
Effects of iterative metal artifact reduction techniques on diagnostic performance in patients with dental artifacts on carotid computed tomography angiography
Introduction
Stroke remains a leading cause of mortality, morbidity, and long-term disability worldwide, with an estimated 12.2 million new cases each year (1). Carotid artery diseases, including atherosclerosis and aneurysms, are major contributors to stroke (2). The global prevalence of carotid plaque among individuals aged 30 to 79 years is 21.1%, while the prevalence of carotid stenosis is 1.5% (3). Carotid computed tomography angiography (CTA) is a widely used imaging technique for vascular imaging because of its multiplanar reconstruction, minimum invasiveness, and ability to diagnose of stenosis (4-6). However, the existence of metal artifacts (MAs) resulting from nonremovable dental prostheses (mainly precious metals and nonprecious metal alloys) can limit the diagnostic performance of CTA. Previous studies have found that MAs could degrade the carotid CTA image quality in 4.7–28% of individuals (7,8). As the population ages, the prevalence of dental prostheses increases, leading to varying degrees of MAs in the CTA images of most patients with vascular diseases in the cervicocephalic region (9). These MAs can lead to errors in assessing the degree of stenosis and may obscure aneurysms, affecting doctors’ ability to make treatment decisions. Therefore, the influence of MAs on image quality must be effectively reduced to enhance the diagnostic performance of CTA.
Currently, metal artifacts reduction (MAR) methods mainly involve the modification of acquisition and reconstruction parameters, projection-based MA reduction techniques, dual energy CT (DECT) or the latest photon-counting detectors, and the combination of these techniques (10-14). DECT, as a hardware approach, can generate virtual monoenergetic images (VMI) and use high energy to reduce MAs (15). Additionally, different vendors have developed their own projection-based MAR algorithms, such as single-energy MAR (SEMAR) by Canon, orthopedic MAR (O-MAR) by Philips, iterative MAR (iMAR) by Siemens Healthineers, and smart MAR (Smart MAR) by GE healthcare (16,17). Among these, iMAR relies on three distinct concepts for MAR, namely normalized frequency-split MAR, beam-hardening correction and sinogram inpainting (18). Several studies have proven that iMAR techniques show encouraging results for substantially reducing MAs for shoulder and hip arthroplasties (19), lower extremity external fixators (20), intracranial coils without increased radiation dose (21). For dental implants, several studies have demonstrated that iMAR, either alone or in combination with VMI, can effectively reduce MAs (22-24). However, most of these studies only evaluate the image quality of mouth and soft tissue without considering angiography. To our knowledge, only one study has explored the performance of iMAR algorithms in carotid CTA images affected by dental implants (17); however, the algorithm was still in the prototype stage at that time and the study primarily focused on the visualization of the carotid artery and evaluation of image quality, while neglecting a comprehensive evaluation of diagnostic effectiveness, particularly in the detection of vessel stenosis and aneurysm.
The objective of this study was to evaluate the effectiveness of the iMAR algorithm in reducing MAs caused by nonremovable dental prostheses in carotid CTA images. This evaluation was conducted through quantitative and qualitative assessments, with a focus on the clinical imaging and detectability of carotid vascular diseases. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2651/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Peking University Third Hospital (No. M2024355) and individual consent for this retrospective analysis was waived.
A cohort of patients suspected of cervicocephalic arteries disease with dental prostheses was consecutively recruited between November 2023 and February 2024. Patients who fulfilled any of the following criteria were excluded from the study: (I) age <18 years, (II) insufficient imaging and clinical data, (III) carotid arteries occlusion. Another control cohort, matched for age-, sex- and body mass, without dental prostheses in carotid CTA images, were included as standard CTA group between March and October 2024 for image quality evaluation. Figure 1 displays the flowchart utilized to ascertain the inclusion of potentially eligible patients.
Image acquisition and postprocessing
CT was conducted using a dual-source CT scanner (Somatom Force, Siemens Healthineers, Munich, Germany). The following scanning protocol were applied: tube voltage, 100 kVp; rotation time, 0.25 s; collimation, 128×0.6; automatic tube current modulation (Care Dose 4D) with reference tube current-time product of 84 mAs; matrix size, 512×512; pitch, 1.2; field of view, 250 mm × 250 mm. The scan ranged from the level of the aortic arch to the skull base. CT data acquisition commenced with bolus tracking in the ascending aorta, utilizing a region of interest (ROI) and a signal attenuation threshold set at 120 Hounsfield units (HUs). The iopromide contrast agent (Ultravist 370, Bayer Schering Pharma, Berlin, Germany) was injected at a flow rate of 4 mL/s (volume, 0.5 mL/kg), followed by a saline flush at a flow rate of 4 mL/s (volume, 40 mL).
Immediately following image acquisition, two image series were reconstructed using a regular workstation (VB10 version, Siemens Healthineers) without and with iMAR (dedicated for dental fillings). Other reconstruction parameters were same: slice thickness of 0.75 mm with increments of 0.5 mm, medium smooth reconstruction kernel (Bv36), and advanced modeled iterative reconstruction (ADMIRE, strength of 3). After reconstruction, non-iMAR-CTA and iMAR-CTA images were transferred to another workstation (syngo.via VB20, Siemens Healthineers) for image analysis.
Intra-arterial digital subtraction angiography (DSA) studies were conducted under the supervision of a neurosurgeon. With the patient under local anesthesia, a 5-F catheter was inserted through a 5-F sheath to access the common carotid artery. Multiple angles, including anteroposterior and lateral projections, were used for carotid angiography. The interventional neuroradiologist interpreted the DSA images without any knowledge of the CTA images.
Image quality analysis
For subjective image quality evaluation, all non-iMAR-CTA and iMAR-CTA images were independently analyzed by three radiologists (radiologist 1 with 4 years, radiologist 2 with 8 years, and radiologist 3 with 22 years of diagnostic experience). Three neuroradiologists were randomly presented with both non-iMAR-CTA and iMAR-CTA images, and each interpretation had at least a 7-day interval. Clinical data were kept hidden from all the radiologists.
Three radiologists subjectively graded the overall image quality for vascular structural discernibility, MA severity, and surrounding soft tissue clarity utilizing the five-point Likert scale (25). The Likert scale was as follows: “excellent” (5 points, almost absent artifacts: vascular structures clearly delineated and abnormalities well shown), “good” (4 points, minor artifacts: vascular structures and abnormalities are depicted but not well described), “moderate” (3 points, moderate artifacts: distinguishability of vascular structures and abnormalities is deteriorating), “unsatisfactory” (2 points, significant artifacts: vascular structures and abnormalities are distinguishable), and “poor” (1 point, extensive artifacts: vascular structures and abnormalities are definitely indistinguishable).
To assess iMAR quantitatively, radiologist 1 set the ROIs (average size, 15 mm2) and measured the mean HU within a specific region (Figure S1). Each measurement was performed three times, and the average HU value was used to calculate the artifact index (AI), signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) (26). Measurements were taken from the vascular lumen near the artifact (ROI1), the vascular lumen located farther from the artifact (ROI2), and the neighboring soft tissue (ROI3), which corresponds to the posterior cervical muscle opposite the oral implant. To guarantee the dimensions and locations of the ROIs were consistent across non-iMAR-CTA and iMAR-CTA images derived from a single patient’s data, the copy–paste tool was employed. For standard CTA, ROIs were placed on similar image slices for comparison.
The SNR was determined using the following formula (27):
The CNR was determined using the following formula (27):
The AI was determined using the following formula (28):
Image diagnostic performance evaluation
The diagnostic capabilities of non-iMAR-CTA and iMAR-CTA was evaluated by three radiologists following three parameters using DSA as gold standard for comparison: (I) stenosis severity is categorized as none, mild (less than 50%), moderate (50% to 69%), or severe (70% to 99%), (II) calcification in the vessel wall (presence or absence), and (III) aneurysm (presence or absence) (29).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 26.0. For descriptive statistics, variables were exhibited as medians and interquartile ranges or means and standard deviations. Subjective evaluation of image quality differences was exhibited utilizing the Wilcoxon signed-rank test and Mann-Whitney U test. Differences in SNR, CNR, and AI among non-iMAR-, iMAR-, and standard CTA were analyzed using t-tests. For assessing the interobserver reliability agreement, the mixed-model two-way single-measure intraclass correlation coefficient (ICC) was utilized. According to Hallgren (30), ICC values were grouped as follows: poor (0–0.49), moderate (0.50–0.74), good (0.75–0.89), and excellent (0.90–1.00). For all analytical procedures, a significance level of P<0.05 was established.
Results
Patient demographics
In total, 172 patients were preliminarily diagnosed with vascular disease in the cervicocephalic region and subsequently underwent carotid CTA. Ultimately, 81 patients with dental hardware (44 males and 37 females; mean age, 63.74±12.26 years; range, 27–87 years) were included in the final patient cohort. An additional 81 age-, sex- and body mass index- matched patients without dental hardware were included as controls cohort. In the final patient cohort, 64 patients underwent DSA. All vascular lesions and structures were confirmed by DSA (luminal stenosis, n=64; aneurysm, n=9; with 9 patients having both luminal stenosis and aneurysms) or by consensus of clinical and imaging information (normal arterial, n=17). Table S1 summarizes the demographic characteristics and DSA outcomes of the study population.
Image quality evaluation results
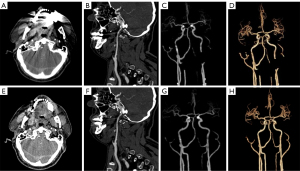
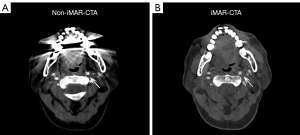
In the subjective image analysis, conducted by applying a five-point Likert scale to evaluate MA strength and image quality, three radiologists unanimously agreed that the image quality of iMAR-CTA surpassed that of non-iMAR-CTA, with statistically significant differences in the ratings [radiologist 1, 5 (5–5) vs. 3 (2–3); radiologist 2, 5 (4–5) vs. 3 (3–3); radiologist 3, 5 (5–5) vs. 2 (2–3), all P<0.001]. There was no significant difference in scores between iMAR-CTA and normal CTA [radiologist 1, 5 (5–5) vs. 5 (5–5), P=0.431; radiologist 2, 5 (4–5) vs. 5 (5–5), P=0.080; radiologist 3, 5 (5–5) vs. 5 (5–5), P=0.694]. Figure 2 presents the CTA images of a patient wherein it can be intuitively understood that iMAR effectively removed MAs without affecting the quality of the normal images. Figure 3 and Table S2 display the outcomes of radiologists’ evaluation of image quality.

In the objective assessment of image quality, iMAR-CTA images exhibited significantly higher SNR (42.90±26.44 vs. 21.07±7.88, P<0.001) and CNR (47.80±28.02 vs. 23.25±8.40, P<0.001) compared to non-iMAR-CTA images. Additionally, the AI of iMAR-CTA was significantly lower than that of non-iMAR-CTA (7.00±3.41 vs. 17.16±5.23, P<0.001). The image quality of iMAR-CTA was comparable to that of standard CTA, with no statistically significant differences in SNR (42.90±26.44 vs. 47.51±31.22, P=0.324) or CNR (47.80±28.02 vs. 45.92±21.23, P=0.109). Table 1 presents the outcomes of objective assessment of iMAR-CTA, non-iMAR-CTA and standard CTA.
Table 1
| Metric | iMAR-CTA | Non-iMAR-CTA | Standard CTA | P value 1 | P value 2 |
|---|---|---|---|---|---|
| SNR | 42.90±26.44 | 21.07±7.88 | 47.51±31.22 | <0.001 | 0.324 |
| CNR | 47.80±28.02 | 23.25±8.40 | 45.92±21.23 | <0.001 | 0.109 |
| AI | 7.00±3.41 | 17.16±5.23 | – | <0.001 | – |
Data are presented as mean ± standard deviation. P value 1: iMAR-CTA vs. non-iMAR-CTA; P value 2: iMAR-CTA vs. standard CTA. P value 1 was obtained using the Wilcoxon signed-rank test to compare iMAR-CTA and non-iMAR-CTA images, while P value 2 was derived from the Mann-Whitney U test to compare standard CTA and iMAR-CTA images. AI, artifact index; CNR, contrast-to-noise ratio; CTA, computed tomography angiography; iMAR, iterative metal artifact reduction; SNR, signal-to-noise ratio.
Diagnostic performance evaluation results
The inter-radiologist evaluation values are enumerated in Table 2. The interobserver reliability for aneurysm identification on non-iMAR-CTA and iMAR-CTA images both showed perfect agreement (ICC: iMAR-CTA, 0.953; non-iMAR-CTA, 0.860). Furthermore, iMAR-CTA images outperformed non-iMAR-CTA images in the visualization of luminal stenosis severity and calcifications. The inter-radiologist evaluation values for luminal stenosis severity and calcification indicated moderate agreement (ICC, 0.694–0.747) on non-iMAR-CTA and perfect agreement (ICC, 0.908–0.910) on iMAR-CTA images.
Table 2
| Vascular abnormality | ICC (95% CI) | |
|---|---|---|
| iMAR-CTA | Non-iMAR CTA | |
| Luminal stenosis severity | 0.910 (0.873, 0.938) | 0.747 (0.659, 0.819) |
| Calcification | 0.908 (0.870, 0.937) | 0.694 (0.595, 0.779) |
| Aneurysm | 0.953 (0.932, 0.968) | 0.860 (0.805, 0.902) |
CI, confidence interval; CTA, computed tomography angiography; iMAR, iterative metal artifact reduction; ICC, intraclass correlation coefficient.
Tables 3-5 present the evaluations of luminal stenosis severity and aneurysm on non-iMAR-CTA and iMAR-CTA images, in contrast to the reference standard DSA. The agreement between CTA and DSA was moderate-to-good for non-iMAR-CTA (ICC, 0.583–0.777) and good-to-excellent for iMAR-CTA (ICC, 0.859–0.946). When evaluating the severity of luminal stenosis, iMAR-CTA demonstrated superior accuracy to non-iMAR-CTA, with accuracy rates ranging from 90.63% to 93.75% (58/64–60/64) across three radiologists compared to the accuracy rates of non-iMAR-CTA ranging from 57.81% to 65.63% (37/64–42/64). Notably, the incidence of luminal stenosis tended to be overestimated in 31.25–37.5% of patients (radiologist 1, 23/64; radiologist 2, 24/64; radiologist 3, 20/64) when non-iMAR-CTA images were used. Furthermore, iMAR-CTA demonstrated higher accuracy rates in aneurysm detection by the three radiologists (7/9–8/9; 77.78–88.89%) than non-iMAR-CTA (4/9–6/9; 44.44–66.67%). Figure 4 shows a case in which streak MAs hindered accurate diagnosis of stenosis severity. Figure 5 shows a case in which the presence of MAs interfered with radiologists’ accurate identification of vascular calcification, causing a false-positive detection, and incorporating iMAR technique significantly enhanced image quality and visualization of the internal carotid artery.
Table 3
| Comparison groups | Radiologist | ICC (95% CI) | |
|---|---|---|---|
| Luminal stenosis severity | Aneurysm | ||
| DSA vs. iMAR-CTA | Radiologist 1 | 0.882 (0.813, 0.927) | 0.933 (0.893, 0.959) |
| Radiologist 2 | 0.917 (0.867, 0.949) | 0.859 (0.779, 0.912) | |
| Radiologist 3 | 0.946 (0.913, 0.967) | 0.933 (0.893, 0.959) | |
| DSA vs. non-iMAR-CTA | Radiologist 1 | 0.610 (0.342, 0.769) | 0.686 (0.529, 0.797) |
| Radiologist 2 | 0.681 (0.410, 0.821) | 0.583 (0.393, 0.725) | |
| Radiologist 3 | 0.624 (0.369, 0.776) | 0.777 (0.658, 0.859) | |
CI, confidence interval; CTA, computed tomography angiography; DSA, digital subtraction angiography; iMAR, iterative metal artifact reduction; ICC, intraclass correlation coefficient.
Table 4
| Modality | Radiologist | Metric | DSA | |||
|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | |||
| iMAR-CTA | Radiologist 1 | None | – | – | – | – |
| Mild | – | 29 | 2 | – | ||
| Moderate | – | 2 | 18 | 1 | ||
| Severe | – | 1 | – | 11 | ||
| Radiologist 2 | None | – | – | – | – | |
| Mild | – | 30 | – | – | ||
| Moderate | – | 2 | 19 | 3 | ||
| Severe | – | – | 1 | 9 | ||
| Radiologist 3 | None | – | – | – | – | |
| Mild | – | 30 | 1 | – | ||
| Moderate | – | 2 | 19 | 1 | ||
| Severe | – | – | – | 11 | ||
CTA, computed tomography angiography; DSA, digital subtraction angiography; iMAR, iterative metal artifact reduction.
Table 5
| Modality | Radiologist | Metric | DSA | |||
|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | |||
| Non-iMAR-CTA | Radiologist 1 | None | – | – | – | – |
| Mild | – | 16 | 1 | – | ||
| Moderate | – | 14 | 12 | 2 | ||
| Severe | – | 2 | 7 | 10 | ||
| Radiologist 2 | None | – | – | – | – | |
| Mild | – | 19 | – | – | ||
| Moderate | – | 13 | 9 | 3 | ||
| Severe | – | – | 11 | 9 | ||
| Radiologist 3 | None | – | – | – | – | |
| Mild | – | 18 | – | – | ||
| Moderate | – | 11 | 14 | 2 | ||
| Severe | – | 3 | 6 | 10 | ||
CTA, computed tomography angiography; DSA, digital subtraction angiography; iMAR, iterative metal artifact reduction.

Discussion
In this study, the iMAR algorithm not only effectively diminishes MAs and enhances the CNR and SNR of carotid angiography images, achieving image quality comparable to standard CTA. Furthermore, it significantly improves diagnostic accuracy for vascular diseases in the cervicocephalic region. For diagnostic performance evaluation, iMAR-CTA exhibited good to excellent agreement with DSA for both luminal stenosis and aneurysm (ICC, 0.859–0.946), exceeding the moderate to good agreement of non-iMAR-CTA (ICC, 0.583–0.777). In particular, iMAR-CTA exhibited superior accuracy to non-iMAR-CTA in assessing the severity of luminal stenosis. Accuracy rates were significantly higher, ranging from 90.63% to 93.75% across three radiologists, compared with the lower accuracy rates of 57.81–65.63% observed with non-iMAR-CTA.
Carotid CTA is a noninvasive and effective vascular imaging technique commonly used for diagnosing vascular lesions, including stenosis, aneurysms and dissections (4,5). However, MAs from dental prostheses can interfere with accurate evaluation of stenosis severity and obscure aneurysms, potentially hindering clinical decision-making. Several studies have explored various techniques in lowering MAs and improving image quality in carotid CTA images. Morsbach et al. (17) evaluated measured CT attenuation values and performed subjective image quality assessments, demonstrating that the MAR algorithm combined with the iterative frequency split method effectively increasing image quality and improving the accuracy of CT attenuation in carotid CTA. Kuya et al. (31) demonstrated the effectiveness of the model-based iterative reconstruction technique in reducing dental MAs by quantifying the reduction in AI index and conducting subjective evaluations of image quality. Nevertheless, the aforementioned studies have not included a comparative analysis with DSA images, which are considered the gold reference standard for assessing vascular lesions. Such a comparison is crucial for validating the diagnostic accuracy and clinical applicability of alternative imaging techniques.
In our study, three experienced radiologists independently assessed the image quality of iMAR-CTA, non-iMAR-CTA and normal CTA using a five-point Likert scale, revealing a statistically significant difference among the groups. In Figure 3, we observed that the spread in image quality scores was notably larger in the non-iMAR CTA group across all three radiologists. This increased variability may be attributed to the presence of MAs, which are more prevalent in non-iMAR images. These MAs can degrade image quality, leading to inconsistencies in the evaluation process. As a result, the presence of MAs could introduce diagnostic uncertainties, influencing radiologists' confidence in their assessments and potentially affecting the overall diagnostic performance. Additionally, objective metrics, including CNR, SNR, and AI index, were calculated for iMAR-CTA and non-iMAR-CTA images and compared to a control group without dental prostheses. The results showed no statistically significant difference in CNR and SNR between iMAR-CTA and standard CTA, confirming the effectiveness of iMAR technology in maintaining image quality comparable to cases without MAs. To evaluate diagnostic performance, this study compared the accuracy of iMAR-CTA and non-iMAR-CTA in diagnosing vascular diseases, using DSA results as the reference standard. In terms of the precision and efficacy of vascular disease diagnosis, iMAR-CTA exhibited superior accuracy to non-iMAR-CTA in assessing the severity of luminal stenosis. Specifically, iMAR-CTA achieved significantly higher accuracy rates (90.63–93.75%) than non-iMAR-CTA (57.81–65.63%). Meanwhile, iMAR-CTA demonstrated superior accuracy in aneurysm detection (77.78–88.89%) than non-iMAR-CTA (44.44–66.67%). MAs can interfere with radiologists’ accurate identification of vascular calcifications; three experienced radiologists all mistakenly identified MAs in some non-iMAR-CTA images (radiologist 1, n=3; radiologist 2, n=2; radiologist 3, n=3) as calcifications, leading to false-positive results. Concerning inter-radiologist reliability, iMAR-CTA demonstrated better diagnostic performance than non-iMAR-CTA.
Compared with standard CTA images, a concern regarding iMAR-CTA is the new and overcorrected artifacts. Several previous studies reported that iMAR may produce algorithm-introduced artifact (18,23). In our study, overcorrected artifacts indeed emerged in the iMAR-CTA images of 36 patients. Notably, these artifacts were primarily observed near the teeth and maxillofacial skeleton, thus not significantly affecting the diagnostic accuracy for vascular diseases. Given the potential emergence of overcorrected artifacts, the hypothesis that the iMAR technique may adversely affect the diagnosis of vascular diseases should be taken with caution. Thus, both conventional CTA and iMAR reconstructions must be assessed in clinical settings. Recently, a novel version of iMAR was reported to overcome these drawbacks and show great improvements in dental fillings (32).
There are several limitations in this study that require consideration. First, this was a single-center study involving a small sample size; therefore, multicenter studies encompassing large sample sizes are required to validate our findings. Second, due to the retrospective analysis, selection bias could not be completely eliminated, even with the stringent exclusion criteria. Finally, emerging imaging technologies, for instance dual-energy CT, have not been compared with the iMAR algorithm in evaluating MAs, thereby providing avenues for further research.
Conclusions
This study used iMAR for reconstructing CTA images in patients with dental joint prostheses. iMAR significantly reduced MAs while achieving image quality comparable to standard CTA, enabling more reliable determination of the carotid anatomy and accurate diagnosis of carotid artery diseases.
Acknowledgments
We would like to thank Medelite for English language editing.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2651/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2651/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Peking University Third Hospital (No. M2024355) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315-81. [Crossref] [PubMed]
- Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, Fowkes FGR, Fowkes FJI, Rudan I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health 2020;8:e721-9. [Crossref] [PubMed]
- Lyu J, Fu Y, Yang M, Xiong Y, Duan Q, Duan C, Wang X, Xing X, Zhang D, Lin J, Luo C, Ma X, Bian X, Hu J, Li C, Huang J, Zhang W, Zhang Y, Su S, Lou X. Generative Adversarial Network-based Noncontrast CT Angiography for Aorta and Carotid Arteries. Radiology 2023;309:e230681. [Crossref] [PubMed]
- van Dam-Nolen DHK, Truijman MTB, van der Kolk AG, Liem MI, Schreuder FHBM, Boersma E, Daemen MJAP, Mess WH, van Oostenbrugge RJ, van der Steen AFW, Bos D, Koudstaal PJ, Nederkoorn PJ, Hendrikse J, van der Lugt A, Kooi ME. Carotid Plaque Characteristics Predict Recurrent Ischemic Stroke and TIA: The PARISK (Plaque At RISK) Study. JACC Cardiovasc Imaging 2022;15:1715-26. [Crossref] [PubMed]
- Hansen NJ. Computed Tomographic Angiography of the Abdominal Aorta. Radiol Clin North Am 2016;54:35-54. [Crossref] [PubMed]
- Kondratyev E, Karmazanovsky G. Low radiation dose 256-MDCT angiography of the carotid arteries: effect of hybrid iterative reconstruction technique on noise, artifacts, and image quality. Eur J Radiol 2013;82:2233-9. [Crossref] [PubMed]
- Kim JJ, Dillon WP, Glastonbury CM, Provenzale JM, Wintermark M. Sixty-four-section multidetector CT angiography of carotid arteries: a systematic analysis of image quality and artifacts. AJNR Am J Neuroradiol 2010;31:91-9. [Crossref] [PubMed]
- Zhao Y, Kim B. The Effects of the Expansion of Dental Care Coverage for the Elderly. Healthcare (Basel) 2024.
- Selles M, van Osch JAC, Maas M, Boomsma MF, Wellenberg RHH. Advances in metal artifact reduction in CT images: A review of traditional and novel metal artifact reduction techniques. Eur J Radiol 2024;170:111276. [Crossref] [PubMed]
- Subhas N, Pursyko CP, Polster JM, Obuchowski NA, Primak AN, Dong FF, Herts BR. Dose Reduction With Dedicated CT Metal Artifact Reduction Algorithm: CT Phantom Study. AJR Am J Roentgenol 2018;210:593-600. [Crossref] [PubMed]
- Kim J, Park C, Jeong HS, Song YS, Lee IS, Jung Y, Lee SM. The Optimal Combination of Monochromatic and Metal Artifact Reconstruction Dual-energy CT to Evaluate Total Knee Replacement Arthroplasty. Eur J Radiol 2020;132:109254. [Crossref] [PubMed]
- Hegazy MAA, Eldib ME, Hernandez D, Cho MH, Cho MH, Lee SY. Dual-energy-based metal segmentation for metal artifact reduction in dental computed tomography. Med Phys 2018;45:714-24. [Crossref] [PubMed]
- Zhu D, Zhang Z, Zou Y, Zhang G, Cheng X, Wan D, Ai S. Experimental and clinical validation of an artificial intelligence metal artifact correction algorithm for low-dose following up CT of percutaneous vertebroplasty. Quant Imaging Med Surg 2024;14:6843-55. [Crossref] [PubMed]
- Katsura M, Sato J, Akahane M, Kunimatsu A, Abe O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018;38:450-61. [Crossref] [PubMed]
- Wagenaar D, van der Graaf ER, van der Schaaf A, Greuter MJ. Quantitative comparison of commercial and non-commercial metal artifact reduction techniques in computed tomography. PLoS One 2015;10:e0127932. [Crossref] [PubMed]
- Morsbach F, Wurnig M, Kunz DM, Krauss A, Schmidt B, Kollias SS, Alkadhi H. Metal artefact reduction from dental hardware in carotid CT angiography using iterative reconstructions. Eur Radiol 2013;23:2687-94. [Crossref] [PubMed]
- Diehn FE, Michalak GJ, DeLone DR, Kotsenas AL, Lindell EP, Campeau NG, Halaweish AF, McCollough CH, Fletcher JG. CT Dental Artifact: Comparison of an Iterative Metal Artifact Reduction Technique with Weighted Filtered Back-Projection. Acta Radiol Open 2017;6:2058460117743279. [Crossref] [PubMed]
- Subhas N, Polster JM, Obuchowski NA, Primak AN, Dong FF, Herts BR, Iannotti JP. Imaging of Arthroplasties: Improved Image Quality and Lesion Detection With Iterative Metal Artifact Reduction, a New CT Metal Artifact Reduction Technique. AJR Am J Roentgenol 2016;207:378-85. [Crossref] [PubMed]
- Brendlin AS, Reinert CP, Baumgartner H, Bongers MN, Thomas C, Afat S, Springer F, Almansour H. CT in Patients With External Fixation for Complex Lower Extremity Fractures: Impact of Iterative Metal Artifact Reduction Techniques on Metal Artifact Burden and Subjective Quality. AJR Am J Roentgenol 2022;218:300-9. [Crossref] [PubMed]
- Eisenhut F, Schmidt MA, Kalik A, Struffert T, Feulner J, Schlaffer SM, Manhart M, Doerfler A, Lang S. Clinical Evaluation of an Innovative Metal-Artifact-Reduction Algorithm in FD-CT Angiography in Cerebral Aneurysms Treated by Endovascular Coiling or Surgical Clipping. Diagnostics (Basel) 2022.
- Bayerl N, May MS, Wuest W, Roth JP, Kramer M, Hofmann C, Schmidt B, Uder M, Ellmann S. Iterative Metal Artifact Reduction in Head and Neck CT Facilitates Tumor Visualization of Oral and Oropharyngeal Cancer Obscured by Artifacts From Dental Hardware. Acad Radiol 2023;30:2962-72. [Crossref] [PubMed]
- Schmidt AMA, Grunz JP, Petritsch B, Gruschwitz P, Knarr J, Huflage H, Bley TA, Kosmala A. Combination of Iterative Metal Artifact Reduction and Virtual Monoenergetic Reconstruction Using Split-Filter Dual-Energy CT in Patients With Dental Artifact on Head and Neck CT. AJR Am J Roentgenol 2022;218:716-27. [Crossref] [PubMed]
- Huang S, Liang Y, Yao X, Qin X, He C, Luo L, Huang L, Lv Y. Comparison of dual-energy computed tomography (DECT) polychromatic and monochromatic images with and without iterative metal artifact reduction algorithm in patients with dental implants. Quant Imaging Med Surg 2024;14:4688-702. [Crossref] [PubMed]
- Jebb AT, Ng V, Tay L. A Review of Key Likert Scale Development Advances: 1995-2019. Front Psychol 2021;12:637547. [Crossref] [PubMed]
- Guziński M, Waszczuk Ł, Sąsiadek MJ, Head CT. Image quality improvement of posterior fossa and radiation dose reduction with ASiR - comparative studies of CT head examinations. Eur Radiol 2016;26:3691-6. [Crossref] [PubMed]
- Willemink MJ, Leiner T, de Jong PA, de Heer LM, Nievelstein RA, Schilham AM, Budde RP. Iterative reconstruction techniques for computed tomography part 2: initial results in dose reduction and image quality. Eur Radiol 2013;23:1632-42. [Crossref] [PubMed]
- Shinohara Y, Ohmura T, Sasaki F, Inomata T, Itoh T, Kinoshita T. Appropriate iMAR presets for metal artifact reduction from surgical clips and titanium burr hole covers on postoperative non-contrast brain CT. Eur J Radiol 2021;141:109811. [Crossref] [PubMed]
- Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, Eliasziw M. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-53. [Crossref] [PubMed]
- Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol 2012;8:23-34. [Crossref] [PubMed]
- Kuya K, Shinohara Y, Kato A, Sakamoto M, Kurosaki M, Ogawa T. Reduction of metal artifacts due to dental hardware in computed tomography angiography: assessment of the utility of model-based iterative reconstruction. Neuroradiology 2017;59:231-5. [Crossref] [PubMed]
- Anhaus JA, Heider M, Killermann P, Hofmann C, Mahnken AH. A New Iterative Metal Artifact Reduction Algorithm for Both Energy-Integrating and Photon-Counting CT Systems. Invest Radiol 2024;59:526-37. [Crossref] [PubMed]


