
Comparison of ectopic versus normal gray matter via quantitative magnetic resonance imaging: a cross-sectional study
Introduction
Gray matter heterotopia (GMH) refers to the ectopic migration of cortical neurons into the white matter, but the etiology of GMH is not fully understood. Genetic factors and neuronal migration impairment, often due to infection, during the 6th to 16th week of pregnancy are considered the primary causes (1).
GMH often exhibits extensive connections with the normal visual cortex, including the hippocampus and contralateral hemisphere, potentially resulting in epileptic-like activity (2,3). Epilepsy in patients with GMH is often focal and resistant to drug treatment. Therapy typically involves resection or minimally invasive multitarget ablation (4). Magnetic resonance imaging (MRI) can identify the anatomical location of the gray matter; however, it cannot ascertain whether functional differences exist between GMH and normal gray matter (NGM) within the cerebral cortex (5). Generally, ectopic gray matter is best visualized with the T1-weighted brain volume (T1 BRAVO) imaging sequence (6). Heterotopia may be manifest as large nodular isointense masses on MRI, sometimes simulating tumors. In such cases, proton magnetic resonance spectroscopy can indicate normal peaks of the metabolites or discrete relative N-acetylaspartate reduction (7).
Arterial spin labeling (ASL) and MRI compilation (MAGiC) sequences in MRI can quantitatively reveal subtle differences that are not observable to the naked eye (8,9). In this study, ASL and MAGiC were used to identify the differences in perfusion and structure between GMH and NGM, thereby laying the groundwork for the further determination of epileptogenic gray matter. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1237/rc).
Methods
Participants
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the ethics committee of the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University (No. O-2024-259). The requirement for informed consent was waived due to the retrospective nature of the study.
This study enrolled all patients diagnosed with GMH at the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, from October 2022 to December 2023 via MRI examinations including T1 BRAVO, T2-weighted imaging (T2WI), MAGiC, and ASL sequences [postlabeling delays (PLDs) of 1.5 s and 2.5 s]. The inclusion criteria were as follows: (I) complete imaging data (T1 BRAVO, T2WI, MAGiC, and ASL, with PLDs of 1.5 s and 2.5 s), (II) the presence of at least one cortical-like signal nodule within the normal brain white matter, and (III) a history of at least three epileptic seizures or abnormal findings on electroencephalography (EEG). Meanwhile, the exclusion criteria included the following: (I) incomplete imaging or clinical data, (II) poor image quality or artifacts, and (III) MRI contraindications.
MRI scanning protocol
In this study, the SIGNA Architect 3.0 T MRI system (GE HealthCare, Chicago, IL, USA) was used to conduct the T1 BRAVO, T2WI, MAGiC, and ASL sequences (with PLDs of 1.5 s and 2.5 s). The scanning parameters for T1 BRAVO were a repetition time (TR) of 7,800 ms, an echo time (TE) 31 of ms, a slice thickness of 1 mm, a field of view (FOV) of 256 mm × 230 mm, a matrix size of 256×256, and a flip angle of 8°; for T2WI, they were a TR of 4,500 ms, a TE of 120 ms, a slice thickness 5 mm, a slice gap of 1.5 mm, an FOV 240 mm × 240 mm, a matrix size of 320×256, and a flip angle of 111°; and for ASL, they were a TR of 4,838 ms, a TE of 57.4 ms, a resolution of 512, 8 arms, a number of excitations (NEX) of 3, and a slice thickness of 3 mm. Meanwhile, the MAGiC sequence (GE HealthCare), which is based on 2D fast spin echo technology, employs the multidynamic multiecho principle, using alternating 120° saturation pulses and a multiecho collection strategy. In a single scan, MAGiC facilitates the simultaneous acquisition of 5 quantitative maps and 10 contrast images, enabling the quantitative measurement of relaxation metrics including T1, T2, and proton density (PD) values. In this study, the parameters for the axial MAGiC sequence were an FOV of 24 cm × 19.2 cm, a TE 21.4 of ms, a TR 4,000 of ms, a matrix size of 320×256, a slice thickness of 5 mm, a slice gap of 1.5 mm, an NEX of 1, and a scan time of 4 min.
Quantitative image measurements
The data were manually measured by two radiologists with 2 years of work experience. GMH was measured as the mean of 1–3 region of interest (ROI) measurements while NGM was measured as the mean of 1–3 ROI measurements in the ipsilateral hemisphere. Normal white matter (NWM) was measured as the mean of 1–3 ROI measurements in the frontal or occipital lobes of the ipsilateral hemisphere. If a patient had more than one GMH, each was recorded separately during quantitative imaging measurements but treated as one patient for clinical data statistics. T1, T2, and PD values of GMH and ipsilateral NGM were measured separately for each patient. The raw cerebral blood flow (CBF) maps from ASL were imported into the Advantage Workstation 4.7 (GE HealthCare), and the GMH and NGM values were measured under PLDs of 1.5 s and 2.5 s, respectively. The quantitative values obtained from measurements of GMH and NGM were normalized against the contralateral white matter visually identified as normal. The resulting relative quantitative values were recorded as relative GMH (rGMH) and relative NGM (rNGM), respectively.
Statistical methods
The statistical analysis of the data was conducted with SPSS 26.0 (IBM Corp., Armonk, NY, USA), OriginPro 2023 (OriginLab, Northampton, MA, USA) with a significance level set at P<0.05. Component concordance between two radiologists was evaluated via Bland-Altman plots. For continuous variables, the Shapiro-Wilk test was used to assess the normality of their distribution. If the data followed a normal distribution, they were recorded as the mean ± standard deviation (). To analyze the differences between the quantitative values of GMH and NGM on the same side before and after standardization, a paired-samples t-test was used. This study evaluated the differences in the quantitative values of GMH before and after standardization as compared to those of NGM on the same side.
Results
Between October 2022 and December 2023, nine patients (32 GMHs) with epilepsy were enrolled according to the inclusion criteria and exclusion criteria (Figure 1).
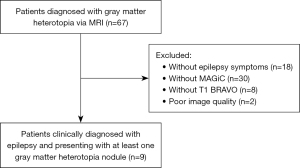
Nine patients, including six males and three females with a median age of 21 years, were enrolled in this study. After EEG testing, aberrant EEG waves were found in six patients. There are also some patients with psychomotor development and other central nervous system malformations. The general condition of the patients is shown in Table 1.
Table 1
| ID | Sex and age at diagnosis | Seizure type | EEG | Heterotopic localization | Psychomotor development | Other CNS malformations |
|---|---|---|---|---|---|---|
| 1 | M/21 y | Clonic seizure | Bilateral hemispheric slow waves, prominent in right frontal region | Adjacent to the peribilateral frontal horns of the lateral ventricles | Normal | None |
| 2 | M/22 y | Tonic–clonic seizure | No abnormalities | Left occipital lobe | Normal | Schizencephaly and polymicrogyria |
| 3 | F/17 y | Myoclonic seizure | Ictal patterns predominantly in the right frontotemporal regions | Adjacent to the peribilateral frontal horns of the lateral ventricles | Motor coordination disorder | None |
| 4 | M/53 y | Absence seizure | Bilateral temporal lobe spike waves and spike-and-slow waves | Adjacent to the left frontal horn | Normal | None |
| 5 | M/60 y | Tonic-clonic seizure | Bilateral cerebral hemispheres with frequent spikes/spike and left predominance | Right lateral ventricular wall | Memory impairment | None |
| 6 | M/17 y | Absence seizure | Interictal epileptiform discharges, with greatest intensity in frontal regions | Adjacent to the left frontal horn of lateral ventricle | Normal | None |
| 7 | M/17 y | Myoclonic seizure | Missing data | Bilateral lateral ventricular walls | Dizziness | Enlarged cisterna magna and cerebellomedullary cistern |
| 8 | F/19 y | Tonic-clonic seizure | No abnormalities | Adjacent to the peribilateral occipital horns of lateral ventricles | Visual hallucinations | None |
| 9 | F/43 y | Absence seizure | Frontal sharp waves and spike-wave complexes | Body of the corpus callosum | Auditory hallucinations | None |
CNS, central nervous system; EEG, electroencephalogram; F, female; M, male; y, years.
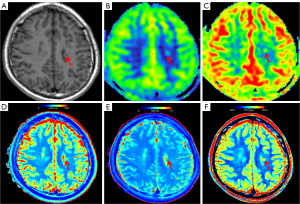
Five patients exhibited bilateral GMH. Consequently, a total of 32 GMH nodules were assessed along with the associated normal gray and white matter. Subsequently, each nodule was statistically evaluated for CBF, T1, T2, and PD via color maps (Figure 2).
The two radiologists measured the CBF, T1, T2, and PD values of GMH and NGM, with high concordance, as illustrated in the Bland-Altman plot shown in Figure S1.
The CBF values of NGM, GMH, with PLDs of 1.5 s and 2.5 s were all normally distributed among patients, as illustrated in Figure 3.

The mean CBF values of GMH with dual PLDs (31.96 and 35.13 mL/100 g/min) were both lower than those of NGM (52.69 and 56.93 mL/100 g/min). The paired-sample t-test (Table 2) indicated that the CBF values of GMH with dual PLDs were statistically different from those of NGM (P<0.001). This result remained significant after standardization (P<0.001). The differences in CBF values between GMH and NGM were calculated separately for each PLD time, with no significant difference attributable to PLD time being observed (P=0.500).
Table 2
| Pair | Mean ± SD | 95% CI | t | P |
|---|---|---|---|---|
| NGM–GMH (1.5) | 20.73±13.09 | (16.01, 25.45) | 8.979 | <0.001 |
| NGM–GMH (2.5) | 21.80±14.12 | (16.71, 26.89) | 8.735 | <0.001 |
| rNGM–rGMH (1.5) | 1.14±0.76 | (0.87, 1.41) | 8.502 | <0.001 |
| rNGM–rGMH (2.5) | 0.97±0.76 | (0.73, 1.21) | 8.199 | <0.001 |
| ΔCBF (2.5)–ΔCBF (1.5) | 1.06±8.82 | (−2.12, 4.24) | 0.682 | 0.500 |
1.5, postlabeling delay of 1.5 s; 2.5, postlabeling delay of 2.5 s; ΔCBF, cerebral blood flow differences between relative gray matter heterotopia and normal gray matter; CI, confidence interval; GMH, gray matter heterotopia; NGM, normal gray matter; rGMH, relative gray matter heterotopia; rNGM, relative normal gray matter; SD, standard deviation.
The T1, T2, and PD values of GMH and NGM all followed a normal distribution, as shown in Figure 4. Paired-sample t-tests were conducted to evaluate the differences between GMH and NGM before and after standardization (Table 3). The T1 values before and after standardization exhibited statistically significant differences (P<0.05) in both GMH and NGM. Additionally, the T1 values of GMH were all lower than those of NGM.

Table 3
| Pair | Mean ± SD | 95% CI | t | P |
|---|---|---|---|---|
| NGM–GMH (T1) | 92.94±79.08 | (64.43, 121.45) | 6.648 | <0.001 |
| NGM–GMH (T2) | −2.00±7.67 | (−4.76, 0.76) | −1.476 | 0.150 |
| NGM–GMH (PD) | 1.45±5.10 | (−0.39, 3.28) | 1.605 | 0.119 |
| rNGM–rGMH (T1) | 0.13±0.11 | (0.09, 0.17) | 6.670 | <0.001 |
| rNGM–rGMH (T2) | −0.03±0.12 | (−0.07, 0.01) | −1.420 | 0.166 |
| rNGM–rGMH (PD) | 0.02±0.08 | (−0.01, 0.05) | 1.602 | 0.119 |
CI, confidence interval; GMH, gray matter heterotopia; NGM, normal gray matter; PD, proton density; rGMH, relative gray matter heterotopia; rNGM, relative normal gray matter; SD, standard deviation; T1, T1 relaxation time; T2, T2 relaxation time.
Discussion
ASL is a noninvasive technique that labels proton spins in arterial blood and uses them as an endogenous tracer, allowing for quantitative depiction of CBF perfusion (10). ASL has a wide range of applications in the central nervous system, with diverse purposes including the identification of perfusion defects in ischemic strokes, the evaluation of CBF perfusion in brain tumors, and the assessment of perfusion during epileptic seizures (11-13). This study found there to be differences in CBF values between GMH and NGM at PLDs of 1.5 s and 2.5 s, which persisted after standardization. The mean CBF values of NGM were higher than those of GMH. Within the heterotopic nodules, there is a decrease in α-CaMKII and N-methyl-D-aspartate receptor NR2A/B subunits, along with immature GABAergic neurons. This collectively contributes to an imbalance in excitability of the GMH (14). All investigations took place during the interictal phase of the patients’ epilepsy, during which GMH typically exhibits hypoexcitability and reduced perfusion. The lack of statistical significance in the CBF differences between NGM and GMH at PLDs of 1.5 and 2.5 s suggests that NGM and GMH exhibit similar perfusion characteristics in both the early and late phases. However, GMH located within the white matter receives less perfusion as compared to the gray matter on the cortical surface.
The MAGiC sequence allows for the quantitative assessment of PD, T1, and T2 values in a single scan and is thus widely used for scans of the central nervous system. Kern et al. found that quantitative T2 values are meaningful for grading brain gliomas and identifying mutations in the isocitrate dehydrogenase gene (15). Additionally, the time of stroke onset is associated with changes in T2 fluid-attenuated inversion recovery (FLAIR) signal and T2 values in the stroke region (16). Quantitative imaging can also aid in the diagnosis of multiple sclerosis and epilepsy (17). Patients with epilepsy often display pathological microstructural tissue remodeling and astrocyte proliferation, which is potentially linked to sustained changes in neuronal activity (18). Subtle gray matter damage has been found in the cortex and thalamus of patients with multiple sclerosis (17). Quantitative PD values often serve as surrogate markers of tissue atrophy, with an increase in PD values indicating enlargement of interstitial spaces and thus a decrease in local tissue volume fraction (19,20). Quantitative T1 values are sensitive to changes in tissue water content, myelin content, and tissue iron deposition (21), typically reflecting reduced interstitial spaces, increased proliferation, and decreased water content, which result in decreased T1 values (22). Quantitative T2 values are often associated with gliosis and disruption of the blood-brain barrier. Changes in gliotic tissue can lead to an increase in tissue T2 values or affect the measurement of T2 values (23-25). Ectopic gray matter arises due to inadequate blood supply, leading to neuronal loss and tissue structure atrophy. The resultant damage from the abnormal discharge of ectopic gray matter may further induce pathological alterations, such as the demyelination of ectopic gray matter neurons and iron deposition in surrounding tissues. Therefore, in this study, we examined the differences between GMH and NGM before and after the normalization of quantitative T1 values. However, due to the limited sample size and minimal changes in PD and T2 values observed in this study, no significant differences were detected between GMH and NGM.
The study, for the first time, used quantitative MRI (ASL and MAGiC) to examine the differences in perfusion and signals between GMH and NGM. Our findings lay a theoretical foundation for the further determination of the true epileptogenic gray matter by studies employing a larger sample size. However, several limitations to this study should be noted. First, we employed a single-center design with a small sample size, which might have introduced potential biases. Second, although differences in perfusion and function between GMH and NGM were identified, we did not determine whether there are functional differences across GMH nodules in different locations. Future studies could analyze whether GMH nodules in different locations differ in their impact on the occurrence of epilepsy. Third, the literature suggests (26) that the occurrence of epilepsy may be associated with multiple GMH nodules or single GMH. In this study, there were five patients with bilateral GMH, but it was not determined which side of the heterotopic gray matter was the true epileptogenic focus.
Conclusions
NGM and GMH demonstrate quantitative differences on ASL and MAGiC sequences. This study offers quantitative data for differentiating GMH from NGM, establishing groundwork for future research in this field.
ASL, which assesses brain perfusion without intravenous injection of any contrast material, might be useful in the evaluation of neuronal activity of subcortical band heterotopia and thus in the detection of the epileptogenic area (27).
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1237/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1237/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the ethics committee of the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University (No. O-2024-259). The requirement for informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012;135:1348-69. [Crossref] [PubMed]
- Shao Y, Ge Q, Yang J, Wang M, Zhou Y, Guo JX, Zhu M, Shi J, Hu Y, Shen L, Chen Z, Li XM, Zhu JM, Zhang J, Duan S, Chen J. Pathological Networks Involving Dysmorphic Neurons in Type II Focal Cortical Dysplasia. Neurosci Bull 2022;38:1007-24. [Crossref] [PubMed]
- Di Matteo F, Bonrath R, Pravata V, Schmidt H, Ayo Martin AC, Di Giaimo R, Menegaz D, Riesenberg S, de Vrij FMS, Maccarrone G, Holzapfel M, Straub T, Kushner SA, Robertson SP, Eder M, Cappello S. Neuronal hyperactivity in neurons derived from individuals with gray matter heterotopia. Nat Commun 2025;16:1737. [Crossref] [PubMed]
- Battaglia G, Chiapparini L, Franceschetti S, Freri E, Tassi L, Bassanini S, Villani F, Spreafico R, D'Incerti L, Granata T. Periventricular nodular heterotopia: classification, epileptic history, and genesis of epileptic discharges. Epilepsia 2006;47:86-97. [Crossref] [PubMed]
- Severino M, Geraldo AF, Utz N, Tortora D, Pogledic I, Klonowski W, Triulzi F, Arrigoni F, Mankad K, Leventer RJ, Mancini GMS, Barkovich JA, Lequin MH, Rossi A. Definitions and classification of malformations of cortical development: practical guidelines. Brain 2020;143:2874-94. [Crossref] [PubMed]
- González G, Vedolin L, Barry B, Poduri A, Walsh C, Barkovich AJ. Location of periventricular nodular heterotopia is related to the malformation phenotype on MRI. AJNR Am J Neuroradiol 2013;34:877-83. [Crossref] [PubMed]
- Pu H, Wang L, Liu W, Tan Q, Wan X, Wang W, Su X, Sun H, Zhang S, Yue Q, Gong Q. Metabolic heterogeneity in different subtypes of malformations of cortical development causing epilepsy: a proton magnetic resonance spectroscopy study. Quant Imaging Med Surg 2023;13:8625-40. [Crossref] [PubMed]
- Nunez-Gonzalez L, van Garderen KA, Smits M, Jaspers J, Romero AM, Poot DHJ, Hernandez-Tamames JA. Pre-contrast MAGiC in treated gliomas: a pilot study of quantitative MRI. Sci Rep 2022;12:21820. [Crossref] [PubMed]
- Rahimzadeh H, Kamkar H, Ghafarian P, Hoseini-Tabatabaei N, Mohammadi-Mobarakeh N, Mehvari-Habibabadi J, Hashemi-Fesharaki SS, Nazem-Zadeh MR. Exploring ASL perfusion MRI as a substitutive modality for 18F-FDG PET in determining the laterality of mesial temporal lobe epilepsy. Neurol Sci 2024;45:2223-43. [Crossref] [PubMed]
- Togao O, Hiwatashi A, Obara M, Yamashita K, Kikuchi K, Kamei R, Nishimura A, Arimura K, Yoshimoto K, Iihara K, Van Cauteren M, Honda H. Acceleration-selective Arterial Spin-labeling MR Angiography Used to Visualize Distal Cerebral Arteries and Collateral Vessels in Moyamoya Disease. Radiology 2018;286:611-21. [Crossref] [PubMed]
- Di Nora A, Costanza G, Pizzo F, Oliva CF, Di Mari A, Greco F, Pavone P. Gray matter heterotopia: clinical and neuroimaging report on 22 children. Acta Neurol Belg 2022;122:153-62. [Crossref] [PubMed]
- Togao O, Obara M, Yamashita K, Kikuchi K, Wada T, Murazaki H, Arimura K, Nishimura A, Horie N, van de Ven K, Van Cauteren M, Ishigami K. Arterial Spin Labeling-Based MR Angiography for Cerebrovascular Diseases: Principles and Clinical Applications. J Magn Reson Imaging 2024;60:1305-24. [Crossref] [PubMed]
- Gennari AG, Gaito L, Cserpan D, Kottke R, Krayenbühl N, Rüegger A, O' Gorman Tuura R, Ramantani G. Brain perfusion imaging by arterial spin labeling predicts postsurgical seizure freedom in pediatric focal lesional epilepsy: A pilot study. Epilepsia 2025; Epub ahead of print. [Crossref] [PubMed]
- Tassi L, Colombo N, Cossu M, Mai R, Francione S, Lo Russo G, Galli C, Bramerio M, Battaglia G, Garbelli R, Meroni A, Spreafico R. Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain 2005;128:321-37. [Crossref] [PubMed]
- Kern M, Auer TA, Picht T, Misch M, Wiener E. T2 mapping of molecular subtypes of WHO grade II/III gliomas. BMC Neurol 2020;20:8. [Crossref] [PubMed]
- Wang Q, Bie F, Lu T, Sun X, Sun Q, Wang G, Li P. The effect of cerebral blood perfusion on the correlation between cerebral stroke onset time and synthetic T2 mapping: a pilot study. Quant Imaging Med Surg 2023;13:3477-88. [Crossref] [PubMed]
- Granziera C, Wuerfel J, Barkhof F, Calabrese M, De Stefano N, Enzinger C, Evangelou N, Filippi M, Geurts JJG, Reich DS, Rocca MA, Ropele S, Rovira À, Sati P, Toosy AT, Vrenken H, Gandini Wheeler-Kingshott CAM, Kappos LMAGNIMS Study Group. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021;144:1296-311. [Crossref] [PubMed]
- Tóth K, Hofer KT, Kandrács Á, Entz L, Bagó A, Erőss L, Jordán Z, Nagy G, Sólyom A, Fabó D, Ulbert I, Wittner L. Hyperexcitability of the network contributes to synchronization processes in the human epileptic neocortex. J Physiol 2018;596:317-42. [Crossref] [PubMed]
- Draganski B, Ashburner J, Hutton C, Kherif F, Frackowiak RS, Helms G, Weiskopf N. Regional specificity of MRI contrast parameter changes in normal ageing revealed by voxel-based quantification (VBQ). Neuroimage 2011;55:1423-34. [Crossref] [PubMed]
- Carey D, Caprini F, Allen M, Lutti A, Weiskopf N, Rees G, Callaghan MF, Dick F. Quantitative MRI provides markers of intra-, inter-regional, and age-related differences in young adult cortical microstructure. Neuroimage 2018;182:429-40. [Crossref] [PubMed]
- Burgetova A, Seidl Z, Krasensky J, Horakova D, Vaneckova M. Multiple sclerosis and the accumulation of iron in the Basal Ganglia: quantitative assessment of brain iron using MRI t(2) relaxometry. Eur Neurol 2010;63:136-43. [Crossref] [PubMed]
- Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 2016;18:89. [Crossref] [PubMed]
- Hasan KM, Walimuni IS, Abid H, Datta S, Wolinsky JS, Narayana PA. Human brain atlas-based multimodal MRI analysis of volumetry, diffusimetry, relaxometry and lesion distribution in multiple sclerosis patients and healthy adult controls: implications for understanding the pathogenesis of multiple sclerosis and consolidation of quantitative MRI results in MS. J Neurol Sci 2012;313:99-109. [Crossref] [PubMed]
- Peixoto-Santos JE, Kandratavicius L, Velasco TR, Assirati JA, Carlotti CG, Scandiuzzi RC, Salmon CE, Santos AC, Leite JP. Individual hippocampal subfield assessment indicates that matrix macromolecules and gliosis are key elements for the increased T2 relaxation time seen in temporal lobe epilepsy. Epilepsia 2017;58:149-59. [Crossref] [PubMed]
- Ahmad R, Maiworm M, Nöth U, Seiler A, Hattingen E, Steinmetz H, Rosenow F, Deichmann R, Wagner M, Gracien RM. Cortical Changes in Epilepsy Patients With Focal Cortical Dysplasia: New Insights With T(2) Mapping. J Magn Reson Imaging 2020;52:1783-9. [Crossref] [PubMed]
- Liu W, An D, Tong X, Niu R, Gong Q, Zhou D. Region-specific connectivity in patients with periventricular nodular heterotopia and epilepsy: A study combining diffusion tensor imaging and functional MRI. Epilepsy Res 2017;136:137-42. [Crossref] [PubMed]
- Xydis VG, Giantsouli A, Styliara E, Alexiou GA, Nakou I, Astrakas LG, Argyropoulou MI. Subcortical Band Heterotopia Shows Increased Perfusion on Arterial Spin Labeling Maps. Can J Neurol Sci 2022;49:703. [Crossref] [PubMed]


