
Hepatic arterial infusion chemotherapy versus systemic chemotherapy in unresectable intrahepatic cholangiocarcinoma: a propensity score-matched analysis of efficacy and safety
Introduction
Intrahepatic cholangiocarcinoma (iCCA), arising from the epithelial cells of intrahepatic bile ducts, ranks as the second most prevalent primary liver cancer following hepatocellular carcinoma (HCC) (1). iCCA accounts for approximately 20% of liver malignancies and 3% of gastrointestinal cancers (2). Notably, the global incidence of iCCA has been rising, with a particularly concerning increase observed in China (3). Currently, surgical resection is the primary curative treatment for iCCA. The majority of patients receive a diagnosis at advanced stages due to nonspecific symptoms, and treatment options are limited (4,5).
For cases of resectable iCCA, the standard treatment typically involves initial surgical resection followed by adjuvant capecitabine. The success of surgical intervention is crucial for achieving favorable oncological outcomes. Approximately 20% of patients have resectable disease, but many will face recurrence or metastasis after curative surgery (6). Unresectable iCCA is classified as either locally advanced or metastatic. The combination of gemcitabine and cisplatin (GEMCIS) remains the recommended first-line treatment for patients with advanced or metastatic iCCA and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 (7-9). The efficacy of systemic chemotherapy (SYS) for increasing the survival rates of patients is relatively limited. Therefore, more effective treatment options need to be developed.
Hepatic arterial infusion chemotherapy (HAIC) has proven to be an effective treatment for locally advanced iCCA (10). This approach involves the targeted delivery of chemotherapy agents directly into the hepatic artery, resulting in increased drug concentrations at the tumor site and minimization of systemic adverse effects. Several studies have shown the superior efficacy of HAIC for individuals with locally advanced iCCA (11-13). Despite the potential benefits of HAIC, comprehensive research specifically targeting patients with iCCA remains limited. This study thus aimed to evaluate and compare the clinical outcomes and safety profiles of patients with unresectable iCCA treated with HAIC with those treated with SYS. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2067/rc).
Methods
Study population
A single-center retrospective study included 146 patients with unresectable iCCA treated with either HAIC or first-line SYS at the National Cancer Center, China, from March 2019 to October 2023. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Ethics Committee of the Cancer Hospital Chinese Academy of Medical Sciences (No. 23/250-3992). Informed consent was obtained from all participants. The inclusion criteria were as follows: age 18 years or older (14), histopathological evidence confirming unresectable iCCA (15), documentation of primary HAIC or first-line SYS treatment (16), an ECOG score of 0–1 (17), and complete medical follow-up data (18). The exclusion criteria included the presence of any other malignant tumors or contraindications to HAIC or SYS treatment (14,15).
Treatment procedures
The resectability of each patient was determined by a multidisciplinary team of specialists, comprising radiologists, surgeons, hepatologists, and oncologists. Treatment strategies were then formulated based on the patient’s desires and after an in-depth conversation with a multidisciplinary team of specialists. To achieve accuracy, HAIC was administered to every patient according to a clearly defined protocol and under careful imaging oversight. The HAIC procedure for cholangiocarcinoma begins with percutaneous puncture of the femoral artery via the Seldinger technique with the patient under local anesthesia. A 5-French catheter is guided into the hepatic artery via the celiac trunk or superior mesenteric artery under fluoroscopic guidance. A 2.7-F microcatheter is then selectively advanced into the segmental, lobar, or whole-liver arteries supplying the tumor. The catheter is connected to an external infusion pump, and chemotherapy is administered, typically following a modified leucovorin, fluorouracil, and oxaliplatin (FOLFOX) regimen, in which oxaliplatin (130 mg/m2) is infused over 2 hours, leucovorin (400 mg/m2) over 2–3 hours, and fluorouracil (400 mg/m2) finally added. Fluorouracil (2,400 mg/m2) is then continuously infused over 23 or 46 hours. HAIC is repeated every 3–4 weeks until tumor progression, unacceptable toxicity, or deterioration in hepatic function or clinical condition.
The mainstay of SYS treatment is GEMCIS. In this study, gemcitabine was administered intravenously on days 1 and 8 at a dose of 1,000 mg/m2, and cisplatin was administered intravenously on days 2 and 3 at a dose of 25 mg/m2. These cycles were repeated every 21 days.
This study reflects real-world research. Depending on individual circumstances and patient preference, HAIC or SYS may be used as part of a treatment strategy in combination with programmed cell death protein 1 (PD-1) inhibitors or tyrosine kinase inhibitors. To improve safety and facilitate treatment completion, HAIC cycle intervals were adjusted to 4 to 6 weeks, minimizing the need for hospitalization and lowering medical costs while maintaining therapeutic efficacy.
Data collection
Clinical data were obtained from the medical records maintained at the National Cancer Center in China. This study focused on examining demographic and clinical characteristics, which are summarized in Table 1. Blood tests and measurements of tumors were conducted within 5 days prior to the initiation of treatment. Following the commencement of treatment, radiological assessments were carried out using magnetic resonance imaging (MRI) or computed tomography (CT) at baseline, followed by evaluations every 6 weeks thereafter.
Table 1
| Characteristic | Before PSM, N (%) | After PSM, N (%) | |||||
|---|---|---|---|---|---|---|---|
| SYS (n=214) | HAIC (n=38) | P | SYS (n=74) | HAIC (n=37) | P | ||
| Sex | 0.021 | 0.892 | |||||
| Male | 133 (62.15) | 16 (42.10) | 31 (41.89) | 16 (43.24) | |||
| Female | 81 (37.85) | 22 (57.89) | 43 (58.11) | 21 (56.76) | |||
| Age (years) | 0.573 | 0.917 | |||||
| ≥65 | 36 (16.82) | 5 (13.16) | 8 (10.81) | 5 (13.51) | |||
| <65 | 178 (83.18) | 33 (86.84) | 66 (89.19) | 32 (86.49) | |||
| No. of lesions | 0.029 | 0.884 | |||||
| Solitary | 103 (48.13) | 11 (28.95) | 23 (31.08) | 11 (29.73) | |||
| Multiple | 111 (51.87) | 27 (71.05) | 51 (68.92) | 26 (70.27) | |||
| Tumor size | 0.051 | >0.999 | |||||
| <5 cm | 110 (51.40) | 13 (34.21) | 26 (35.13) | 13 (35.17) | |||
| ≥5 cm | 104 (48.60) | 25 (65.79) | 48 (64.87) | 24 (64.87) | |||
| Distant metastasis | 0.542 | 0.868 | |||||
| Yes | 159 (74.30) | 30 (78.95) | 59 (79.73) | 29 (78.38) | |||
| No | 55 (25.70) | 8 (21.05) | 15 (20.27) | 8 (21.62) | |||
| Combined targeted/immunotherapy | 0.637 | 0.776 | |||||
| Yes | 149 (69.63) | 25 (65.79) | 50 (67.57) | 24 (64.87) | |||
| No | 65 (30.37) | 13 (34.21) | 24 (32.43) | 13 (35.14) | |||
HAIC, hepatic arterial infusion chemotherapy; PSM, propensity score matching; SYS, systemic chemotherapy.
The primary endpoint of this research was overall survival (OS), which was assessed from the initiation of treatment until death from any cause or the last follow-up conducted on June 30, 2024. Data regarding survival were collected through follow-up telephone calls. The secondary aims included the evaluation of tumor response rates and adverse event (AE) frequencies. Tumor responses were classified according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (19). The objective response rate (ORR) was defined as the sum of complete response (CR) and partial response (PR); meanwhile, the disease control rate (DCR) was defined as the sum of CR, PR, and stable disease (SD), representing cases achieving clinical benefits. Concurrent treatment with molecular targeted therapy was permitted. Two independent radiologists, each possessing over 10 years of clinical experience, conducted tumor response evaluations. The first follow-up evaluation was suggested to occur 3 months posttreatment, via either contrast-enhanced CT or dynamic contrast-enhanced MRI. AEs were characterized as any unintended or negative medical occurrences arising during or after the treatment period. Assessments of AEs within 1 month following the completion of treatment were carried out in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 guidelines.
Data that deviated from a normal distribution are displayed as the median and range. For comparisons of parametric continuous variables, the unpaired Student t test was used, while the Mann-Whitney test was used for nonparametric continuous variables. Categorical variables were analyzed with the Pearson correlation coefficient, the chi-squared test with continuity correction, or the Fisher exact test depending on the specific conditions required. OS was visualized via Kaplan-Meier curves, and differences between groups were assessed with the log-rank test. Furthermore, propensity score matching (PSM) was employed, with factors including sex, age (≥65 vs. <65 years), number of lesions, tumor size (5 vs. ≤5 cm), presence of extrahepatic spread, and the application of combination targeted immunotherapy. Matching was carried out at a 1:2 ratio with a caliper set to 0.1, without replacement. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify potential prognostic factors for OS. Variables with P<0.1 in the univariate analysis were entered into the multivariate analysis. Results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The threshold for statistical significance was established at a P value of less than 0.05. Data analyses were conducted with SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.1 (The R Foundation for Statistical Computing).
Results
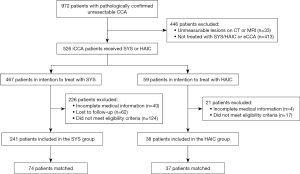
From March 2019 to October 2023, a total of 972 patients with pathologically confirmed unresectable CCA were screened at the National Cancer Center in China (Figure 1). A total of 446 patients were excluded due to unmeasurable lesions on CT or MRI or because they were diagnosed with extrahepatic CCA or not treated with SYS/HAIC. Of the remaining 526 patients, 467 were allocated to the SYS group and 59 to the HAIC group. In the SYS group, 226 patients were excluded for reasons such as incomplete medical records (40 patients), loss to follow-up (62 patients), or failure to meet the study’s eligibility criteria (124 patients). This resulted in 241 patients remaining in the SYS group, of whom 74 were matched for analysis. In the HAIC group, 21 patients were excluded due to incomplete medical records (4 patients) or failure to meet eligibility criteria (17 patients), leaving 38 patients in the HAIC group, 37 of whom were matched for analysis (Figure 1).
The specific characteristics of each group are outlined in Table 1. Prior to PSM, the proportion of male patients was notably higher in the SYS group (62.15%) compared to the HAIC group (42.10%), with a statistically significant P value of 0.021. The age distribution was similar between the groups, with patients aged 65 years or older accounting for 16.82% of patients in the SYS group and 13.16% in the HAIC group (P=0.573). A significantly larger proportion of patients in the HAIC group had multiple lesions (71.05%) as compared to the SYS group (51.87%) (P=0.029). The mean tumor size was slightly smaller in the SYS group, with 65.79% of patients in the HAIC group having tumors ≥5 cm as compared to 48.60% in the SYS group (P=0.051). The occurrence of distant metastasis was similar between the SYS and HAIC groups, at 74.30% and 78.95%, respectively (P=0.542). The use of combined targeted/immunotherapy was also similar between groups, with 67.57% in the SYS group and 64.87% in the HAIC group receiving treatment (P=0.776).
Other baseline characteristics between the two groups were also compared (Table 2). Regarding etiology, the hepatitis B virus infection distribution was similar between the HAIC and SYS groups, with 22 and 39 patients, respectively, and not significantly different (P=0.55). Among liver function indicators, the aspartate aminotransferase (AST) level in the HAIC group (median 33.5 U/L) was significantly higher than that in the SYS group (median 28.1 U/L) (P=0.03), while the difference in alanine aminotransferase (ALT), total bilirubin (TBIL), albumin (ALB), creatinine (Cr), and C-reactive protein (CRP) did not differ significantly between the two groups (P>0.05). Tumor markers, such as median alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) levels, were also comparable (P>0.05). Finally, complete blood count (CBC) parameters, including median levels of white blood cells (WBC), neutrophils (Neus), lymphocytes (Lyms), and platelets (PLTs), were not significantly different between the SYS and HAIC groups (P>0.05).
Table 2
| Characteristic | SYS (n=74) | HAIC (n=37) | P value |
|---|---|---|---|
| HBV | 0.55 | ||
| Positive | 39 (52.7) | 22 (59.5) | |
| Negative | 35 (47.3) | 15 (40.5) | |
| Liver function | |||
| ALT (U/L) | 23.8 (14.5–53.7) | 32.0 (17.6–61.0) | 0.07 |
| AST (U/L) | 28.1 (22.6–55.3) | 33.5 (25.1–66.4) | 0.03 |
| TBIL (μmol/L) | 15.2 (11.4–20.1) | 16.1 (13.8–25.4) | 0.40 |
| ALB (g/L) | 42.7 (40.6–46.2) | 42.0 (38.5–44.5) | 0.51 |
| Cr (μmol/L) | 60.5 (52.1–73.7) | 64.3 (53.5–73.7) | 0.37 |
| CRP (mg/L) | 0.3 (0.1–1.5) | 0.7 (0.2–2.7) | 0.52 |
| Tumor markers | |||
| AFP (ng/mL) | 3.01 (2.2–5.3) | 3.59 (2.6–4.7) | 0.27 |
| CEA (ng/mL) | 3.46 (1.7–7.6) | 3.35 (1.8–6.2) | 0.60 |
| CA19-9 (U/mL) | 0.09 | ||
| <1,000 | 61 (82.4) | 25 (67.6) | |
| ≥1,000 | 13 (17.6) | 12 (32.4) | |
| Complete blood count | |||
| WBC (×109/L) | 6.5 (5.8–8.0) | 6.3 (5.2–8.0) | 0.83 |
| Neu (×109/L) | 4.1 (3.6–5.4) | 4.8 (3.5–6.0) | 0.13 |
| Lym (×109/L) | 1.6 (1.3–2.0) | 1.5 (1.2–1.7) | 0.71 |
| PLT (×109/L) | 240.5 (189.0–285.5) | 218.0 (172.0–265.0) | 0.20 |
Categorical variables are presented as n (%) and continuous variables are presented as median (interquartile range). AFP, alpha-fetoprotein; ALB, serum albumin; ALT, alanine transaminase; AST, aspartate transaminase; CA19-9, cancer antigen 19-9; CEA, carcinoembryonic antigen; Cr, creatinine; CRP, C-reactive protein; HAIC, hepatic arterial infusion chemotherapy; HBV, hepatitis B virus; Lym, lymphocyte; Neu, neutrophil; PLT, platelet; PSM, propensity score matching; SYS, systemic chemotherapy; TBIL, total bilirubin; WBC, white blood cell.
Tumor response and patient survival
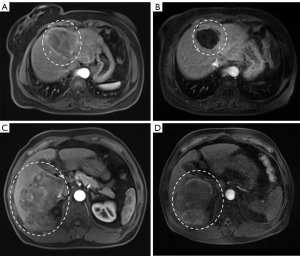
The median follow-up time for all patients was 14.6 months [interquartile range (IQR): 9.55–26.83 months]. In the SYS group, no patients achieved CR, 9 patients (12.16%) had PR, 39 patients (52.70%) had SD, and 26 patients (35.14%) experienced progressive disease (PD). In the HAIC group, there were also no patients with CR, but 13 patients (35.13%) achieved PR, 17 patients (45.95%) had SD, and 7 patients (18.92%) experienced PD (Table 3). The ORR and DCR were significantly higher in the HAIC group than in the SYS group (P<0.05). Dynamic contrast-enhanced MRI demonstrated tumor response to HAIC in two representative patients, with reductions in tumor volume and enhancement observed at follow-up imaging (Figure 2).
Table 3
| Tumor response | Patients, No. (%) | P value | |
|---|---|---|---|
| SYS (n=74) | HAIC (n=37) | ||
| CR | 0 | 0 | |
| PR | 9 (12.16) | 13 (35.13) | |
| SD | 39 (52.70) | 17 (45.95) | |
| PD | 26 (35.14) | 7 (18.92) | |
| ORR | 9 (12.16) | 13 (35.13) | 0.005 |
| DCR | 48 (64.86) | 30 (81.08) | 0.029 |
CR, complete response; DCR, disease control rate; HAIC, hepatic arterial infusion chemotherapy; ORR, objective response rate; PD, progressive disease; PR, partial response; PSM, propensity score matching; SD, stable disease; SYS, systemic chemotherapy.
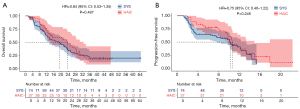
After PSM, the median OS was 23.7 months (95% CI: 13.8–32.7) in the HAIC group and 19.3 months (95% CI: 12.4–23.6) in the SYS group. Kaplan-Meier curves showed no significant difference in OS between the HAIC and SYS groups (HR =0.84, 95% CI: 0.53–1.35; P=0.487) (Figure 3A). The median progression-free survival (PFS) was comparable between groups, at 10.7 months (95% CI: 9.8–15.0) in the HAIC group and 10.3 months (95% CI: 7.7–13.4) in the SYS group. The HR for PFS in the comparison between the SYS and HAIC groups was 0.75 (95% CI: 0.46–1.22; P=0.246), indicating no significant difference between the treatment approaches (Figure 3B).
To identify the prognostic factors associated with OS, univariate and multivariate Cox regression analyses were performed (Table 4). In the univariate analysis, tumor number (HR =1.71, 95% CI: 1.03–2.85; P=0.039), serum ALB level (HR =1.66, 95% CI: 1.01–2.70; P=0.044), and CA19-9 level (HR =1.81, 95% CI: 1.01–3.23; P=0.046) were significantly associated with OS. Additionally, tumor size (HR =1.55, 95% CI: 0.97–2.49; P=0.069) and CEA level (HR =1.57, 95% CI: 0.99–2.47; P=0.051), with a value near the threshold of significance (P<0.1). In the multivariate Cox regression analysis, multiple tumors (HR =2.16, 95% CI: 1.19–3.94; P=0.011), elevated CA19-9 level (HR =1.95, 95% CI: 1.03–3.70; P=0.041), and elevated CEA level (HR =1.75, 95% CI: 1.06–2.91; P=0.030) were independent prognostic factors for poor OS.
Table 4
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Group (HAIC vs. SYS) | 0.84 (0.53–1.35) | 0.487 | |||
| Age (≥65 vs. <65 years) | 1.41 (0.61–3.25) | 0.419 | |||
| Sex (female vs. male) | 0.92 (0.59–1.45) | 0.730 | |||
| Tumor number (multiple vs. single) | 1.71 (1.03–2.85) | 0.039 | 2.16 (1.19–3.94) | 0.011 | |
| Tumor size (≥5 vs. <5 cm) | 1.55 (0.97–2.49) | 0.069 | 1.39 (0.80–2.42) | 0.248 | |
| Extrahepatic metastasis (present vs. absent) | 1.48 (0.82–2.68) | 0.197 | |||
| Combined T/I therapy (yes vs. no) | 1.25 (0.78–2.01) | 0.358 | |||
| HBV infection (positive vs. negative) | 0.89 (0.57–1.40) | 0.618 | |||
| ALT (≥40 vs. <40 U/L) | 0.80 (0.48–1.31) | 0.370 | |||
| AST (≥35 vs. <35 U/L) | 1.17 (0.74–1.83) | 0.503 | |||
| TBIL (≥22 vs. <22 μmol/L) | 0.75 (0.44–1.29) | 0.306 | |||
| ALB (≤40 vs. >40 g/L) | 1.66 (1.01–2.70) | 0.044 | 1.40 (0.83–2.36) | 0.213 | |
| Cr (≥73 vs. <73 μmol/L) | 0.69 (0.40–1.19) | 0.180 | |||
| CRP (≥0.06 vs. <0.06 mg/L) | 1.17 (0.65–2.08) | 0.601 | |||
| CA19-9 (≥27 vs. <27 U/mL) | 1.81 (1.01–3.23) | 0.046 | 1.95 (1.03–3.70) | 0.041 | |
| AFP (≥7 vs. <7 ng/mL) | 1.51 (0.83–2.75) | 0.173 | |||
| CEA (≥5 vs. <5 ng/mL) | 1.57 (0.99–2.47) | 0.051 | 1.75 (1.06–2.91) | 0.030 | |
AFP, alpha-fetoprotein; ALB, serum albumin; ALT, alanine transaminase; AST, aspartate transaminase; CA19-9, cancer antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; Cr, creatinine; CRP, C-reactive protein; HAIC, hepatic arterial infusion chemotherapy; HBV, hepatitis B virus; HR, hazard ratio; SYS, systemic chemotherapy; T/I, targeted/immunotherapy; TBIL, total bilirubin.
AEs and safety
Comparison of treatment-related AEs showed several significant differences between groups (Table 5). The SYS group, as compared with HAIC group, had higher rates of vomiting (39.2% vs. 18.9%; P=0.03), fatigue (41.9% vs. 10.8%; P<0.01), anemia (48.6% vs. 18.9%; P<0.01), thrombocytopenia (28.4% vs. 10.8%; P=0.01), and elevated ALT level (44.6% vs. 24.3%; P=0.04) as compared to the HAIC group. Grade 3–4 vomiting was also significantly more frequent in the SYS group than in the HAIC group (14.8% vs. 0%; P=0.04). No significant differences were observed in the occurrence of rash, fever, abdominal pain, leukopenia, elevated AST, hyperbilirubinemia, hypoalbuminemia, elevated creatinine, or sensory neuropathy between the groups.
Table 5
| Adverse event | Any grade | Grades 3–4 | |||||
|---|---|---|---|---|---|---|---|
| HAIC (n=37) | SYS (n=74) | P value | HAIC (n=37) | SYS (n=74) | P value | ||
| Rash | 5 (13.5%) | 25 (33.8%) | 0.08 | 0 | 0 | – | |
| Fever | 11 (29.7%) | 23 (31.1%) | 0.88 | 0 | 0 | – | |
| Abdominal pain | 9 (24.3%) | 10 (13.5%) | 0.15 | 1 (2.7%) | 3 (4.1%) | >0.99 | |
| Vomiting | 7 (18.9%) | 29 (39.2%) | 0.03 | 0 | 11 (14.8%) | 0.04 | |
| Fatigue | 4 (10.8%) | 31 (41.9%) | <0.01 | 0 | 0 | – | |
| Leukopenia | 3 (8.1%) | 10 (13.5%) | 0.31 | 0 | 7 (9.5%) | 0.12 | |
| Anemia | 7 (18.9%) | 36 (48.6%) | <0.01 | 0 | 9 (12.2%) | 0.07 | |
| Thrombocytopenia | 4 (10.8%) | 21 (28.4%) | 0.01 | 0 | 2 (2.7%) | 0.48 | |
| Elevated ALT | 9 (24.3%) | 33 (44.6%) | 0.04 | 1 (2.7%) | 3 (4.1%) | >0.99 | |
| Elevated AST | 13 (35.1%) | 28 (37.8%) | 0.78 | 1 (2.7%) | 4 (5.4%) | 0.60 | |
| Hyperbilirubinemia | 7 (18.9%) | 12 (16.2%) | 0.72 | 2 (5.4%) | 3 (4.1%) | >0.99 | |
| Hypoalbuminemia | 5 (13.5%) | 16 (21.6%) | 0.30 | 1 (2.7%) | 0 | 0.48 | |
| Elevated creatinine | 2 (5.4%) | 9 (12.2%) | 0.39 | 0 | 0 | – | |
| Sensory neuropathy | 4 (10.8%) | 18 (24.3%) | 0.18 | 0 | 0 | – | |
ALT, alanine transaminase; AST, aspartate transaminase; HAIC, hepatic arterial infusion chemotherapy; SYS, systemic chemotherapy.
Discussion
iCCA is a highly aggressive tumor that originates from the epithelium of the intrahepatic bile ducts and often involves a poor prognosis. Due to being at an advanced stage at diagnosis, most patients with iCCA are ineligible for surgery and typically receive chemotherapy to control tumor growth. In recent years, GEMCIS and gemcitabine-plus-oxaliplatin (GEMOX) regimens have emerged as standard first-line therapies (20,21). Immune checkpoint inhibitors have also demonstrated breakthrough efficacy in the treatment of biliary tract tumors. In the KEYNOTE-966 trial, a regimen of pembrolizumab and GEMCIS demonstrated significant survival benefit compared to placebo plus GEMCIS, with a median OS of 12.7 vs. 10.9 months (HR =0.83; P=0.0034) and a manageable toxicity profile (grade ≥3 treatment-related AEs: 79% vs. 75%) (22). Meanwhile, in the TOPAZ-1 trial, a regimen of durvalumab combined with GEMCIS compared to placebo plus GEMCIS showed improved ORR (26.7% vs. 18.7%) and OS (12.8 vs. 11.5 months; HR =0.80; P=0.021), confirming durable clinical benefit (23). For second-line therapy, molecular profiling guides treatment selection. In the TAS-120-101 trial, futibatinib demonstrated a 42% ORR and a 9.7-month median duration of response (DOR) (24), while pemigatinib showed comparable efficacy in FIGHT-202 trial (25). In the ROAR basket trial, patients with BRAF V600E mutations responded to dabrafenib plus trametinib, achieving a 51% ORR and 9.1-month median PFS (26). For patients without molecular targeting, mFOLFOX remains the standard second-line chemotherapy, and in the ABC-06 trial, it improved the 6-month survival to 50.6% (27).
However, the high incidence of severe AEs with these systemic combinations underscores the need for alternative therapeutic approaches with better tolerability. Emerging localized arterial therapies, such as HAIC, transarterial chemoembolization (TACE), and transarterial radioembolization (TARE), have demonstrated promising results in the treatment of advanced cholangiocarcinoma (28-30). Cai et al. reported that HAIC could provide superior survival outcomes as compared to TACE, with 1-year OS rates of 60.2% and 42.9%, respectively (P=0.028) (31). Additionally, a retrospective study indicated that applying the mFOLFOX regimen in HAIC could offer a novel therapeutic strategy for patients with iCCA (31). Although most of the studies on HAIC with mFOLFOX have primarily focused on HCC, the relatively low toxicity observed in these analyses suggests that it might also serve as a safe and promising treatment alternative for patients with iCCA (32-34).
In our study, we compared HAIC with standard first-line SYS. The findings revealed that the HAIC group had a higher ORR and DCR than did the SYS group. One possible explanation for these outcomes is that HAIC allows for higher concentrations of chemotherapeutic agents to be delivered directly to the liver, which may improve tumor control. The liver’s dual blood supply is well understood: the hepatic artery predominantly supplies blood to the tumor, while the portal vein primarily supports the noncancerous liver tissue. By delivering chemotherapy preferentially through the hepatic artery, HAIC may achieve better liver tumor control as compared to SYS.
We further found that patients with unresectable iCCA experienced comparable OS and PFS outcomes regardless of whether they received HAIC or SYS, suggesting similar clinical efficacy between the two approaches. Although HAIC provided better local control of intrahepatic tumors than did SYS, this advantage did not lead to significantly improved overall outcomes. This may be attributable to the fact that most participants were in advanced stages of the disease and had extrahepatic metastases. Progression of these extrahepatic lesions was the leading cause of death, and HAIC was less effective at managing them. Consequently, combining HAIC with immunotherapy, targeted therapy, or SYS might offer a more effective treatment approach for patients with extrahepatic metastasis.
In addition to therapeutic efficacy, AE rates are key metrics for assessing chemotherapy regimens. Notably, regimens combining immunotherapy and chemotherapy combination are associated with a 40–50% incidence of grade 3–4 AEs, which emphasizes the need for alternative options that can achieve comparable survival rates while minimizing side effects (35). In our study, the HAIC group exhibited a lower overall incidence of AEs as compared to the SYS group. Specifically, the rates of rash, vomiting, fatigue, leukopenia, anemia, and sensory neuropathy were reduced in the HAIC group. Hematologic toxicity and liver function impairment were the most common grade 3–4 AEs observed. Furthermore, the HAIC group experienced fewer grade 3–4 AEs. One possible reason for this is that HAIC administers chemotherapy directly to the liver, which leads to a relatively lower systemic drug concentration. On the other hand, SYS involves intravenous delivery, requiring higher systemic drug levels to achieve sufficient tumor control in the liver, resulting in increased systemic toxicity. Additionally, the liver’s first-pass metabolism may further minimize systemic side effects by metabolizing the drugs before they circulate throughout the body (36). The majority of AEs in the HAIC group were effectively managed with symptomatic treatment and did not disrupt the continuation of therapy. As a result, HAIC may be considered a safe and viable treatment option for patients with unresectable iCCA.
Li et al.’s study demonstrated that combining HAIC with SYS significantly improves OS and reduces liver failure-related mortality in patients with iCCA and extrahepatic oligometastasis as compared to the administration of SYS alone (37). Whereas Li et al. examined the combined impact of HAIC and SYS, we focused on clarifying the individual contributions of each agent and comparing the efficacy of each treatment modality. Yang et al.’s study found that HAIC, as compared to SYS, provided better intrahepatic tumor control in patients with unresectable iCCA, with a median intrahepatic progression-free survival (IPFS) of 13.7 vs. 11.4 months, respectively (P=0.035) (38). However, by employing PSM, we were able to reduce interpatient variability, strengthening the validity of our comparisons.
This study involved several limitations which should be noted. First, as we employed a retrospective, single-center design, the results may lack generalizability, necessitating further prospective, large-scale, randomized trials to confirm our findings. Second, the relatively small sample size limits the applicability of the results and raises the potential for a type II error. Finally, further laboratory-based research is needed to clarify the mechanisms through which HAIC provides benefits to patients with iCCA.
This study found that HAIC provided superior local tumor control as compared to SYS in patients with iCCA, while also resulting in fewer side effects. These findings suggest that HAIC may serve as an effective and better-tolerated treatment option for the iCCA patient population, offering enhanced tumor management with reduced toxicity.
Conclusions
HAIC demonstrated superior local tumor control and a better safety profile as compared to SYS in unresectable patients with iCCA, although OS outcomes were comparable. These findings suggest that HAIC is a promising alternative treatment, warranting further investigation in combination with systemic therapies.
Acknowledgments
We thank the patients and their families for participating in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2067/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2067/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Ethics Committee of the Cancer Hospital Chinese Academy of Medical Sciences (No. 23/250-3992). Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin 2023;73:198-222. [Crossref] [PubMed]
- Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, Bray F, McGlynn KA, Petrick JL. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020;126:2666-78. [Crossref] [PubMed]
- Bupathi M, Ahn DH, Bekaii-Saab T. Therapeutic options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:91-100. [Crossref] [PubMed]
- Ilyas SI, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. [Crossref] [PubMed]
- Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol 2022;27:100737. [Crossref] [PubMed]
- Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux MESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:127-40. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw 2023;21:694-704. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. [Crossref] [PubMed]
- Zheng Z, Wang J, Wu T, He M, Pan Y, Wang J, Chen J, Hu D, Xu L, Zhang Y, Chen M, Zhou Z. Hepatic arterial infusion chemotherapy plus targeted therapy and immunotherapy versus systemic chemotherapy for advanced intrahepatic cholangiocarcinoma: a retrospective cohort study. Int J Surg 2025;111:1552-7. [Crossref] [PubMed]
- Yamashita S, Koay EJ, Passot G, Shroff R, Raghav KP, Conrad C, Chun YS, Aloia TA, Tao R, Kaseb A, Javle M, Crane CH, Vauthey JN. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: A comprehensive analysis of 362 consecutive patients. Cancer 2017;123:1354-62. [Crossref] [PubMed]
- Holster JJ, El Hassnaoui M, Franssen S, IJzermans JNM, de Jonge J, Mostert B, Polak WG, de Wilde RF, Homs MYV, Groot Koerkamp B. Hepatic Arterial Infusion Pump Chemotherapy for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Ann Surg Oncol 2022;29:5528-38. [Crossref] [PubMed]
- Franssen S, Soares KC, Jolissaint JS, Tsilimigras DI, Buettner S, Alexandrescu S, et al. Comparison of Hepatic Arterial Infusion Pump Chemotherapy vs Resection for Patients With Multifocal Intrahepatic Cholangiocarcinoma. JAMA Surg 2022;157:590-6. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Sirica AE, Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wei Wang X, Zhu AX. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology 2019;69:1803-15. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, Nam AR, Oh KS, Kim JM, Lee Y, Guthrie V, McCoon P, Li W, Wu S, Zhang Q, Rebelatto MC, Kim JW. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022;7:522-32. [Crossref] [PubMed]
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-80. [Crossref] [PubMed]
- Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306. [Crossref] [PubMed]
- Grenader T, Nash S, Plotkin Y, Furuse J, Mizuno N, Okusaka T, Wasan H, Valle J, Bridgewater J. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol 2015;26:1910-6. [Crossref] [PubMed]
- Fiteni F, Nguyen T, Vernerey D, Paillard MJ, Kim S, Demarchi M, Fein F, Borg C, Bonnetain F, Pivot X. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med 2014;3:1502-11. [Crossref] [PubMed]
- Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853-65. [Crossref] [PubMed]
- Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol 2024;9:694-704. [Crossref] [PubMed]
- Gandhy SU, Casak SJ, Mushti SL, Cheng J, Subramaniam S, Zhao H, et al. FDA Approval Summary: Futibatinib for Unresectable Advanced or Metastatic, Chemotherapy Refractory Intrahepatic Cholangiocarcinoma with FGFR2 Fusions or Other Rearrangements. Clin Cancer Res 2023;29:4027-31.
- Vogel A, Sahai V, Hollebecque A, Vaccaro GM, Melisi D, Al Rajabi RM, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Lihou CF, Zhen H, Veronese ML, Abou-Alfa GK. An open-label study of pemigatinib in cholangiocarcinoma: final results from FIGHT-202. ESMO Open 2024;9:103488. [Crossref] [PubMed]
- Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med 2023;29:1103-12. [Crossref] [PubMed]
- Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 2021;22:690-701. [Crossref] [PubMed]
- Mosconi C, Solaini L, Vara G, Brandi N, Cappelli A, Modestino F, Cucchetti A, Golfieri R. Transarterial Chemoembolization and Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma-a Systemic Review and Meta-Analysis. Cardiovasc Intervent Radiol 2021;44:728-38. [Crossref] [PubMed]
- Schaarschmidt BM, Kloeckner R, Dertnig T, Demircioglu A, Müller L, Auer TA, Santos DPD, Steinle V, Miederer M, Gebauer B, Radunz S, Kasper S, Weber M, Theysohn J. Real-Life Experience in the Treatment of Intrahepatic Cholangiocarcinoma by (90)Y Radioembolization: A Multicenter Retrospective Study. J Nucl Med 2023;64:529-35. [Crossref] [PubMed]
- Ishii M, Itano O, Morinaga J, Shirakawa H, Itano S. Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PLoS One 2022;17:e0266707. [Crossref] [PubMed]
- Cai Z, He C, Zhao C, Lin X. Survival Comparisons of Hepatic Arterial Infusion Chemotherapy With mFOLFOX and Transarterial Chemoembolization in Patients With Unresectable Intrahepatic Cholangiocarcinoma. Front Oncol 2021;11:611118. [Crossref] [PubMed]
- Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, Deng HJ, He M, Mu LW, Zhao M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J Clin Oncol 2022;40:468-80. [Crossref] [PubMed]
- Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang CK, et al. Postoperative Adjuvant Hepatic Arterial Infusion Chemotherapy With FOLFOX in Hepatocellular Carcinoma With Microvascular Invasion: A Multicenter, Phase III, Randomized Study. J Clin Oncol 2023;41:1898-908. [Crossref] [PubMed]
- Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol 2022;40:150-60. [Crossref] [PubMed]
- Olkus A, Tomczak A, Berger AK, Rauber C, Puchas P, Wehling C, Longerich T, Mehrabi A, Chang DH, Liermann J, Schäfer S, Pfeiffenberger J, Jäger D, Michl P, Springfeld C, Dill MT. Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: An Exploratory Analysis of Real-World Data. Target Oncol 2024;19:213-21. [Crossref] [PubMed]
- Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination With Systemic Gemcitabine and Oxaliplatin in Patients With Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2020;6:60-7. [Crossref] [PubMed]
- Li Z, Xu R, Chang X, Sun P. Systemic Chemotherapy with or without Hepatic Arterial Infusion Chemotherapy for Intrahepatic Cholangiocarcinoma with Extrahepatic Oligometastasis: A Propensity Score-Matched Analysis. J Vasc Interv Radiol 2024;35:416-427.e17. [Crossref] [PubMed]
- Yang Z, Fu Y, Wu W, Hu Z, Pan Y, Wang J, Chen J, Hu D, Zhou Z, Chen M, Zhang Y. Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front Pharmacol 2023;14:1234342. [Crossref] [PubMed]


