
The association between volume reduction ratio and time after ultrasound-guided microwave ablation for benign thyroid nodules of different compositions
Introduction
The incidence of thyroid nodules has increased annually. The proportion of thyroid nodules detected by ultrasound (US) is as high as 70%, of which 90% are benign nodules (1). Most benign nodules have no obvious clinical symptoms and follow-up observations are required. However, patients seek treatment for some nodules because of their large size or special location, causing local compression symptoms or affecting aesthetics. Traditional clinical treatment involves surgery (2,3). Although thyroid nodule resection can completely remove lesions, there are problems such as surgical trauma, neck scars that affect appearance, and other postoperative complications (4).
US-guided thermal ablation (TA) can be performed under local anesthesia with percutaneous needle penetration, which is less traumatic to the tissue and does not affect aesthetics; the US instrument monitors and guides in real time during the operation, which can avoid damaging blood vessels, nerves, and other tissues, thus reducing the incidence of postoperative complications. Currently, US-guided TA for the treatment of benign thyroid nodules (BTNs) is recommended by several guidelines (3,5,6).
Microwave ablation (MWA) is a type of TA. Compared with other TA methods such as radiofrequency ablation and laser ablation, it has the advantages of a strong coagulation ability, and a large ablation zone, and has become a promising therapeutic method (7-10). In addition, most studies have shown that MWA is effective in the treatment of BTNs; however, there are large individual differences in the degree of lesion absorption and shrinkage speed. Although the internal components of nodules have been reported as important influencing factors, most inferences have been made from timepoint studies, such as comparing the volume reduction ratio (VRR) at 1, 3, 6, and 12 months (11-14). Few studies have reported temporal changes in the VRR in BTNs of different compositions (15). Therefore, this study evaluated the efficacy of US-guided MWA in BTNs of different compositions, analyzed temporal changes in the VRR, and determined when the VRR increases most significantly. We present this article in accordance with the STROBE reporting checklist (16) (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2096/rc).
Methods
Patients
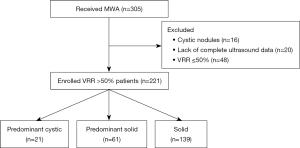
This is a retrospective study. We consecutively collected patients with BTNs of different compositions that underwent US-guided MWA between July 2020 and June 2021 in The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital. Patients with BTNs performed MWA because of their large size or special location, causing local compression symptoms or affecting aesthetics. To protect patient privacy, all patients’ details were de-identified. The inclusion criteria were as follows: (I) the US examination showed that the thyroid nodule was benign, namely Chinese Thyroid Imaging Reporting and Data System 3 categories (no obvious hypoechoic signal, vertical orientation, lobulated or irregular margin, and microcalcification) and (II) fine-needle aspirations or core needle biopsy confirmed that the nodules were benign, namely Bethesda II (17). The exclusion criteria were as follows: (I) cystic thyroid nodules or (II) lack of complete US data. BTNs were classified into three categories: predominantly cystic (51–90% fluid), predominantly solid (11–50% fluid), and solid (≤10% fluid). Schematic diagrams are shown in Figure 1. A total of 221 treatment effective nodules (216 patients) were included in this study. Among the effective nodules, 21 were predominantly cystic, 61 were predominantly solid, and 139 were solid. This study was conducted in accordance with the tenets of the Declaration of Helsinkiand its subsequent amendments (18). This retrospective study was approved by the Ethics Committee of The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital (No. 20220802018), and informed consent was obtained from all patients prior to the procedure.
Equipment and operational tools
US, contrast-enhanced ultrasound (CEUS), and US-guided MWA were performed using MyLab Twice (Esaote, Genoa, Italy). A linear array probe (LA533) with 4.0–13.0 MHz was used for US and CEUS examinations. An MTI-5A MWA therapy instrument (Nanjing Changcheng Medical Equipment, Nanjing, China) was used for MWA, including a microwave generator (frequency 2,450 MHz, output power 30 W), microwave cable, and XR-A1610W liquid-cooled circulation MWA needle (diameter 16 G, length 10 cm). For predominantly cystic nodules and predominantly solid nodules, a 20 mL syringe was used to extract cystic fluid.
CEUS of nodules was performed using 1.5 mL of SonoVue (Bracco Imaging, Milan, Italy).
US and CEUS examination
CEUS of nodules was performed using 1.5 mL of SonoVue. US, CEUS and MWA were performed by two physicians with 7-year experience in thyroid interventional US. Conventional transverse and longitudinal images of each target nodule were obtained using US. The volume of the nodule was calculated using the following equation based on diameter measurement with US: V= πabc/6 (V: volume; a: the largest diameter; b and c: the other two perpendicular diameters). Dynamic CEUS examinations were then performed for each BTN. BTNs were observed continuously for 2 min to determine their enhancement range. After US-guided MWA, CEUS was again performed to evaluate the ablation effect. The lesion was not completely ablated if there was an area of enhancement. Supplemental ablation was required until CEUS showed no enhancement of the lesion. The US data and characteristics of each BTN were collected after MWA. All detection data were stored for subsequent analysis.
Ablation technique
The patient was placed in a supine position with the neck fully exposed. After routine disinfection and draping, the patient was locally anesthetized with 2% lidocaine hydrochloride at the puncture site, puncture route, and anterior thyroid capsule under US guidance. Multiple injections of 0.9% normal saline were used to form a liquid isolation zone around the thyroid tissue (large blood vessels, nerves, trachea, esophagus, and muscles) with a width of approximately 5–10 mm to avoid puncture and ablation damage to the surrounding tissue. The 16-G MWA needle punctured through the isthmus to the inside of the BTN. The “moving shot technique” was used to perform ablation from inside to outside (deep to shallow) (7-10), until the nodules were completely covered by the hyperechoic gasification area. Before ablation, in predominantly cystic nodules and predominantly solid nodules, cystic fluid was aspirated as much as possible, after which the solid portion and cyst wall were ablated. MWA was performed directly for solid nodules. If there were multiple nodules in the thyroid gland at the same time, each nodule was ablated separately. After MWA, CEUS was performed to evaluate the ablation effect. Before completing the MWA of a BTN, the MWA needle remained inserted within the nodule. The following data were recorded: ablation time, energy, and possible complications such as hoarseness, hematoma, and pain.
Data collection and follow-up evaluation
The following data were recorded before and after MWA: (I) data and characteristics of each BTN using US examination, including size, nodule position, proximity to the trachea or recurrent laryngeal nerve, shape, margin, nodular composition, and homogeneity; (II) side effects; (III) VRR at the 12-month follow-up. VRR was calculated as follows: VRR = (initial volume − follow-up volume)/initial volume ×100%. A VRR >50% within 12 months was considered effective and a VRR ≤50% was considered ineffective (19).
Statistical analysis
All analyses were performed using EmpowerStats statistical software (X&Y Solutions, Boston, MA, USA). Demographic and US characteristics were compared between the effective and ineffective groups. Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using Fisher’s exact test. Continuous data were described as median (25th percentile, 75th percentile) and categorical variables as frequencies and percentages. Moreover, we used a generalized additive mixed model to identify non-linear relationships. If a non-linear correlation was observed, a two-piecewise linear regression model was used to calculate the threshold effect of time on VRR in terms of the smoothing plot. When the ratio between the VRR and time appeared obvious in the smoothed curve, the recursive method calculated the inflection point and the maximum model likelihood was used (20). Intraclass correlation coefficient (ICC) was used to evaluate the consistency of two operators in MWA of BTNs. Statistical significance was set at P<0.05.
Results
Baseline characteristics
A total of 645 examinations were performed in 216 patients. The mean examination ± standard deviation in each patient was 2.92±0.85. The baseline characteristics of the nodules included in this study are shown in Table 1.
Table 1
| Characteristics | Predominantly cystic nodules | Predominantly solid nodules | Solid nodules | Total |
|---|---|---|---|---|
| Number of cases | 21 | 61 | 139 | 221 |
| Volume (mL) | ||||
| Median (25th percentile, 75th percentile) | 3.5 (1.6, 9.4) | 5.7 (2.8, 11.9) | 3.7 (1.6, 7.9) | 4.6 (1.8, 9.0) |
| Mean | 6.9 | 8.2 | 6.1 | 6.8 |
| Minimum | 0.2 | 0.1 | 0.1 | 0.1 |
| Maximum | 34.0 | 32.9 | 53.7 | 53.7 |
| Position (n) | ||||
| Left lobe | 15 | 22 | 59 | 96 |
| Right lobe | 5 | 39 | 76 | 120 |
| Isthmus | 1 | 0 | 4 | 5 |
| Location, close to trachea (n) | ||||
| No | 6 | 25 | 67 | 98 |
| Yes | 15 | 36 | 72 | 123 |
| Location, close to recurrent laryngeal nerve (n) | ||||
| No | 9 | 30 | 79 | 118 |
| Yes | 12 | 31 | 60 | 103 |
| Shape (n) | ||||
| Regular | 14 | 45 | 120 | 179 |
| Irregular | 7 | 16 | 19 | 42 |
| Margin (n) | ||||
| Circumscribed | 14 | 51 | 116 | 181 |
| Not circumscribed | 7 | 10 | 23 | 40 |
| Homogeneity (n) | ||||
| Homogeneous | 2 | 8 | 16 | 26 |
| Heterogeneous | 19 | 53 | 123 | 195 |
Follow-up results
The relationship between VRR and time was non-linear and had a threshold effect, with the thresholds (inflection points) of VRR growth at 2.0, 2.3, and 4.3 months for predominantly cystic, predominantly solid, and solid nodules, respectively. In predominantly cystic nodules, the VRR increased by 36% per month until 2.0 months (P<0.001) and by 1.4% per month after 2.0 months (Figure 2A). In predominantly solid nodules, the VRR increased by 30.6% per month until 2.3 months (P<0.001) and by 0.7% per month after 2.3 months (Figure 2B). In solid nodules, the VRR increased by 17.4% per month until 4.3 months (P<0.001) and by 0.3% per month after 4.3 months (Figure 2C). Threshold effect for the relationship of follow-up time with VRR using piece-wise linear regression as shown in Table 2. There was no significant difference in inflection points among the three groups (P=0.37). The differences in VRR changes were statistically significant among the three groups (P<0.001).

Table 2
| Categories | β (95% CI) | P |
|---|---|---|
| VRR in predominant cystic group | ||
| Time ≤2.0 m | 36.0 (28.8, 43.2) | <0.001 |
| Time >2.0 m | 1.4 (−1.0, 3.7) | 0.26 |
| Log-likelihood ratio test | – | <0.001 |
| VRR in predominant solid group | ||
| Time ≤2.3 m | 30.6 (27.3, 33.9) | <0.001 |
| Time >2.3 m | 0.7 (−0.5, 2.0) | 0.25 |
| Log-likelihood ratio test | – | <0.001 |
| VRR in predominant solid group | ||
| Time ≤4.3 m | 17.4 (16.2, 18.5) | <0.001 |
| Time >4.3 m | 0.3 (−1.3, 3.6) | 0.63 |
| Log-likelihood ratio test | – | <0.001 |
CI, confidence interval; m, month; VRR, volume reduction ratio.
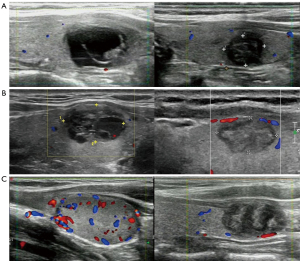
Figure 3 illustrates the US images of each group of nodes before MWA and with VRR >50%, respectively.
Consistency between the two operators
ICC was 0.839 (95% confidence interval: 0.773, 0.887), indicating good consistency between the two operators in MWA of BTNs.
Discussion
Clinical observation without intervention is an option for most BTNs, but appropriate treatment should be administered when the nodule has recently grown rapidly, is affecting aesthetics or causing symptoms of pressure, and if the patient is in a state of anxiety due to the nodule (21). Surgery is an effective treatment modality for BTNs. However, surgical procedures have shortcomings such as high trauma, possible serious complications, and damage to thyroid function, and need to be performed under general anesthesia; therefore, there is a clinical need for a minimally invasive, safe, and effective treatment modality (1,22).
US-guided MWA has become the first-line treatment modality for BTNs in recent years as it is minimally invasive, safe, and effective (3,5,23). The theory behind MWA for BTN is that polar molecules in human tissues are shocked by ultra-high frequency electromagnetic waves and high temperatures is generated within a short period of time, causing coagulative necrosis in the target tissues. The resulting necrotic tissues are eventually cleared and absorbed by the body’s immune system to form a physical local inactivation for treatment purposes (5).
For predominantly cystic or predominantly solid nodules, although US-guided chemical ablation is a minimally invasive treatment modality that can be applied, energy-based MWA can achieve better treatment outcomes (11). Previous studies have identified different internal nodule components as important factors affecting nodule shrinkage rates (5,11). However, there are few reports on temporal changes in the VRR in BTNs with different compositions. Therefore, in this study, BTNs were divided into predominantly cystic, predominantly solid, and solid nodules to explore how the VRR changes over time (7-10).
Figure 2 shows that the temporal changes in VRR for BTNs with different components are different. Previous studies on VRR mostly include timepoint studies, such as at 1, 3, 6, and 12 months (11,12). Few studies have reported how the VRR changes over time in BTNs with different compositions. In addition, in actual clinical practice, although the patient can be followed up by thyroid US, it can be difficult to access the patient at the timepoint specified by the doctor due to factors like patient compliance, disease severity, and public health measures [such as those seen during the coronavirus disease 2019 (COVID-19) pandemic]. Therefore, this study adopted a method more suitable for the actual clinical situation; that is, the generalized additive mixed model, which can be used to analyze changes in VRR over time and is not limited by specific timepoints. The log-likelihood ratio test, using a piecewise regression model for comparison, revealed the existing threshold.
In this study, the VRR of BTNs with three different components first increased significantly and then tended to increase slowly, which is consistent with the efficacy of radiofrequency ablation and Laser photocoagulation in the treatment of BTNs (7,8,19). The VRR of predominantly cystic nodules was significantly higher than that of the other two groups in the first few months and at 12 months, which is consistent with the conclusions of Khanh et al. and Fu et al. (13,15). Predominantly cystic nodules showed the most significant increase in VRR at 0–2.0 months, whereas predominantly solid nodules and solid nodules showed the most significant increases at 0–2.3 and 0–4.3 months, respectively. The most significant increase in VRR was found in predominantly cystic nodules, likely because the proportion of cystic components was greater than 50%. Before MWA, cystic fluid was extracted, and MWA was performed on the solid portion and cystic wall. After the operation, the nodule volume was significantly reduced compared with that before the operation; however, the VRR calculation was based on the data before the operation, and thus, the VRR increased faster (24,25). Predominantly solid nodules had the second highest VRR, possibly due to the cystic component accounting for ≤50%. The proportion of cystic components was lower than that of predominantly cystic nodules, and the proportion of solid components was higher. Compared to predominantly cystic and predominantly solid nodules, solid nodules had the slowest VRR, and the reasons for this may be as follows. First, it is extremely clear that MWA easily overcame smaller remnant solid portions compared to larger solid portions of the completely solid nodules. Second, solid nodules are ablated at multiple levels during the ablation process (which may affect the operator’s field of view), the timing of nodule ablation may be inaccurate, and the ablation needle may not ablate at each level of the nodule for the right amount of time. If the ablation is not sufficient, the nodule is not completely inactivated, and the treatment effect is not significant (26). If ablation is excessive, it may cause carbonization, and carbonized tissue is usually difficult to absorb, thus showing a slow shrinkage of the nodule after treatment (27). This study will help to explore the pattern of shrinkage of BTNs of different compositions after US-guided MWA therapy, which will help to explain to patients in clinical practice that the shrinking process of nodules of different compositions is not analogous.
There are certain limitations in this study. First, this was a retrospective study with inevitable bias. Second, this study was not a timepoint study due to compliance and time constraints of the follow-up population; however, this study used the generalized additive mixed model to fit the VRR data at different time points and calculated the thresholds. Third, similar to various studies on MWA and radiofrequency ablation, we could not histologically confirm BTNs without surgery. Fourth, this study did not compare the efficacy of MWA with other treatments like ablation and ethanol ablation for treating thyroid nodules. Fifth, we noted that the best tool to calculate these volumes of predominantly cystic nodules and predominantly solid nodules was CEUS, compared to conventional ultrasonography. In future research, we will use CEUS to calculate the enhanced remnant solid portion after aspiration of nodule’s fluid for the partially liquid BTNs.
Conclusions
In the present study, the VRR after US-guided MWA for different components of BTN was non-linearly related with the follow-up time, and there was a threshold effect. The VRR of BTNs of three different compositions after MWA increased with time. The rate of growth was most significant before the threshold and demonstrated a slow growth after the threshold.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2096/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2096/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the tenets of the Declaration of Helsinki and its subsequent amendments. This retrospective study was approved by the Ethics Committee of The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital (No. 20220802018), and informed consent was obtained from all patients prior to the procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li S, Yang M, Guo H, Liu M, Xu S, Peng H. Microwave Ablation Vs Traditional Thyroidectomy for Benign Thyroid Nodules: A Prospective, Non-Randomized Cohort Study. Acad Radiol 2022;29:871-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J 2020;9:172-85. [Crossref] [PubMed]
- Zhi X, Zhao N, Liu Y, Liu JB, Teng C, Qian L. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia 2018;34:644-52. [Crossref] [PubMed]
- Mauri G, Pacella CM, Papini E, Solbiati L, Goldberg SN, Ahmed M, Sconfienza LM. Image-Guided Thyroid Ablation: Proposal for Standardization of Terminology and Reporting Criteria. Thyroid 2019;29:611-8. [Crossref] [PubMed]
- Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol 2018;19:632-55. [Crossref] [PubMed]
- Jin H, Fan J, Lu L, Cui M. A Propensity Score Matching Study Between Microwave Ablation and Radiofrequency Ablation in Terms of Safety and Efficacy for Benign Thyroid Nodules Treatment. Front Endocrinol (Lausanne) 2021;12:584972. [Crossref] [PubMed]
- Yue WW, Wang SR, Lu F, Sun LP, Guo LH, Zhang YL, Li XL, Xu HX. Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: a propensity score matching study. Endocrine 2017;55:485-95. [Crossref] [PubMed]
- Zheng BW, Wang JF, Ju JX, Wu T, Tong G, Ren J. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine 2018;62:307-17. [Crossref] [PubMed]
- Honglei G, Shahbaz M, Farhaj Z, Ijaz M, Kai SY, Davrieux CF, Cheng SZ. Ultrasound guided microwave ablation of thyroid nodular goiter and cystadenoma: A single center, large cohort study. Medicine (Baltimore) 2021;100:e26943. [Crossref] [PubMed]
- Luo F, Huang L, Gong X, Han Z, Liu F, Cheng Z, Dou J, Yu X, Liang P, Yu J. Microwave ablation of benign thyroid nodules: 3-year follow-up outcomes. Head Neck 2021;43:3437-47. [Crossref] [PubMed]
- Su C, Liu YJ, Qian LX. Modified percutaneous ethanol injection method combined with microwave ablation for the treatment of symptomatic, predominantly cystic, benign thyroid nodules: a retrospective study of 201 cases. Int J Hyperthermia 2021;38:995-1001. [Crossref] [PubMed]
- Fu QQ, Kang S, Wu CP, Wang SY, Liu YY, Tian JW, Jiang SQ. A study on the efficacy of microwave ablation for benign thyroid nodules and related influencing factors. Int J Hyperthermia 2021;38:1469-75. [Crossref] [PubMed]
- Liu YJ, Qian LX, Liu D, Zhao JF. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med (Maywood) 2017;242:1515-23. [Crossref] [PubMed]
- Khanh HQ, Hung NQ, Vinh VH, Khoi NV, Vuong NL. Efficacy of Microwave Ablation in the Treatment of Large (≥3 cm) Benign Thyroid Nodules. World J Surg 2020;44:2272-9. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. [Crossref] [PubMed]
- Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023;33:1039-44. [PubMed]
- Nicogossian A, Kloiber O, Stabile B. The Revised World Medical Association's Declaration of Helsinki 2013: Enhancing the Protection of Human Research Subjects and Empowering Ethics Review Committees. World Medical & Health Policy 2014;6:1-3. [Crossref]
- Magri F, Chytiris S, Molteni M, Croce L, Coperchini F, Rotondi M, Fonte R, Chiovato L. Laser photocoagulation therapy for thyroid nodules: long-term outcome and predictors of efficacy. J Endocrinol Invest 2020;43:95-100. [Crossref] [PubMed]
- Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis 2018;17:130. [Crossref] [PubMed]
- Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018;319:914-24. [Crossref] [PubMed]
- Yan J, Qiu T, Lu J, Wu Y, Yang Y. Microwave ablation induces a lower systemic stress response in patients than open surgery for treatment of benign thyroid nodules. Int J Hyperthermia 2018;34:606-10. [Crossref] [PubMed]
- Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, Vitti P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest 2010;33:51-6. [Crossref] [PubMed]
- Baek JH, Ha EJ, Choi YJ, Sung JY, Kim JK, Shong YK. Radiofrequency versus Ethanol Ablation for Treating Predominantly Cystic Thyroid Nodules: A Randomized Clinical Trial. Korean J Radiol 2015;16:1332-40. [Crossref] [PubMed]
- Deandrea M, Garino F, Alberto M, Garberoglio R, Rossetto R, Bonelli N, Spiezia S, De Santis M, Monti S, Deiana MG, Vincenzo T, Cugini C, El Dalati G, Limone PP. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol 2019;180:79-87. [Crossref] [PubMed]
- Sim JS, Baek JH. Long-Term Outcomes Following Thermal Ablation of Benign Thyroid Nodules as an Alternative to Surgery: The Importance of Controlling Regrowth. Endocrinol Metab (Seoul) 2019;34:117-23. [Crossref] [PubMed]
- Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 2013;23:1044-9. [Crossref] [PubMed]


