
Diagnosing placenta accreta spectrum in different placental locations using a combination of magnetic resonance imaging (MRI) features and diffusion-weighted MRI parameters
Introduction
Placenta accreta spectrum (PAS) is a serious pregnancy complication where the chorionic villi invade the myometrium. As the depth of the chorionic villi invasion increases, PAS is classified as placenta accreta, increta, and percreta. The incidence of PAS has increased 5-fold over the past three decades (1), and it is now a major health concern. In developed countries, the incidence of PAS is approximately 3/1,000 pregnancies (1), while in developing countries, the incidence of PAS is unclear, but it is estimated to have increased 5- to 10-fold (2).
Prior cesarean section (CS), placenta previa, advanced maternal age, and the use of assisted reproduction technologies are well-recognized risk factors for PAS (3-6). The continuing high CS rate, prior uterine surgery, delayed childbearing, and the need for subsequent reproductive assistance account for the increasing incidence of PAS. PAS can be life-threatening, and is associated with postpartum hemorrhage, local organ damage, hysterectomy, and even death (7). Compared with diagnosis at delivery, the early and accurate prenatal diagnosis of PAS is associated with improved maternal outcomes, as it allows multidisciplinary team management, which in turn can result in reduced blood loss and surgical complications, and a reduced need for transfusions (8).
Ultrasound (US) is the main prenatal modality used in the diagnosis of PAS. However, magnetic resonance imaging (MRI) is an important adjunct in the setting of posterior placenta, or large maternal body habitus, or when US findings are equivocal (9,10). MRI and US have comparable predictive accuracy in the diagnosis of PAS, but MRI is purported to be better at detecting PAS in the posterior placenta (11).
Various features have been investigated in the diagnosis of PAS. However, in patients with posterior placenta, PAS can be easily missed even with prenatal MRI, as the well-described MRI features used for diagnosis are mainly applicable to PAS in patients with anterior placenta. Cases of posterior PAS may not show the classical MRI features described in anterior PAS, making the detection of posterior PAS difficult even with MRI.
Our previous studies showed that MRI is capable of detecting both morphologic and functional changes in the placenta (12-14). Diffusion-weighted imaging (DWI) is commonly used to measure water molecular movement within tissue. Intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) are advanced DWI models that can be used to quantify the perfusion, diffusion, and heterogeneity of tissue in a non-invasive way. Our previous studies showed that diffusion coefficient (D) and pseudo-diffusion coefficient (D*) measured by IVIM are elevated in PAS and placenta percreta, respectively (12-14). These quantitative MRI techniques have proven useful in and could improve the diagnosis of PAS.
The timely diagnosis of PAS could help to identify a sub-set of women who require further assessment and management. However, the diagnostic criteria have yet to be established. Therefore, this study sought to determine whether certain MRI features and quantitative parameters from IVIM and DKI could be used to diagnose PAS in different placental locations. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2450/rc).
Methods
This cross-sectional study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board of Sichuan Provincial People’s Hospital (No. 2021288), and written informed consent was provided by all participants.
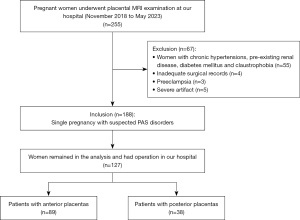
In total, 255 women referred for placental MRI, including a DWI sequence, from November 2018 to May 2023 were initially included in the study. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have a singleton pregnancy; (II) have suspected PAS based on clinical risk factors, including placenta previa, prior uterine surgery, prior CS, prior miscarriage, an advanced maternal age, in vitro fertilization, or uncertain US results; and (III) have PAS confirmed either by surgery or pathological analysis. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had any maternal pathology, including chronic hypertension, pre-existing renal disease, or diabetes mellitus; (II) had inadequate surgical records; (III) had preeclampsia; and/or (IV) had severe artifacts on MRI images (Figure 1).
Analysis of clinical characteristics
Patients’ information, including maternal age, gravidity, parity, previous CS, previous miscarriage, gestational age at MRI, and delivery, was obtained from the patients’ charts.
MRI protocols
All the MRI examinations were performed with a 1.5T magnetic resonance scanner (Aera, Siemens Healthineers, Erlangen, Germany). The images were acquired using a 16-channel body matrix coil using the following parameters: (I) Halffourier acquisition single-shot turbo spin echo (HASTE) in the three orthogonal planes: field of view (FOV): 420×80 mm, repetition time (TR)/time to echo (TE): 1,300/91 ms, section thickness: 5 mm, gap: 20%, matrix: 272×320, scan duration: 50 s; (II) true fast imaging in steady-state precession in the three orthogonal planes: FOV: 420×80 mm, TR/TE: 4.05/1.63 ms, section thickness: 5 mm, gap: 30%, matrix: 234×384, and scan duration: 48 s; (III) three-dimensional volumetric interpolated breath-hold examination: FOV: 400 mm, TR/TE: 3.79/1.42 ms, section thickness: 5 mm, gap: 20%, matrix: 180×320, scan duration: 8 s; and (IV) DWI: FOV: 390 mm, TR/TE: 5,200/83 ms, section thickness: 5 mm, matrix: 192×120, parallel imaging acceleration factor: 2, and b values: 11 (b=0, 50, 100, 150, 200, 400, 600, 800, 1,000, 1,200, and 1,600 s/mm2). The scan time for this sequence was about 7 min 29 s.
Imaging analysis
A mono-exponential model was used to calculate the apparent diffusion coefficient (ADC) using the following formula: Sb/S0 = exp (–b × ADC), where Sb and S0 are the signal intensities in the diffusion gradient factors of b and 0, respectively.
A polynomial model was used to calculate mean diffusivity (MD) and mean diffusion kurtosis (MK) using the following formula (15,16): Sb/S0 = exp (−b × MD + b2⋅MD2 × MK/6).
A bi-exponential model was used to calculate the perfusion fraction (f), diffusion coefficient (D) and pseudo-diffusion coefficient (D*) using the following formula (17,18): Sb/S0 = (1 – f) exp (−b × D) + f exp [−b × (D + D*)].
The regions of interest (ROIs) were manually drawn by a radiologist with 3 years of experience in obstetric imaging using IMAgenGINE (Vusion Tech, Hefei, China) (19). All the ROIs were drawn on axial b=0 s/mm2 images, and automatically copied to all diffusion parameter maps (Figure 2). In normal placentas, the entire placenta was delineated, and in PAS, the lower one-third part of the placenta was included with a reference to T2-weighted images; areas with infarcts, hemorrhage, and great vessels near the insertion of the umbilical cord were excluded. The parameters including ADC, MD, MK, D, D*, and f were then calculated.
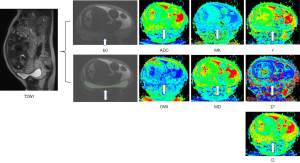
Two radiologists (with 5 and 10 years of experience, respectively), blinded to the US diagnosis and surgical and pathological findings, reviewed the images using the following previously described criteria: T2 dark bands, placental heterogeneity, abnormal intraplacental vascularity, focal exophytic mass, placental bulge, abnormal vascularization of the placental bed, and myometrial interruption (20,21). If any issues arose, a third reader was consulted to resolve any discrepancies in image interpretation. Placental location was categorized as posterior or anterior (including cases with both anterior placenta and posterior placenta).
Reference standard
The diagnosis was primarily determined using gross intraoperative findings based on the criteria of the Federation International of Gynecology and Obstetrics (FIGO) (22). The surgical diagnoses were confirmed by the pathological examination of placental tissue obtained from invasive sites or uterine specimens in hysterectomy cases. According to the reference standard, the patients were grouped as with or without PAS.
Statistical analysis
The results for the categorical variables are expressed as the absolute number (proportion, %). Differences in the clinical features between the patients with and without PAS were compared using the Mann-Whitney U test and χ2 test. Differences in the DWI parameters were also compared using the Mann-Whitney U test. The interobserver agreement for the MRI features was evaluated by kappa statistics. Stepwise logistic regression analyses with a forward procedure were performed to identify the MRI features and DWI parameters predictive of PAS, respectively. In addition, receiver operating characteristic (ROC) analyses were performed to determine the discriminative ability of significant DWI parameters and MRI features for PAS. The statistically significant DWI parameters and MRI features in the univariate analyses were included in the logistic regression analyses. A P value <0.05 was considered statistically significant. The analyses were performed using SPSS 27.0 (IBM Inc.).
Results
In total, 127 patients, of whom 38 had posterior placentas and 89 had anterior placentas, were included in the final analysis. The mean maternal age of the patients was 31.45 years (range, 22–45 years), and the mean gestational age at examination was 31 weeks (range, 18–38 weeks) (Figure 1). Compared to those without PAS, the patients with PAS, were older (P<0.05), had a higher frequency of prior CSs and placenta previa (P<0.05), and also delivered earlier (P<0.05). No significant differences were found between the two groups in terms of the number of prior CSs, prior miscarriages, gravidity, and parity (all P>0.05).
The clinical characteristics of the two groups are set out in Table 1. Of the 38 patients with posterior placentas, 10 (26.32%) underwent vaginal delivery and 28 (73.68%) underwent CS, and 19 (50%) were classified as having PAS (4 with FIGO grade 1 PAS, 14 with FIGO grade 2 PAS, and 1 with FIGO grade 3 PAS). Of the 19 PAS patients, one patient underwent hysterectomy and the other patients had their uterus preserved, including eight patients with abdominal artery balloon occlusion, seven patients with ligation of the uterine artery, and one patient with uterine balloon tamponade.
Table 1
| Diagnosis-related measures | Patients without PAS (n=39) | Patients with PAS (n=88) | P value |
|---|---|---|---|
| Age (years) | 29.32±4.13 | 32.44±4.23 | <0.001 |
| Gestational age at examination (weeks) | 31 [4] | 31 [4.75] | 0.558 |
| Gestational age at the time of delivery (weeks) | 38 [2] | 36 [3] | <0.001 |
| Previous cesarean section | <0.001 | ||
| Yes | 18 (46.15) | 65 (73.86) | |
| No | 21 (53.85) | 23 (26.14) | |
| Number of previous cesarean sections | 0.034 | ||
| 0 | 21 (53.85) | 27 (30.68) | |
| 1 | 16 (41.03) | 49 (55.68) | |
| 2 | 2 (5.12) | 12 (13.64) | |
| Previous miscarriage | 0.09 | ||
| Yes | 25 (64.10) | 69 (78.41) | |
| No | 14 (35.90) | 19 (21.59) | |
| Number of previous miscarriages | 0.26 | ||
| 0 | 14 (35.90) | 15 (17.05) | |
| 1 | 11 (28.20) | 29 (32.95) | |
| 2 or more | 14 (35.90) | 44 (50) | |
| Placenta previa | <0.001 | ||
| Yes | 14 (35.90) | 82 (93.18) | |
| No | 25 (64.10) | 6 (6.82) | |
Data are presented as mean ± standard deviation, median [interquartile range] or n (%). PAS, placenta accreta spectrum.
Of the 89 patients with anterior placentas, 5 (5.62%) underwent vaginal delivery and 84 (94.38%) underwent CS, and 68 (79.40%) were diagnosed as having PAS (13 with FIGO grade 1 PAS, 42 with FIGO grade 2 PAS, and 13 with FIGO grade 3 PAS). Of the 68 PAS patients, five patients underwent hysterectomy, and the other patients had their uterus preserved, including 37 patients with abdominal artery balloon occlusion, 40 patients with ligation of the uterine artery, and 11 patients with uterine balloon tamponade.
Performance of DWI parameters
The DWI parameter comparisons showed that D and D* were significantly higher (P=0.002 and 0.017, respectively), while MK was significantly lower (P=0.032) in the patients with posterior PAS (Table 2 and Figure 3A). In the logistic regression analysis, D was found to be an independent risk factor for predicting posterior PAS (P=0.002). The area under the curve (AUC) in diagnosing PAS was 0.789 [95% confidence interval (CI): 0.638–0.941] with a cut-off value of 1.59×10–3 mm2/s, a sensitivity of 84%, and a specificity of 68%.
Table 2
| Parameters | Patients without PAS | Patients with PAS | P value |
|---|---|---|---|
| Standard DWI parameters | |||
| ADC mean (×10–3 mm2/s) | |||
| Δ | 1.51 (0.09) | 1.53 (0.09) | 0.389 |
| # | 1.53 (0.12) | 1.55 (0.13) | 0.757 |
| DKI parameters | |||
| MD mean (×10–3 mm2/s) | |||
| Δ | 3.03 (0.32) | 3.17 (0.61) | 0.109 |
| # | 2.96 (0.30) | 3.14 (0.47) | 0.03 |
| MK mean | |||
| Δ | 0.55 (0.03) | 0.54 (0.04) | 0.032 |
| # | 0.52 (0.04) | 0.53 (0.04) | 0.954 |
| IVIM parameters | |||
| f mean (%) | |||
| Δ | 42.67 (4.49) | 44.54 (10.75) | 0.343 |
| # | 41.23 (5.16) | 43.09 (6.80) | 0.03 |
| D mean (×10–3 mm2/s) | |||
| Δ | 1.55 (0.11) | 1.65 (0.11) | 0.002 |
| # | 1.60 (0.14) | 1.65 (0.12) | 0.057 |
| D* mean (×10–3 mm2/s) | |||
| Δ | 30.54 (5.99) | 37.21 (13.19) | 0.017 |
| # | 33.26 (7.95) | 39.08 (9.84) | 0.001 |
Data are presented as the median (interquartile range). Δ, comparison between posterior placenta subjects; #, comparison between anterior placenta subjects. ADC, apparent diffusion coefficient; D, diffusion coefficient; D*, pseudo-diffusion coefficient; DKI, diffusion kurtosis imaging; DWI, diffusion weighted imaging; f, perfusion fraction; IVIM, intravoxel incoherent motion; MD, mean diffusivity; MK, mean diffusion kurtosis; PAS, placenta accreta spectrum.
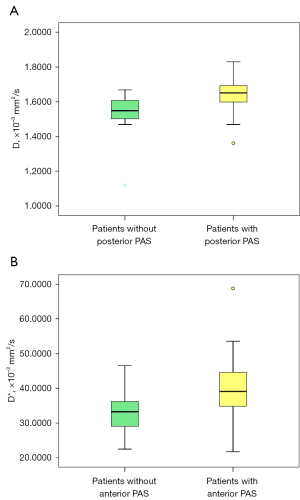
The DWI parameter comparisons showed that MD, f, and D* were significantly higher (P=0.03, 0.03, and 0.001, respectively) in patients with anterior placenta PAS (Table 2 and Figure 3B). The logistic regression analysis showed that D* was an independent risk factor for predicting anterior PAS (P=0.002). The AUC in diagnosing PAS was 0.751 (95% CI: 0.641–0.861) with a cut-off value of 37.26×10–3 mm2/s, a sensitivity of 65%, and a specificity of 86%.
Performance of MRI features
The diagnostic accuracy of different MRI features is presented in Table 3. The logistic regression analysis showed that T2 dark bands and abnormal intraplacental vascularity were independent risk factors for predicting PAS for posterior placentas (P=0.027 and P=0.006) and anterior placentas (P=0.04 and P=0.006). T2 dark bands had an AUC of 0.711, a sensitivity of 47.4%, and a specificity of 94.7% in posterior PAS, and an AUC of 0.680, a sensitivity of 45.6%, and a specificity of 90.5% in anterior PAS. Abnormal intraplacental vascularity had an AUC of 0.763, a sensitivity of 63.2%, and a specificity of 89.5% in posterior PAS, and an AUC of 0.728, a sensitivity of 64.7%, and a specificity of 81% in anterior PAS (Figure 4).
Table 3
| MRI signs | Pts PAS– | Pts PAS+ | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | P |
|---|---|---|---|---|---|---|---|---|
| T2 dark bands | ||||||||
| Δ | 5.26 (1/19) | 47.37 (9/19) | 47.4 | 94.7 | 89.94 | 64.29 | 0.711 | 0.003 |
| # | 9.52 (2/21) | 45.59 (31/68) | 45.6 | 90.5 | 82.76 | 62.46 | 0.680 | 0.003 |
| Placental heterogeneity | ||||||||
| Δ | 0 (0/19) | 26.32 (5/19) | 26.3 | 100 | 100 | 57.57 | 0.632 | 0.016 |
| # | 4.76 (1/21) | 25 (17/68) | 25 | 95.2 | 83.89 | 55.93 | 0.601 | 0.044 |
| Abnormal intraplacental vascularity | ||||||||
| Δ | 10.53 (2/19) | 63.16 (12/19) | 63.2 | 89.5 | 85.75 | 70.86 | 0.763 | 0.001 |
| # | 19.05 (4/21) | 64.71 (44/68) | 64.7 | 81 | 77.30 | 69.65 | 0.728 | <0.001 |
| Focal exophytic mass | ||||||||
| Δ | 0 (0/19) | 26.32 (5/19) | 26.3 | 100 | 100 | 57.57 | 0.632 | 0.016 |
| # | 0 (0/21) | 14.71 (10/68) | 27.9 | 100 | 100 | 54.73 | 0.640 | 0.006 |
| Placental bulge | ||||||||
| Δ | 5.26 (1/19) | 10.53 (2/19) | 10.5 | 94.7 | 66.46 | 57.41 | 0.526 | 0.547 |
| # | 0 (0/21) | 27.94 (19/68) | 23.5 | 97.5 | 100 | 56.66 | 0.618 | 0.014 |
| Abnormal vascularization of the placental bed | ||||||||
| Δ | 0 (0/19) | 10.53 (2/19) | 10.5 | 100 | 100 | 52.77 | 0.553 | 0.008 |
| # | 0 (0/21) | 7.35 (5/68) | 27.9 | 90.5 | 74.60 | 56.89 | 0.592 | 0.082 |
| Myometrial interruption | ||||||||
| Δ | 5.26 (1/19) | 42.11 (8/19) | 42.1 | 94.7 | 88.82 | 62.06 | 0.684 | 0.008 |
| # | 0 (0/21) | 23.53 (16/68) | 33.8 | 90.5 | 78.06 | 57.75 | 0.621 | 0.049 |
Data are presented as the percentage (number). Δ, comparison between posterior placenta subjects; #, comparison between anterior placenta subjects. AUC, area under the curve; MRI, magnetic resonance imaging; NPV, negative predictive value; PAS, placenta accreta spectrum; PPV, positive predictive value; pts, patients.
Interobserver agreement
Interobserver agreement was moderate to perfect for the seven MRI features. Interobserver agreement was substantial to perfect for T2 dark bands and abnormal intraplacental vascularity (k=0.895 and 0.776, respectively). Detailed k values are listed in Table 4.
Table 4
| MRI features | Kappa |
|---|---|
| T2 dark bands | 0.895 |
| Placental heterogeneity | 0.573 |
| Abnormal intraplacental vascularity | 0.776 |
| Focal exophytic mass | 0.771 |
| Placental bulge | 0.659 |
| Abnormal vascularization of the placental bed | 0.826 |
| Myometrial interruption | 0.659 |
MRI, magnetic resonance imaging.
Performance of the combination model of DWI parameters and MRI features
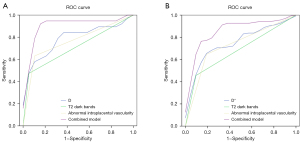
D, T2 dark bands, and abnormal intraplacental vascularity were combined to predict posterior PAS, and had a sensitivity of 90%, a specificity of 89%, and an AUC of 0.903 (95% CI: 0.791–1) (Figure 5A). D*, T2 dark bands, and abnormal intraplacental vascularity were combined to predict anterior PAS, and had a sensitivity of 77%, a specificity of 90.9%, and an AUC of 0.858 (95% CI: 0.767–0.948) (Figure 5B).
Discussion
The results of this study showed that T2 dark bands and abnormal intraplacental vascularity are associated with PAS, as are D and D* in cases of posterior placenta and anterior placenta, respectively. When the placental villi invade the myometrium, the abnormal adherence leads to a change in the morphology and function of the placenta that can be observed on MRI and functional MRI, respectively. Therefore, a model that combines MRI features and DWI parameters can evaluate the placenta more comprehensively, and could thus improve the diagnosis of PAS.
Similar to previous studies (3), our study of 38 posterior placentas, of which 19 had PAS (50%) and 89 anterior placentas, of which 68 had PAS (79.40%), showed that the frequency of placenta previa and prior CSs was high in both anterior and posterior PAS. Tinari et al. suggested that in addition to placenta previa, prior uterine surgery is also a common risk factor for posterior PAS (11). Morgan et al. reported that compared to anterior PAS, posterior PAS is associated with assisted reproductive technology and lower numbers of prior CSs (23). MRI is usually adopted as a secondary tool in prenatal imaging, and only patients suspected of having PAS after US are referred for MRI. Therefore, the incidence of PAS detected by MRI was high in our selected cohort of women, and large and adequately powered studies need to be conducted to fully elucidate the incidence and risks of posterior PAS.
A prior CS scar or any other surgical interventions potentially affecting the integrity of the uterine wall may lead to invasion, and subsequent diffusion and perfusion changes in the placenta. New quantitative MRI techniques have improved our understanding and diagnosis of PAS. DWI is a non-invasive technique for quantifying placental function, and IVIM is an advanced DWI model that separates diffusivity and microperfusion in the tissue from the signal intensity at different b values. Generally, D represents slow diffusion outside the microvasculature, and D* represents fast diffusion within the capillaries (24). The higher D values in posterior PAS and the higher D* values in anterior PAS represent the significant increase of both slow and fast diffusion related to the intravascular and extravascular components in the accreta lesions, resulting from local disruption of the endometrium. Therefore, the detection of an elevated D or D* value may extend our understanding of the pathophysiological change of PAS.
T2 dark bands, placental heterogeneity, abnormal intraplacental vascularity, focal exophytic mass, placental bulge, abnormal vascularization of the placental bed, and myometrial interruption are well-known MRI features suggestive of PAS. However, in our multivariate analysis, only T2 dark bands and abnormal intraplacental vascularity were significantly associated with PAS in both anterior placenta and posterior placenta. T2 dark bands are thick, nodular and disorganized T2 hypointense bands (25,26). This is the most common feature for PAS, and is also associated with poor maternal outcomes, including massive postpartum hemorrhage and the need for cesarean hysterectomy (27,28). Abnormal intraplacental vascularity refers to an abnormal pathologic intraplacental vascular pattern with a fetal origin (29). A previous study showed that these abnormal vessels are more elongated and wider with deficient branching, surrounded by sparse chorionic tissue compared with intraplacental vascularity in normal placenta (30). We speculate that the aberrant intraplacental vascular pattern and their sparse surrounding tissue may result in an increase of water molecular movement and microcirculatory perfusion in the capillary network, and thus higher D and D* values can be anticipated.
When PAS develops, the placental morphology and function change accordingly. The use of both MRI features and DWI parameters has great potential in aiding in the diagnosis of PAS. When combinations of the morphological and functional risk factors were examined, the AUC of the combined model was 0.903 for predicting posterior PAS (sensitivity: 90% and specificity: 89%), and the AUC of the combined model was 0.858 for predicting anterior PAS (sensitivity: 77% and specificity: 90.9%). The combined model yielded better results than any single risk factor, implying that it was more accurate and comprehensive in evaluating the placenta. The early and accurate prenatal diagnosis of PAS could improve maternal outcomes by allowing patients to receive well-planned treatment at centers with expertise in the surgical management of the disease, and reducing surgical complications, such as massive bleeding, the need for transfusions, and admissions to intensive care unit.
This study had some limitations. First, it was a retrospective study with a small sample size. The small sample size might limit the generalizability of the findings of the current study. MRI is not a screening tool used in clinical practice for PAS. Only 38 patients with posterior placenta, 19 of whom had PAS, were enrolled in the study. Our study may serve as a pilot study for improving the diagnosis of posterior PAS. Only women highly likely to have PAS based on US findings are referred for MRI to confirm the diagnosis. The retrospective nature of the study might have also introduced a selection bias. Therefore, future prospective studies with larger cohorts need to be conducted to validate the diagnostic performance of the combined model. Second, histopathologic findings are essential and remain the gold standard for the diagnosis of PAS. As most of our patients had their uterus preserved, the intraoperative findings were used as the primary reference standard; however, this standard might be subject to the surgical expertise of the obstetricians and lack accuracy. Nonetheless, it has been agreed that clinical descriptions are acceptable as the reference standard for PAS (31). Third, the scanning time (7 min 29 s) of the current DWI sequence was relatively long for pregnant women as 11 b values were employed, which limits its applicability in clinical practice. Given the more significant contribution of IVIM in the evaluation of the placenta, a future sequence without high b values could be employed to reduce the scanning time. Fourth, IVIM is heavily affected by T2 tissue that may influence the measurements of D and D*. Previous research has shown that IVIM parameters calculated from standard modeling may not represent the physiological measures of different organs (32). For example, Ma et al. showed the measured f in hepatocellular carcinoma was underestimated due to T2 relaxation time elongation (33). Therefore, a more appropriate model for measuring the placental tissue may be considered in future research.
Conclusions
A combined model of morphological and functional markers showed better performance in the diagnosis of posterior PAS and anterior PAS than models of morphological or functional markers alone; however, our findings were affected by the small number of included cases of posterior PAS. Using these risk factors, posterior PAS could be detected early. The establishment of risk scores for each type of suspicious PAS could improve the diagnostic confidence of clinicians and assist in the proper management of patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2450/rc
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2450/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board of Sichuan Provincial People’s Hospital (No. 2021288), and written informed consent was provided by all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Belfort MA, Shamshirsaz AA, Fox KA. The diagnosis and management of morbidly adherent placenta. Semin Perinatol 2018;42:49-58. [Crossref] [PubMed]
- Solheim KN, Esakoff TF, Little SE, Cheng YW, Sparks TN, Caughey AB. The effect of cesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. J Matern Fetal Neonatal Med 2011;24:1341-6. [Crossref] [PubMed]
- Silver RM, Branch DW. Placenta Accreta Spectrum. N Engl J Med 2018;378:1529-36. [Crossref] [PubMed]
- Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Placenta Accreta Spectrum. Am J Obstet Gynecol 2018;219:B2-16. [Crossref] [PubMed]
- Thurn L, Lindqvist PG, Jakobsson M, Colmorn LB, Klungsoyr K, Bjarnadóttir RI, Tapper AM, Børdahl PE, Gottvall K, Petersen KB, Krebs L, Gissler M, Langhoff-Roos J, Källen K. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: results from a large population-based pregnancy cohort study in the Nordic countries. BJOG 2016;123:1348-55. [Crossref] [PubMed]
- Klar M, Michels KB. Cesarean section and placental disorders in subsequent pregnancies--a meta-analysis. J Perinat Med 2014;42:571-83. [Crossref] [PubMed]
- O'Brien JM, Barton JR, Donaldson ES. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol 1996;175:1632-8. [Crossref] [PubMed]
- Buca D, Liberati M, Calì G, Forlani F, Caisutti C, Flacco ME, Manzoli L, Familiari A, Scambia G, D'Antonio F. Influence of prenatal diagnosis of abnormally invasive placenta on maternal outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;52:304-9. [Crossref] [PubMed]
- D'Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, Bhide A. Prenatal identification of invasive placentation using magnetic resonance imaging: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014;44:8-16. [Crossref] [PubMed]
- Clark EA, Silver RM. Long-term maternal morbidity associated with repeat cesarean delivery. Am J Obstet Gynecol 2011;205:S2-10. [Crossref] [PubMed]
- Tinari S, Buca D, Cali G, Timor-Tritsch I, Palacios-Jaraquemada J, Rizzo G, Lucidi A, Di Mascio D, Liberati M, D'Antonio F. Risk factors, histopathology and diagnostic accuracy in posterior placenta accreta spectrum disorders: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2021;57:903-9. [Crossref] [PubMed]
- Lu T, Wang Y, Guo A, Cui W, Chen Y, Wang S, Wang G. Monoexponential, biexponential and diffusion kurtosis MR imaging models: quantitative biomarkers in the diagnosis of placenta accreta spectrum disorders. BMC Pregnancy Childbirth 2022;22:349. [Crossref] [PubMed]
- Lu T, Li M, Wang Y, Li H, Wu M, Wang G. Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion in differentiating invasive placentas. Arch Gynecol Obstet 2024;309:503-14. [Crossref] [PubMed]
- Li H, Lu T, Li M, Wang Y, Zhang F, Yuan Y, Zhu M, Zhao X. Differentiation of placenta percreta through MRI features and diffusion-weighted magnetic resonance imaging. Insights Imaging 2023;14:93. [Crossref] [PubMed]
- Xiao Z, Zhong Y, Tang Z, Qiang J, Qian W, Wang R, Wang J, Wu L, Tang W, Zhang Z. Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of sinonasal malignancies: correlations with Ki-67 proliferation status. Eur Radiol 2018;28:2923-33. [Crossref] [PubMed]
- Cui Y, Yang X, Du X, Zhuo Z, Xin L, Cheng X. Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: Correlation with clinical pathologic prognostic factors. Eur Radiol 2018;28:1485-94. [Crossref] [PubMed]
- Ding Y, Tan Q, Mao W, Dai C, Hu X, Hou J, Zeng M, Zhou J. Differentiating between malignant and benign renal tumors: do IVIM and diffusion kurtosis imaging perform better than DWI? Eur Radiol 2019;29:6930-9. [Crossref] [PubMed]
- Wan Q, Deng YS, Lei Q, Bao YY, Wang YZ, Zhou JX, Zou Q, Li XC. Differentiating between malignant and benign solid solitary pulmonary lesions: are intravoxel incoherent motion and diffusion kurtosis imaging superior to conventional diffusion-weighted imaging? Eur Radiol 2019;29:1607-15. [Crossref] [PubMed]
- Yang M, Yan Y, Wang H. IMAge/enGINE: a freely available software for rapid computation of high-dimensional quantification. Quant Imaging Med Surg 2019;9:210-8. [Crossref] [PubMed]
- Jha P, Pōder L, Bourgioti C, Bharwani N, Lewis S, Kamath A, Nougaret S, Soyer P, Weston M, Castillo RP, Kido A, Forstner R, Masselli G. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur Radiol 2020;30:2604-15. [Crossref] [PubMed]
- Ueno Y, Kitajima K, Kawakami F, Maeda T, Suenaga Y, Takahashi S, Matsuoka S, Tanimura K, Yamada H, Ohno Y, Sugimura K. Novel MRI finding for diagnosis of invasive placenta praevia: evaluation of findings for 65 patients using clinical and histopathological correlations. Eur Radiol 2014;24:881-8. [Crossref] [PubMed]
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, Fox KA, Collins S. FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet 2019;146:20-4. [Crossref] [PubMed]
- Morgan EA, Sidebottom A, Vacquier M, Wunderlich W, Loichinger M. The effect of placental location in cases of placenta accreta spectrum. Am J Obstet Gynecol 2019;221:357.e1-5. [Crossref] [PubMed]
- León RL, Brown BP, Persohn SA, Norris CD, Steinhardt NP, Lin C, Territo PR. Intravoxel incoherent motion MR imaging analysis for diagnosis of placenta accrete spectrum disorders: A pilot feasibility study. Magn Reson Imaging 2021;80:26-32. [Crossref] [PubMed]
- Derman AY, Nikac V, Haberman S, Zelenko N, Opsha O, Flyer M. MRI of placenta accreta: a new imaging perspective. AJR Am J Roentgenol 2011;197:1514-21. [Crossref] [PubMed]
- Lax A, Prince MR, Mennitt KW, Schwebach JR, Budorick NE. The value of specific MRI features in the evaluation of suspected placental invasion. Magn Reson Imaging 2007;25:87-93. [Crossref] [PubMed]
- Lim PS, Greenberg M, Edelson MI, Bell KA, Edmonds PR, Mackey AM. Utility of ultrasound and MRI in prenatal diagnosis of placenta accreta: a pilot study. AJR Am J Roentgenol 2011;197:1506-13. [Crossref] [PubMed]
- Clark HR, Ng TW, Khan A, Happe S, Dashe J, Xi Y, Twickler DM. Placenta Accreta Spectrum: Correlation of MRI Parameters With Pathologic and Surgical Outcomes of High-Risk Pregnancies. AJR Am J Roentgenol 2020;214:1417-23. [Crossref] [PubMed]
- Konstantinidou AE, Bourgioti C, Fotopoulos S, Souka E, Nikolaidou ME, Zafeiropoulou K, Moulopoulos LA. Stripped fetal vessel sign: a novel pathological feature of abnormal fetal vasculature in placenta accreta spectrum disorders with MRI correlates. Placenta 2019;85:74-7. [Crossref] [PubMed]
- Bourgioti C, Konstantinidou AE, Zafeiropoulou K, Antoniou A, Fotopoulos S, Theodora M, Daskalakis G, Nikolaidou ME, Tzavara C, Letsika A, Martzoukos EA, Moulopoulos LA. Intraplacental Fetal Vessel Diameter May Help Predict for Placental Invasiveness in Pregnant Women at High Risk for Placenta Accreta Spectrum Disorders. Radiology 2021;298:403-12. [Crossref] [PubMed]
- Collins SL, Alemdar B, van Beekhuizen HJ, Bertholdt C, Braun T, Calda P, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol 2019;220:511-26. [Crossref] [PubMed]
- Yu WL, Xiao BH, Ma FZ, Zheng CJ, Tang SN, Wáng YXJ. Underestimation of the spleen perfusion fraction by intravoxel incoherent motion MRI. NMR Biomed 2023;36:e4987. [Crossref] [PubMed]
- Ma FZ, Wáng YXJ. T(2) relaxation time elongation of hepatocellular carcinoma relative to native liver tissue leads to an underestimation of perfusion fraction measured by standard intravoxel incoherent motion magnetic resonance imaging. Quant Imaging Med Surg 2024;14:1316-22. [Crossref] [PubMed]


