU90 keV, HU100 keV, NIC, and NEffZ among the three positions; artifacts had the second highest, whereas embolism showed the lowest (all P<0.05). For slope, an opposite trend was observed, showing the highest for embolism, the second highest for artifacts, and the lowest for normal arteries (all P<0.05). As shown in the representative case (Figure 2), on the 100 keV VMI, the HU100 keV of the artifact (the ROI named A) is a little lower than the normal artery (the ROI named N), which were 205.80 and 269.44 HU, respectively. Meanwhile, the difference between HU100 keV (artifact) and HU100 keV (embolism, ROI named T) is relatively large. We obtained similar results on 90 keV VMIs, iodine maps, effective atomic number images, and spectral curves, as shown in Figure 3, which was consistent with the statistical results.
Table 2
| Parameters | Normal | Artifact | Embolism | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| HU90 keV (HU) | 215.17±63.04 | 163.28±56.55 | 32.51±49.90 | <0.001 | <0.001 | <0.001 |
| HU100 keV (HU) | 172.80±50.07 | 128.05±45.96 | 13.90±49.40 | <0.001 | <0.001 | <0.001 |
| NIC | 0.98±0.12 | 0.80±0.15 | 0.46±0.26 | <0.001 | <0.001 | <0.001 |
| NEffZ | 0.99±0.03 | 0.95±0.04 | 0.84±0.17 | <0.001 | <0.001 | <0.001 |
| Slope | 0.81±0.02 | 0.82±0.03 | 0.91±0.10 | <0.001 | 0.003 | <0.001 |
Data are presented as mean ± standard deviation. HU90 keV and HU100 keV CT values measured on virtual monochromatic images with energy of 90 and 100 keV, respectively. P1 evaluated the difference between normal and embolism; P2 for artifact and embolism; P3 for normal and artifact. CT, computed tomography; DECT, dual-energy computed tomography; HU, Hounsfield unit; NEffZ, normalized effective atomic numbers; NIC, normalized iodine concentration; Slope, slope of Hounsfield unite curve between 40 and 90 keV.
Efficacy of DECT quantitative parameters in differentiating artifact, embolism, and normal artery
The multivariable logistic regression analysis showed the involved parameters for constructing multivariable model to differentiate different statuses (Table 3). The efficacy of DECT quantitative parameters in differentiating artifact and embolism, normal and embolism, and normal and artifact is shown in Tables 4-6 and Figure 4. For the identification between artifact and embolism (Table 4, Figure 4A,4D), all parameters had an AUC higher than 0.800 (0.808–0.963), with model quality ranging from 0.73 to 0.93. Among single parameters, HU100 keV held the best efficacy with an AUC of 0.963, a model quality of 0.93, and a sensitivity of 100%, followed by HU90 keV with an AUC of 0.960, a model quality of 0.93, and a sensitivity of 94.1%. The multi-variables had an AUC of 0.968, comparable to that of HU100 keV (P>0.05). For the differentiation between normal arteries and artifacts (Table 5, Figure 4B,4E), the AUC for all parameters ranged from 0.675 to 0.865. NIC and NEffZ showed good efficacy with AUCs of 0.865 and 0.841 and model qualities of 0.79 and 0.76, respectively, whereas HU90 keV, HU100 keV, and Slope held worse efficacy with both AUC and model quality. After a multivariable analysis combining HU100 keV, NIC, and Slope, the AUC was increased to 0.937 and the model quality increased to 0.90 (P<0.05), giving excellent performance with a sensitivity of 91.2% and a specificity of 86.6%. For distinguishing embolism and normal location (Table 6, Figure 4C,4F), all DECT parameters showed good efficacy (AUC: 0.849–0.991, model quality: 0.78–0.98). Therein, HU90 keV and HU100 keV had the highest AUC (0.99 for HU90 keV and 0.991 for HU100 keV), showing sensitivities of 97.1% and 100%, specificities of 95.6% and 95.6%, and overall model quality of 0.98 and 0.98, respectively. After a multivariable analysis combining HU90 keV, NIC, and Slope, the AUC was increased slightly to 0.995, comparable to those of HU90 keV and HU100 keV (P>0.05).
Table 3
| Parameters | Odds ratio (95% CI) | P value |
|---|---|---|
| Model 1: embolism vs. artifacts (backward_LR) | ||
| HU100 keV | 1.060 (1.032–1.090) | <0.001 |
| NIC* | 0.801 (0.629–1.020) | 0.072 |
| NEffZ# | 2.221 (0.977–5.045) | 0.057 |
| Model 2: artifacts vs. normal (forward_LR) | ||
| HU100 keV | 1.016 (1.005–1.028) | 0.004 |
| NIC* | 1.133 (1.078–1.192) | 0.002 |
| Slope& | 0.732 (0.580–0.924) | <0.001 |
| Model 3: embolism vs. normal (forward_LR) | ||
| HU90 keV | 1.041 (1.009–1.074) | 0.012 |
| NIC* | 1.094 (1.017–1.177) | 0.016 |
| Slope& | 0.636 (0.396–1.022) | 0.061 |
*, the value for NIC is a product of actual NIC and 100. #, the value for NEffZ is a product of actual NEffZ and 100. &, the value for Slope is a product of actual Slope and 100. HU90 keV and HU100 keV, CT values measured on virtual monochromatic images with energy of 90 and 100 keV. CI, confidence interval; HU, Hounsfield unit; LR, logistic regression; NEffZ, normalized effective atomic numbers; NIC, normalized iodine concentration; Slope, slope of Hounsfield unit curve between 40 and 90 keV.
Table 4
| Parameters | AUC | 95% CI | Cut-off | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| HU90 keV | 0.960 | 0.93–0.989 | 78.89 | 94.1% | 88.2% | 0.823 |
| HU100 keV | 0.963 | 0.934–0.992 | 43.60 | 100% | 82.4% | 0.824 |
| NIC | 0.856 | 0.784–0.927 | 0.58 | 97.1% | 75.0% | 0.721 |
| NEffZ | 0.819 | 0.739–0.898 | 0.90 | 97.1% | 72.1% | 0.692 |
| Slope | 0.808 | 0.726–0.89 | 0.85 | 72.1% | 92.6% | 0.647 |
| Multi-variables | 0.968 | 0.943–0.993 | 0.35 | 97.1% | 85.3% | 0.824 |
HU90 keV and HU100 keV, CT values measured on virtual monochromatic images with energy of 90 and 100 keV, respectively; Multi-variables, combined HU100 keV, NIC and NEffZ. AUC, area under the curve; CI, confidence interval; DECT, dual-energy computed tomography; HU, Hounsfield unit; NEffZ, normalized effective atomic numbers; NIC, normalized iodine concentration; Slope, slope of Hounsfield unite curve between 40 and 90 keV.
Table 5
| Parameters | AUC | 95% CI | Cut-off | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| HU90 keV | 0.733 | 0.650–0.817 | 196.6 | 61.8% | 76.1% | 0.379 |
| HU100 keV | 0.746 | 0.664–0.827 | 151.3 | 66.2% | 73.1% | 0.393 |
| NIC | 0.865 | 0.802–0.928 | 0.88 | 86.8% | 76.1% | 0.629 |
| NEffZ | 0.841 | 0.773–0.909 | 0.97 | 83.8% | 73.1% | 0.569 |
| Slope | 0.675 | 0.584–0.767 | 0.83 | 79.4% | 95.5% | 0.749 |
| Multi-variables | 0.937 | 0.896–0.978 | 0.57 | 91.2% | 86.6% | 0.778 |
HU90 keV and HU100 keV, CT values measured on virtual monochromatic images with energy of 90 and 100 keV, respectively; Multi-variables, combined HU100keV, NIC and Slope. AUC, area under the curve; CI, confidence interval; DECT, dual-energy computed tomography; HU, Hounsfield unit; NEffZ, normalized effective atomic numbers; NIC, normalized iodine concentration; Slope, slope of Hounsfield unite curve between 40 and 90 keV.
Table 6
| Parameters | AUC | 95% CI | Cut-off | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| HU90 keV | 0.99 | 0.978–1.002 | 124.23 | 97.1% | 95.6% | 0.927 |
| HU100 keV | 0.991 | 0.979–1.003 | 93.45 | 100% | 95.6% | 0.956 |
| NIC | 0.945 | 0.902–0.988 | 0.85 | 94.1% | 91.2% | 0.853 |
| NEffZ | 0.912 | 0.859–0.965 | 0.96 | 95.6% | 80.9% | 0.765 |
| Slope | 0.849 | 0.773–0.924 | 0.83 | 79.4% | 95.6% | 0.75 |
| Multi-variables | 0.995 | 0.989–1.002 | 0.50 | 98.5% | 95.6% | 0.941 |
HU90 keV and HU100 keV, CT values measured on virtual monochromatic images with energy of 90 and 100 keV, respectively; Multi-variables, combined HU90keV, NIC and Slope. AUC, area under the curve; CI, confidence interval; DECT, dual-energy computed tomography; HU, Hounsfield unit; NEffZ, normalized effective atomic numbers; NIC, normalized iodine concentration; Slope, slope of Hounsfield unite curve between 40 and 90 keV.

Discussion
Contrast injection-induced BHA may hinder the accurate diagnosis of PE (4). Recently, many methods focusing on the improvement of image quality have been used to reduce artifacts. Correction algorithms, VMIs, and optimal contrast injection administrators (injection rate, contrast dose, and contrast concentration) have been demonstrated to be effective for artifact reduction. However, researchers have not been able to completely eliminate the artifacts, and the benefit of improved image quality for PE diagnosis has not been fully discussed (6-8). In another perspective, this study explored the feasibility of DECT spectral quantitative parameters in differentiating artifacts, embolism, and normal arteries. Non-thrombotic PE is an uncommon condition (16), this study only involved thrombotic embolism.
Our study showed that quantitative parameters were significantly different among normal arteries, artifacts, and embolism, which could be utilized to differentiate normal arteries, artifacts, and embolism with desirable efficacy. In particular, 90 and 100 keV VMIs and their HU measurements would be more valuable. Moreover, the efficiency and model quality in identifying normal arteries and artifacts could be improved by multivariable analysis combining HU100 keV, NIC, and Slope. However, the improvement of AUC for multivariable analysis in differentiating other status is limited.
There was a significant difference in spectral parameters between normal and embolism, and spectral parameters had excellent efficiency in the diagnosis of PE. HU90 keV and HU100 keV had the highest AUC among the VMIs. Some studies have shown that lower-kV CT imaging may assist in CTPA diagnostic interpretation (9,17). Meanwhile, another study found significantly higher attenuation values of the pulmonary arteries than embolism when using kV of 100–110 without obvious compromise of image quality (taking noise into consideration) (18). In our study, the best parameters were shown to be HU90 keV and HU100 keV with the highest AUC, which is accordant with previous studies. Moreover, in our study, combining quantitative measurements on 90 keV VMI and iodine maps and Slope did not significantly enhance diagnostic performance, when comparing with HU90 keV and HU100 keV. Notably, a previous study on computer-aided PE detection using variable keV VMIs showed that 60–65 keV VMIs provided the optimal balance between sensitivity and false-positive rates (9). The differences may be derived from the distinct diagnosis mechanisms between radiologist-judgment-based measurements and computer-based algorithms. Additionally, DECT spectral imaging provides both higher-contrast anatomical information and composition quantification. The quantification added value over CTPA in the diagnosis of both apparent and occult PE. Moreover, iodine-based simulated lung perfusion imaging showed a higher application value in the evaluation of PE, with a lower radiation dose and comparable efficacy to true lung CT perfusion (19,20).
For differentiating artifact and embolism, HU100 keV and HU90 keV had the best efficacies, indicating that 90–100 keV may be the best single energy for separating embolism and artifacts. One study verified the use of virtual high-keV VMIs in reducing BHA (15). Stolzmann et al. asserted that BHA reduction was greatest on VMIs at an optimal keV of 108±17 keV (21). Another study pointed out that significant streaking and shadowing can be seen on 40- and 70-keV VMIs, but not on 100- and 120-keV VMIs (22). These results revealed a general trend: high-keV VMIs could reduce beam hardening, whereas lower keV has been reported to substantially increase the contrast enhancement of the pulmonary arteries (23). The balance in contrast and BHA reduction may be responsible for HU100 keV and HU90 keV being the best parameters in PE diagnosis.
For the differentiation between normal artery and artifact, NIC and NEffZ had better performance, which is different from the other differentiation pairs. Compared to the differentiations between normal versus artifact and embolism versus artifact, HU90 keV (AUC: 0.733 vs. 0.960), HU100 keV (AUC: 0.746 vs. 0.963), and Slope (AUC: 0.675 vs. 0.808) had better efficiency in distinguishing embolism from artifacts, whereas NIC and NeffZ had comparable performance in two differentiation pairs, with AUCs ranging from 0.819 to 0.865. It might be explained by the closer iodine concentration or EffZ value between normal and artifact (actually normal artery buried by artifact). However, the VMIs near 90–100 keV can reduce BHA and make the CT values closer between the artifact area affected by BHA and the normal. Nevertheless, by collaborating different variables, the diagnostic efficiency has been improved. However, clinically, radiologists focus more on the differentiation between artifacts and embolism in CTPA, whereas the artifacts and normal artery would both be regarded as normality (as we did not find an individual with artifacts burying on the embolism). Thus, HU90 keV and HU100 keV would be more clinically valuable.
In our study, nearly half of the patients (33/68) had a history of malignant tumors, including ovarian cancer and cervical carcinoma; they also had elevated D-dimer levels, as well as CTPA-diagnosed PE. It should be emphasized that patients with malignant tumors are prone to having higher D-dimer levels due to the inherent activation of coagulation, which is a higher risk for thrombosis and a worse prognosis (24). Therefore, the novel and powerful quantitative tool for improving PE diagnosis in CTPA raised by this study may benefit high-risk patients, including those with malignant tumors.
This study has several limitations. First, the retrospective design inevitably introduced bias, as there was a higher proportion of hospitalized cancer patients, which may influence the results. Cancer patients often exhibit different thrombosis and embolism characteristics compared to other populations. Second, we did not differentiate between non-neoplastic and neoplastic thrombi, which may have distinct characteristics, including their mechanisms of formation, structural features, and responses to imaging modalities. The lack of differentiation between these two thrombus types in our analysis of embolism and artifact may complicate the results, making it difficult to assess the independent impact of each thrombus type on diagnosis. Third, the size, density, and location of artifacts and emboli vary across patients, despite normalization of the DECT parameters. When the density of artifacts is elevated or the embolus is situated near areas affected by BHA, identification may be more challenging, increasing the risk of misdiagnosis as an artifact. Lastly, patients with overlapping artifacts and embolisms were not included in this study. As a result, our findings may not fully address how to manage these complex cases, thereby limiting the generalizability of our conclusions. Prospective studies that subclassify thrombus types, increase sample sizes, and include cases with overlapping artifacts and emboli could enhance diagnostic accuracy and clinical relevance.
Conclusions
In CTPA for PE diagnosis, DECT provides quantitative parameters feasible to differentiate normal artery, embolism, and the artifact interference of contrast medium in the superior vena cava. In particular, 90 and 100 keV VMIs and their HU measurements would be more valuable.
Acknowledgments
We thank Duxing Wan (GE HealthCare) for providing technique assistance.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1385/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1385/coif). S.C. reports that she is employed by GE HealthCare, the manufacturer of the CT equipment used in the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Zhongshan Hospital (Xiamen), Fudan University (No. B2023-109R) and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pulmonary Embolism and Pulmonary Vascular Disease Group, CTS, Pulmonary Embolism and Pulmonary Vascular Disease Committee, CACP, CMDA, National Pulmonary Embolism and Pulmonary Vascular Disease Prevention and Treatment Collaboration Group. Guidelines for the Diagnosis, Treatment, and Prevention of Pulmonary Embolism. Chin Med J 2018;98:1060-87.
- Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics 2004;24:1679-91. [Crossref] [PubMed]
- He T, Qian X, Zhai R, Yang Z. Computed Tomography Number Measurement Consistency Under Different Beam Hardening Conditions: Comparison Between Dual-Energy Spectral Computed Tomography and Conventional Computed Tomography Imaging in Phantom Experiment. J Comput Assist Tomogr 2015;39:981-5. [Crossref] [PubMed]
- Taslakian B, Latson LA, Truong MT, Aaltonen E, Shiau MC, Girvin F, Alpert JB, Wickstrom M, Ko JP. CT pulmonary angiography of adult pulmonary vascular diseases: Technical considerations and interpretive pitfalls. Eur J Radiol 2016;85:2049-63. [Crossref] [PubMed]
- Weiss J, Schabel C, Othman AE, Bier G, Nikolaou K, Bamberg F, Bongers MN. Impact of dual-energy CT post-processing to differentiate venous thrombosis from iodine flux artefacts. Eur Radiol 2018;28:5076-82. [Crossref] [PubMed]
- Coleman AJ, Sinclair M. A beam-hardening correction using dual-energy computed tomography. Phys Med Biol 1985;30:1251-6. [Crossref] [PubMed]
- Elbakri IA, Fessler JA. Statistical image reconstruction for polyenergetic X-ray computed tomography. IEEE Trans Med Imaging 2002;21:89-99. [Crossref] [PubMed]
- Wang W, Liu B, Zhou Y, Wu X, Yu Y, Wang J, Wang L, Zhang S, Shen Y. Selecting optimal monochromatic images of high-definition gemstone spectral CT for beam-hardening artifacts and image noise reduction with phantom. Chin J Med Imaging Technol 2011;27:2349-52.
- Vlahos I, Jacobsen MC, Godoy MC, Stefanidis K, Layman RR. Dual-energy CT in pulmonary vascular disease. Br J Radiol 2022;95:20210699. [Crossref] [PubMed]
- Cao Y, Zhang J, Bao H, Zhang G, Yan X, Wang Z, Ren J, Chai Y, Zhao Z, Zhou J. Development of a Nomogram Combining Clinical Risk Factors and Dual-Energy Spectral CT Parameters for the Preoperative Prediction of Lymph Node Metastasis in Patients With Colorectal Cancer. Front Oncol 2021;11:689176. [Crossref] [PubMed]
- Deng L, Yang J, Ren T, Jing M, Han T, Zhang B, Zhou J. Can spectral computed tomography (CT) replace perfusion CT to assess the histological classification of non-small cell lung cancer? Quant Imaging Med Surg 2023;13:4960-72. [Crossref] [PubMed]
- Elsherif SB, Zheng S, Ganeshan D, Iyer R, Wei W, Bhosale PR. Does dual-energy CT differentiate benign and malignant ovarian tumours? Clin Radiol 2020;75:606-14. [Crossref] [PubMed]
- Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med 2012;33:138-43. [Crossref] [PubMed]
- Farag A, Fielding J, Catanzano T. Role of Dual-energy Computed Tomography in Diagnosis of Acute Pulmonary Emboli, a Review. Semin Ultrasound CT MR 2022;43:333-43. [Crossref] [PubMed]
- Otrakji A, Digumarthy SR, Lo Gullo R, Flores EJ, Shepard JA, Kalra MK, Dual-Energy CT. Spectrum of Thoracic Abnormalities. Radiographics 2016;36:38-52. [Crossref] [PubMed]
- Han D, Lee KS, Franquet T, Müller NL, Kim TS, Kim H, Kwon OJ, Byun HS. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics 2003;23:1521-39. [Crossref] [PubMed]
- Ma G, Dou Y, Dang S, Yu N, Guo Y, Yang C, Lu S, Han D, Jin C. Influence of Monoenergetic Images at Different Energy Levels in Dual-Energy Spectral CT on the Accuracy of Computer-Aided Detection for Pulmonary Embolism. Acad Radiol 2019;26:967-73. [Crossref] [PubMed]
- Matsuoka S, Hunsaker AR, Gill RR, Oliva IB, Trotman-Dickenson B, Jacobson FL, Hatabu H. Vascular enhancement and image quality of MDCT pulmonary angiography in 400 cases: comparison of standard and low kilovoltage settings. AJR Am J Roentgenol 2009;192:1651-6. [Crossref] [PubMed]
- Zhang J, Cai J, Liu S, Zhang X. Value of Dual-energy Lung Perfusion Imaging Using a Dual-source CT System for the Pulmonary Embolism. Open Life Sci 2018;13:107-11. [Crossref] [PubMed]
- Foldyna B, Zangeneh FA, Wagner M, Doktorov K, Basmagi S, Matveeva A, Denecke T, Gohmann R, Gutberlet M, Lehmkuhl L. Pulmonary perfusion defect volume on dual-energy CT: prognostic marker of adverse events in patients with suspected pulmonary embolism. Int J Cardiovasc Imaging 2023;39:1333-41. [Crossref] [PubMed]
- Stolzmann P, Winklhofer S, Schwendener N, Alkadhi H, Thali MJ, Ruder TD. Monoenergetic computed tomography reconstructions reduce beam hardening artifacts from dental restorations. Forensic Sci Med Pathol 2013;9:327-32. [Crossref] [PubMed]
- Yu L, Leng S, McCollough CH. Dual-energy CT-based monochromatic imaging. AJR Am J Roentgenol 2012;199:S9-S15. [Crossref] [PubMed]
- Wannasopha Y, Leesmidt K, Srisuwan T, Euathrongchit J, Tantraworasin A. Value of low-keV virtual monoenergetic plus dual-energy computed tomographic imaging for detection of acute pulmonary embolism. PLoS One 2022;17:e0277060. [Crossref] [PubMed]
- Lubetsky A. Pulmonary Embolism in Cancer Patients: A Review. Isr Med Assoc J 2022;24:179-82. [PubMed]


