
Increased optic nerve stiffness can aid in localizing ipsilateral brain or eye lesions in patients with secondary headaches
Introduction
Headaches are prevalent nervous system disorders and significant contributors to disability (1). They account for up to 5% of visits to emergency departments and acute medical units (2). According to the International Classification of Headache Disorders diagnostic criteria, headache disorders are classified into primary, secondary, and other types (3). Primary headaches are idiopathic pain conditions, whereas secondary headaches result from serious underlying conditions and are associated with high morbidity and mortality (4). Secondary headaches can be caused by brain lesions such as subarachnoid hemorrhage, intracranial mass lesions, and intracranial hypertension or by non-brain-related conditions, including lesions of the eye such as acute angle-closure glaucoma (2,5). Accurate localization and diagnosis of the underlying causes of secondary headaches are crucial for effective management and treatment.
Neuroimaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and digital subtraction angiography (DSA) have be shown to be effective for localizing and diagnosing headache causes, particularly in cases of suspected elevated intracranial pressure (6). Recently, ocular ultrasound (US) has emerged as an alternative neuroimaging method for assessing headache etiology and increased intracranial pressure by measuring the optic nerve sheath diameter in patients with suspected elevated intracranial pressure (7,8). Previous studies have established correlations between increased intracranial pressure and both the optic nerve sheath diameter and the perineural subarachnoid space-to-optic nerve sheath ratio (9-12).
More recently, shear wave elastography (SWE), an advanced US elastographic imaging technique that assesses tissue stiffness or elasticity based on changes in tissue deformation after compression, has been employed to assess tissue stiffness and evaluate optic nerve stiffness for diagnosing increased intracranial and intraocular pressures (13-18). A relationship between increased optic nerve stiffness and elevated intracranial and intraocular pressures has been reported (13-18). However, the relationship between the values of stiffness of the optic nerve, determined by SWE, and the presence of ipsilateral intracranial lesions or eye lesions in patients with secondary headaches is still uncertain. This study aimed to explore the association between optic nerve stiffness values and the existence of these lesions in patients experiencing secondary headaches. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2760/rc).
Methods
Patients
This observational and retrospective study received approval from the Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University (No. KY2024-R214). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and written informed consent was provided by all patients.
A total of 54 patients diagnosed with headaches who underwent optic nerve US from January 2023 to April 2024 in our hospital were retrospectively studied. Patients were included based on the following criteria: (I) optic nerve US (gray-scale and elastography) was performed; (II) patients were hospitalized; (III) they had secondary headache attributed to intracranial lesions or lesions of the eyes; (IV) they underwent cranial CT; (V) they had unilateral intracranial lesions (such as aneurysms, intracranial hematomas, and intracranial tumors) or unilateral eye lesions (such as vitreous hemorrhage, optic nerve injury, and acute angle-closure glaucoma), which were confirmed through surgery, angiography [including DSA, CTA, or magnetic resonance angiography (MRA)], or follow-up after treatment, with the CT and US conducted within 1 week. Patients with only 1 eye US were excluded. The patient selection flowchart is presented in Figure 1.
This study defined intracranial lesions as focal lesions (including vascular and non-vascular ones), regardless of whether they are accompanied by diffuse brain lesions such as subarachnoid hemorrhage. Lesions of the eyes were defined as conditions affecting the eyeball or optic nerve, as these can easily lead to elevated intraocular pressure or pathological changes in the optic nerve. Neuroimaging results are considered the diagnostic standard for patients with brain or eye lesions. For individuals suspected of having vascular diseases, angiography results were utilized; for those with non-vascular diseases, CT scans and/or MRI results were used. The diagnostic criteria for secondary headaches attributed to intracranial lesions or secondary headaches related to eye lesions were based on the guidelines from the International Classification of Headache Disorders (3). Body mass index was calculated as weight in kilograms divided by the square of height in meters. The optic nerves were divided into 2 categories based on the presence or absence of ipsilateral brain or eye lesions: the target group (which has ipsilateral lesions) and the contralateral group (which lacks ipsilateral lesions).
US imaging and analysis
All US examinations, including gray-scale and elastography, were performed in our hospital’s ultrasonographic department. The elastographic examination utilized a LOGIQ E11 US system (GE Healthcare, Milwaukee, WI, USA) with elastographic software and a 6–15 MHz multifrequency linear array transducer. Patients were positioned supine, with their heads at 0 degrees, and instructed to keep their eyes closed and still. US was conducted with the probe in the axial plane. Coupling gel was applied between the transducer and the eyelids to obtain clear images. The optic nerve sheath diameter was measured approximately 3 mm behind the globe, between the outer hypoechoic borders, with the axis perpendicular to the optic nerve (11,12,19). SWE was performed in the transverse imaging plane (11). A color-coded box, adjusted to the size of the posterior triangle of the eye globe, was superimposed on the image. The color spectrum ranged from blue (softer tissues) to red (stiffer tissues). After freezing the images (with the probe held stationary for 3 seconds), a circular region of interest (ROI) with a diameter of 1–4 mm was placed within the nerve based on its diameter to measure SWE stiffness. The average SWE value from 5 ROIs was calculated manually, with an interquartile range (IQR)/median ratio of <30%, and reported in kiloPascals (kPa) (20,21). All US were performed by a radiologist (S.P.C., who has 12 years of experience in elastography US and 30 years of experience in conventional US). The US imaging interpretations were determined through consensus by 3 radiologists (S.P.C., Y.S.X., and L.Y.; Y.S.X. has 10 years of experience in elastography and 26 years in conventional US, whereas L.Y. has 9 years of experience in both elastography and conventional US). All three radiologists were blind to the clinical diagnostic results at the time of the interpretation of US imaging.
Statistical analysis
Statistical analyses were conducted using the software SPSS 27.0 (IBM Corp., Armonk, NY, USA) and MedCalc 12.0 (MedCalc Software, Ostend, Belgium). Continuous variables were presented as means ± standard deviations (SD), whereas categorical variables were presented as percentages. Comparisons between study groups utilized the paired Student’s t-test for normally distributed continuous variables. Statistical significance was set at P<0.05. The receiver operating characteristic (ROC) curve for SWE stiffness values was generated to identify the optimal cutoff for lesion localization in the brain or eye. The optimal cutoff was determined by the highest accuracy, corresponding to the maximum Youden index.
Results
Clinical characteristics of the study cohort
A total of 18 patients with headaches were included in the study, with headache duration ranging from 4 to 216 hours (median 20; IQR: 8–126 hours). The cohort comprised 7 males and 11 females, with a mean age of 52.28±11.78 years (range: 33–71 years) (Table 1). Among these patients, 5 had a history of injury, whereas the remaining 13 had no such history. All patients underwent cranial CT, and 11 had DSA examinations, whereas 12 underwent CT angiography. The interval between cranial imaging and optic nerve US ranged from 1 to 15 days, with a median of 3 days (IQR: 2–4 days). Headache etiologies included unilateral aneurysm rupture with subarachnoid hemorrhage (n=9; 1 with eye involvement), spontaneous subarachnoid hemorrhage with vitreous hemorrhage (n=2), traumatic subarachnoid hemorrhage (n=3; 1 with subdural hematoma, 1 with unilateral optic nerve injury, and 1 with unilateral oculomotor nerve injury), simple right subdural hematoma (n=1), cerebral infarction due to posterior cerebral artery occlusion (n=1), cerebral falx meningioma (n=1), and acute angle-closure glaucoma (n=1).
Table 1
| Parameters | Values (N=18) |
|---|---|
| Age (years) | 33–71(52.28±11.78) |
| Male | 7 (38.9) |
| Height (cm) | 150–175 (162±7.50) |
| Weight (kg) | 46–92.5 (63.79±13.47) |
| Body mass index (kg/m2) | 18.7–31.1 (24.03±3.76) |
| Systolic blood pressure (mmHg) | 100–218 (143.06±28.78) |
| Diastolic blood pressure (mmHg) | 36–106 (84.78±16.08) |
| Optic nerve sheath diameter (mm) | |
| Target side | 2.7–6.8 (5.24±0.11) |
| Contralateral side | 2.5–6.7 (5.06±0.93) |
| Optic nerve stiffness values (kPa)* | |
| Target side | 42.06–221.30 (55.09±12.99) |
| Contralateral side | 21.81–102.93 (22.63±5.33) |
Data are presented as range (mean ± standard deviation) or number (percentage). *, a significant difference (P<0.001) in optic nerve stiffness values between the target and contralateral sides. No significant difference (P>0.05) was observed in optic nerve sheath diameters between the target and contralateral sides.
Among these causes, 5 involved the eye, whereas 13 involved unilateral cerebral lesions, with 3 lesions on the right side and 15 on the left. Of the 18 patients, 13 received interventional therapy or surgery, whereas 5 underwent conservative treatment.
A total of 17 (94.4%, 17/18) patients were followed up in September 2024 via telephone interview or our electronic medical record system by one of the authors (L.Y.). The median interval between SWE examination and the follow-up was 8.23 months (range: 3–13 months). Except for 1 patient who died of unknown cause after discharged from our hospital, no patients complained any symptoms suggestive of injury associated with US examination.
Optic nerve US findings of the study cohort and localization of the lesions
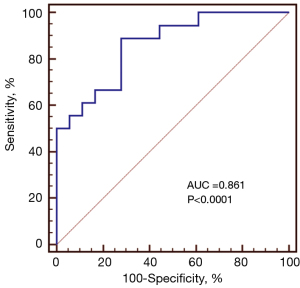
The 36 optic nerves were categorized into two groups based on the presence or absence of ipsilateral brain or eye lesions: a target group (n=18) and a contralateral group (n=18). No significant difference was observed in optic nerve sheath diameter between the groups (P=0.353). However, SWE stiffness values were significantly higher in the target group compared to the contralateral group (P<0.001) (Figure 2). ROC curve analysis showed that the AUC for SWE optic nerve stiffness was 0.861 [95% confidence interval (CI): 0.653–0.986, P<0.0001] (Figure 3). Using a SWE cutoff value of 59.2 kPa for ipsilateral lesion localization, the sensitivity was 88.89% (95% CI: 65.3–98.6%), specificity was 72.22% (95% CI: 46.5–90.3%), positive predictive value (PPV) was 76.2% (95% CI: 52.8–91.8%), negative predictive value (NPV) was 86.7% (95% CI: 59.5–98.3%), and accuracy was 83.3% (95% CI: 67.19–93.63%).
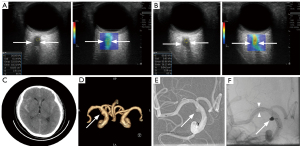
Discussion
The study reveals a significant association between increased optic nerve stiffness and ipsilateral brain or eye lesions in patients with secondary headaches. Optic nerve elastographic US proves valuable for localizing lesions in these patients, with these findings being reported for the first time.
Prior research has indicated elevated optic nerve stiffness in conditions such as idiopathic intracranial hypertension, papilledema, preeclampsia, and glaucoma compared to control groups (13-18). The current results support the findings of recent studies (13-18), suggesting that SWE is effective for assessing optic nerve stiffness to aid in diagnosing intracranial hypertension or elevated intraocular pressure. Given that 83% (15/18) of patients in this study had subarachnoid hemorrhage or related brain injuries, which are often associated with intracranial hypertension, increased optic nerve stiffness would be anticipated. In this study, 17% (3/18) of patients presented with vitreous hemorrhage or acute angle-closure glaucoma, conditions often linked to elevated intraocular pressure and increased optic nerve stiffness. The exact mechanisms connecting optic nerve stiffness with intracranial hypertension or elevated intraocular pressure remain only partially elucidated. When intracranial or intraocular pressure rises, the optic nerve sheath—an extension of the meninges—may become distended with cerebrospinal fluid (22), potentially disrupting its dynamics. Furthermore, both intracranial hypertension and elevated intraocular pressure can cause axoplasmic flow stasis in optic nerve fibers, leading to swelling (23,24). These mechanical alterations likely explain the higher SWE stiffness values observed in patients with these conditions.
In contrast, our findings diverge from previous research (25), which indicated that SWE measurements of the optic nerve are not useful for evaluating optic neuritis in patients with multiple sclerosis. The absence of acute optic neuritis cases in our study might explain this discrepancy, although further investigation is needed to clarify this difference. In addition to differing from the results of previous studies (25), our findings also contrast with earlier research (13,15-18) that compared bilateral optic nerve stiffness values in patients with those of a control group. Specifically, our study took a self-controlled approach by comparing optic nerve stiffness on the affected side with the contralateral side within the same patient. Typically, increased intracranial hypertension is bilateral and symmetric but can present asymmetrically or unilaterally, as seen in asymmetric or unilateral papilledema (14). In our cohort, all patients (18/18) had unilateral lesions, potentially resulting in more pronounced intracranial hypertension or elevated intraocular pressure on the affected side compared to the contralateral side. This disparity may be attributed to the mass effect on the targeted side, resulting in significantly higher optic nerve stiffness values on the target side. This hypothesis warrants further validation.
Furthermore, although differences were noted between the target and control groups in SWE, no significant disparity was observed in the optic nerve sheath diameter measurements. This finding is consistent with a previous study (26) that showed patients with brain injuries leading to increased intracranial hypertension tend to have similar optic nerve sheath diameter measurements on both sides. Several factors contribute to this observation: (I) the majority of patients in this study had a subarachnoid hemorrhage, which can result in increased intracranial hypertension. (II) The US measurement of the optic nerve sheath diameter is a reliable predictor of intracranial hypertension, which is positively associated with the optic nerve sheath diameter. (III) The optic nerve is located in the cerebrospinal fluid of the subarachnoid space; pressure changes on one side can be transmitted to the opposite side, maintaining a balance in the cerebrospinal fluid pressure. As a result, the optic nerve sheath diameter measurements were similar for both groups for these reasons. However, this hypothesis requires further validation.
This study holds significant clinical value. Firstly, it facilitates the localization of brain or eye lesions in patients with secondary headaches, which is particularly useful in bedside or emergency settings, or for those who cannot undergo DSA examinations or are allergic to contrast agents. Secondly, SWE of the optic nerve can be utilized for ongoing monitoring during treatment. Lastly, optic nerve elastographic US offers a novel, non-invasive method for evaluating patients with headaches and may serve as a predictive tool for subarachnoid hemorrhage resulting from unilateral intracranial aneurysm rupture.
Several limitations are acknowledged. This study was retrospective and conducted at a single institution, introducing potential biases in data collection. Not all patients underwent surgery, although all were subjected to imaging, including DSA or follow-up assessments, which confirmed the diagnoses. Additionally, the small size of the patient cohort may have introduced bias. Furthermore, the secondary headaches in our study were caused by several factors, including intracranial aneurysms, intracranial hematomas, intracranial tumors, vitreous hemorrhage, optic nerve injuries, and acute angle-closure glaucoma. It is crucial to approach our SWE findings cautiously when locating other intracranial or intraocular lesions in patients experiencing secondary headaches. Despite these limitations, this study was the first to explore SWE as a new imaging modality for lesion localization in patients with headaches. As a pioneering effort, it demonstrates the feasibility of SWE in localizing brain or eye lesions non-invasively. Further multicenter, larger-scale retrospective, or prospective studies are needed to validate the effectiveness of SWE for lesion localization in patients with secondary headaches.
Conclusions
Increased optic nerve stiffness correlates with the presence of ipsilateral brain or eye lesions in patients with secondary headaches. Optic nerve elastography can aid in localizing lesions in the brain or eye in patients with secondary headaches.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2760/rc
Funding: This research was supported by
Conflicts of Interests: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2760/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University (No. KY2024-R214). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and written informed consent was provided by all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459-80. [Crossref] [PubMed]
- Chinthapalli K, Logan AM, Raj R, Nirmalananthan N. Assessment of acute headache in adults - what the general physician needs to know. Clin Med (Lond) 2018;18:422-7. [Crossref] [PubMed]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders 3rd edition. Cephalalgia 2018;38:1-211.
- Hernandez J, Molina E, Rodriguez A, Woodford S, Nguyen A, Parker G, Lucke-Wold B. Headache Disorders: Differentiating Primary and Secondary Etiologies. J Integr Neurosci 2024;23:43. [Crossref] [PubMed]
- Murthy HD, Mowry SE. The Role of the Otolaryngologist in the Evaluation and Management of Headache. Otolaryngol Clin North Am 2022;55:493-9. [Crossref] [PubMed]
- Wang LL, Mahammedi A, Vagal AS. Imaging of Headache Attributed to Vascular Disorders. Neurol Clin 2022;40:507-30. [Crossref] [PubMed]
- Canakci Y, Koksal O, Durak VA. The value of bedside ocular ultrasound assessment of optic nerve sheath diameter in the detection of increased intracranial pressure in patients presenting to the emergency room with headache. Niger J Clin Pract 2018;21:778-82. [Crossref] [PubMed]
- Aletreby W, Alharthy A, Brindley PG, Kutsogiannis DJ, Faqihi F, Alzayer W, Balhahmar A, Soliman I, Hamido H, Alqahtani SA, Karakitsos D, Blaivas M. Optic Nerve Sheath Diameter Ultrasound for Raised Intracranial Pressure: A Literature Review and Meta-analysis of its Diagnostic Accuracy. J Ultrasound Med 2022;41:585-95. [Crossref] [PubMed]
- Şık N, Ulusoy E, Çitlenbik H, Öztürk A, Er A, Yılmaz D, Duman M. The role of sonographic optic nerve sheath diameter measurements in pediatric head trauma. J Ultrasound 2022;25:957-63. [Crossref] [PubMed]
- Netteland DF, Aarhus M, Smistad E, Sandset EC, Padayachy L, Helseth E, Brekken R. Noninvasive intracranial pressure assessment by optic nerve sheath diameter: Automated measurements as an alternative to clinician-performed measurements. Front Neurol 2023;14:1064492. [Crossref] [PubMed]
- Berhanu D, Carneiro I, Antunes AP, Abegão Pinto L, Fragata I, Tavares Ferreira J, Lucas Neto L. Dimensions of Arachnoid Bulk Ratio: A Superior Optic Nerve Sheath Index for Intracranial Pressure. Radiology 2024;312:e240114. [Crossref] [PubMed]
- Kerscher SR, Zipfel J, Bevot A, Sollmann N, Haas-Lude K, Tellermann J, Schuhmann MU. Non-Invasive Quantitative Approximation of Intracranial Pressure in Pediatric Idiopathic Intracranial Hypertension Based on Point-of-Care Ultrasound of the Optic Nerve Sheath Diameter. Brain Sci 2023;14:32. [Crossref] [PubMed]
- Elsaid N, Belal T, Batouty N, Razek AAKA, Azab A. Effect of changes in optic nerve elasticity on central retinal artery blood flow in patients with idiopathic intracranial hypertension. J Neuroradiol 2022;49:357-63. [Crossref] [PubMed]
- Zhou B, Chen JJ, Kazemi A, Sit AJ, Zhang X. An Ultrasound Vibro-Elastography Technique for Assessing Papilledema. Ultrasound Med Biol 2019;45:2034-9. [Crossref] [PubMed]
- Su S, Zhong H, Wang X, Huang Y, Su Q. Shear wave elastography combined with two-dimensional ultrasonography for detecting optic nerve sheath: An effective tool for assessing preeclampsia. J Clin Ultrasound 2023;51:1412-8. [Crossref] [PubMed]
- Qian X, Li R, Lu G, Jiang L, Kang H, Kirk Shung K, Humayun MS, Zhou Q. Ultrasonic elastography to assess biomechanical properties of the optic nerve head and peripapillary sclera of the eye. Ultrasonics 2021;110:106263. [Crossref] [PubMed]
- Razek AAKA, Elsaid N, Belal T, Batouty N, Azab A. Combined accuracy of optic nerve sheath diameter, strain ratio, and shear wave elastography of the optic nerve in patients with idiopathic intracranial hypertension. Ultrasonography 2022;41:106-13. [Crossref] [PubMed]
- Xu G, Wu X, Yu J, Ding H, Ni Z, Wang Y. Non-invasive intracranial pressure assessment using shear-wave elastography in neuro-critical care patients. J Clin Neurosci 2022;99:261-7. [Crossref] [PubMed]
- Şahan MH, Doğan A, İnal M, Alpua M, Asal N. Evaluation of the Optic Nerve by Strain and Shear Wave Elastography in Patients With Migraine. J Ultrasound Med 2019;38:1153-61. [Crossref] [PubMed]
- Xu JH, Wu ZZ, Tao FY, Zhu ST, Chen SP, Cai C, Liang ZH, Shi BB, Chen B, Xie YP. Ultrasound Shear Wave Elastography for Evaluation of Diaphragm Stiffness in Patients with Stable COPD: A Pilot Trial. J Ultrasound Med 2021;40:2655-63. [Crossref] [PubMed]
- Chen SP, Ye TT, Hong J, Zhu H. Evaluation of Sciatic Nerve Stiffness Using Shear Wave Elastography in Patients with Unilateral Diabetic Foot Ulcers. Diagnostics(Basel) 2023;13:547. [Crossref] [PubMed]
- Malayeri AA, Bavarian S, Mehdizadeh M. Sonographic evaluation of optic nerve diameter in children with raised intracranial pressure. J Ultrasound Med 2005;24:143-7. [Crossref] [PubMed]
- Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res 2016;50:108-44. [Crossref] [PubMed]
- Unal O, Cay N, Yulek F, Taslipinar AG, Bozkurt S, Gumus M. Real-Time Ultrasound Elastographic Features of Primary Open Angle Glaucoma. Ultrasound Q 2016;32:333-7. [Crossref] [PubMed]
- Sahin Ediz S, Atalay B, Aydin Canturk I, Kabaalioglu A. Assessment of the optic nerve, optic disc, and perineural area using shear-wave elastography in patients with multiple sclerosis. Int J Clin Pract 2021;75:e14736. [Crossref] [PubMed]
- Tian J, Wu GB, Liu XB, Wang ZY, Guo JY. The Effect of Different Optic Nerve Sheath Diameter Measurements Using Ultrasound to Assess Intracranial Pressure in Patients With Acute Brain Injury. J Neuroophthalmol 2024;44:201-5. [Crossref] [PubMed]



