
Predicting the regrowth of residual uterine fibroids after high-intensity focused ultrasound treatment: an interpretable magnetic resonance imaging radiomics model
Introduction
Uterine fibroids are monoclonal neoplasms that derive their origin from the smooth muscle tissue of the uterus (1,2). Clinical manifestations affect nearly half of uterine fibroid cases, with symptom burden demonstrating substantial correlation with diminished health-related quality of life metrics (3). As a mainstream therapeutic modality, high-intensity focused ultrasound (HIFU) is widely used for treating uterine fibroids due to its safety, efficacy, and short postoperative recovery period (4,5). Studies have indicated that the long-term outcomes of HIFU treatment and laparoscopic myomectomy for symptomatic uterine fibroids are comparable in terms of patient satisfaction and re-intervention rates (6). Symptomatic improvement and complication resolution in patients correlate with fibroid volume diminishment following HIFU therapy (7). Notwithstanding, emerging evidence suggests potential uterine fibroid regrowth, which may precipitate symptomatic recurrence (8,9). Among re-intervention rates, 70.1–84.6% of cases are attributed to the regrowth of residual uterine fibroids (RFs) (10-12).
Magnetic resonance imaging (MRI), owing to its superior soft-tissue resolution, serves as an indispensable tool for assessing treatment outcomes of HIFU in uterine fibroids (13,14). Previous studies have shown that T2-weighted imaging (T2WI) signal intensity (SI) classification reflects different histopathological components (15), particularly hyperintense fibroids, which are more prone to regrowth postoperatively (16-19). Contrast-enhanced T1-weighted imaging (CE-T1WI) can directly reflect the blood supply within fibroids, with significantly enhanced fibroids being more likely to regrow (10).
Radiomics represents a methodology for deriving high-dimensional features from medical imagery, thereby quantifying tumor heterogeneity and capturing subtle structural differences that are undetectable by the human eye (20). In the past, radiomics has been widely applied in HIFU pre-evaluation and post-treatment (11,12,21,22). However, the complex, fuzzy, and heterogeneous nature of medical data used in modeling presents challenges in interpretation (23). The “black box” nature of machine learning models makes it difficult to elucidate specific prognostic forecasts formulated for patients. To better interpret medical artificial intelligence (AI) systems, it is necessary to explain how internal features influence outcomes, enabling clinicians to trust the decisions made by the model. Lundberg and Lee pioneered the SHapley Additive exPlanations (SHAP) framework to augment the interpretability of machine learning models (24). Positive or negative SHAP values indicate the direction of influence, whereas the magnitude denotes the “weight” or “significance” of the feature. This technique allows for understanding the role of each feature in the prediction process, both at the global sample level and the individual sample level. The integration of SHAP and radiomics provides an interpretable model representation, thus enhancing the clinical credibility of radiomics-based predictions for both clinicians and patients.
This study sought to construct an MRI-derived radiomics framework to forecast the prognosis of fibroids treated with HIFU. Furthermore, SHAP technology was employed to visually illustrate the decision-making mechanisms of the model, enhancing the understanding of radiomic features in predicting HIFU treatment outcomes and thereby increasing the model’s reliability for clinicians and patients. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1844/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was conducted with approval from the Chongqing Haifu Hospital (No. 2023-011), and the requirement for informed consent was waived for this single-center retrospective study.
Study population
A total of 1,455 patients with uterine fibroids who underwent HIFU treatment between January 2016 and December 2021 were included in this study. These patients underwent pelvic MRI examinations before and after treatment, as well as approximately 1 year postoperatively, with the follow-up ranging from 9 to 17 months, as determined by previous studies and the consensus of two experienced radiologists (12).
The inclusion criteria were as follows: (I) aged ≥18 years and pre-menopausal; (II) availability of pre-treatment, immediate post-HIFU, and 1-year follow-up (9–17 months) MRI datasets; (III) a single-session HIFU treatment, not preceded by HIFU treatment in the past 5 years and without subsequent treatments or drug interventions; and (IV) in patients with multiple fibroids, only the largest fibroid was selected for analysis. The exclusion criteria were as follows: (I) MRI artifacts, poor contrast enhancement, low resolution, or incomplete magnetic resonance (MR) sequences; (II) absolute contraindications to MRI; (III) suspected malignancy of uterine tumors; (IV) preoperative MRI evidence of severe spontaneous necrosis, calcification, or red degeneration; and (V) postoperative MRI demonstrating intraprocedural hemorrhage.
MRI examination and ultrasound-guided HIFU treatment
MRI examinations were performed using uMR570 (1.5 Tesla; China United Imaging Company, Shanghai, China), with acquisition parameters detailed in Table 1. Treatment devices used were the JC200 or JC-focused ultrasound tumor treatment systems (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). Sagittal MRI sequences were exported from the Picture Archiving and Communication System (PACS) in Digital Imaging and Communications in Medicine (DICOM) format for further radiomics feature extraction.
Table 1
| MRI type | T1-weighted | T2-weighted | CE-T1WI |
|---|---|---|---|
| Repetition time (ms) | 214 | 5,300 | 3.94 |
| Echo time (ms) | 10 | 88 | 1.84 |
| Number of excitations | 1.3 | 2 | 1 |
| Field of view (cm × cm) | 25.2×36 | 24×24 | 35×28 |
| Matrix size (mm × mm) | 320×70 | 320×75 | 288×75 |
| Pixel spacing (mm) | 0.74/0.74 | 0.52/0.52 | 0.56/0.56 |
| Slice thickness (mm) | 5 | 5 | 4 |
| Slice gap (mm) | 1 | 1 | 0.5 |
| Flip angle (°) | 20 | 150 | 10 |
| Acquisition duration (s) | 99.2 | 70.4 | 16.35 |
| Imaging planes | T | T, S | T, S, C |
The parameters used for MRI are illustrated in the table. MRI imaging of uterine fibroid patients was performed using a 16-channel body coil/abdominal coil. C, coronal plane; S, sagittal plane; T, transverse plane; CE-T1WI, contrast-enhanced T1-weighted imaging; MRI, magnetic resonance imaging.
A HIFU tumor treatment system was used. The ultrasound treatment beam is generated by a single-element transducer operated at a frequency of 0.8 MHz ultrasound, featuring a 15-cm focal length and 20-cm diameter. The patient was placed in a prone position on the HIFU platform, with the anterior abdominal wall submerged in degassed water. A degassed water balloon was positioned between the abdominal wall and the probe to compress the intestines and displace them from the sound path. Sagittal ultrasound scanning mode was employed for both pretreatment planning and treatment execution. Fibroid and adjacent tissue localization was performed on ultrasound images, with the target fibroid segmented into multiple sub-volumes in real-time at 5-mm intervals. Point scanning was performed with power settings ranging from 300 to 400 watts.
Tumor region of interest delineation, clinical data, and MRI features
RFs were defined by the degree of enhancement observed on CE-T1WI following HIFU treatment with the internal SI ratio of the uterine fibroid calculated as (25,26): internal SI ratio = (post-CE-T1WI SI of the fibroid/post-CE-T1WI SI of the iliacus muscle)/(pre-CE-T1WI SI of the fibroid/pre-CE-T1WI SI of the iliacus muscle). An internal SI ratio of the fibroid >1% indicated persistent enhancement postoperatively, defining the fibroid as residual fibroid volume (RFV), whereas ≤1% indicated minimal/no enhancement, categorizing the area as non-perfused volume (NPV). RFV and NPV were delineated 1 day and approximately 1 year after the treatment, respectively, with the RFV on day one subtracted from the RFV 1 year postoperatively to categorize patients into RF regrowth and non-regrowth groups.
The clinical data collected included the patient’s age, body mass index (BMI), uterine fibroid volume, RFV, NPV and NPV ratio (NPVR), number and size of fibroids, RF thickness, T2WI SI types (hypointensity, isointensity, hyperintensity), and CE-T1WI SI enhancement (slight enhancement, moderate enhancement, significant enhancement).
Radiomic feature extraction
Figure 1 illustrates the radiomics workflow of this study. All images were processed with ‘N4ITK’ bias field correction to enhance grayscale uniformity and minimize artifacts, followed by Z-score normalization to standardize intensities (27). B-spline interpolation was employed to resample images to an isotropic voxel size of 1×1×1 mm3.

Two radiologists with 6 years of experience in pelvic imaging manually delineated the uterine fibroids on T2WI and CE-T1WI arterial phase images, acquired 1 day post-treatment, using 3D-Slicer software (version 5.5.0; https://www.slicer.org/), with regions of interest (ROIs) delineating the contours layer by layer. Discrepancies were adjudicated by a senior radiologist with 32 years of experience.
Radiomic features were then extracted from the ROIs based on the shape and texture of the fibroids on T2WI and CE-T1WI images using the radiomics package (PyRadiomics version 3.0.1; https://pypi.org/project/pyradiomics/) in Python (version 3.11.5; https://www.python.org/downloads/release/python-3115/). Low-dimensional features included shape and first-order histogram features, whereas the high-dimensional features encompassed texture features such as Gray Level Co-occurrence Matrix (GLCM), Gray Level Run Length Matrix (GLRLM), Gray Level Size Zone Matrix (GLSZM), Gray Level Dependence Matrix (GLDM), Neighborhood Gray Tone Difference Matrix (NGTDM), as well as features derived from texture matrices in both Gaussian-Laplacian and wavelet filtering domains.
Assessment of radiomic feature reproducibility
The intraclass correlation coefficient (ICC) was used to assess the consistency of features extracted from ROIs independently delineated by two observers on the same image. Features with an ICC greater than 0.75 were considered consistent, whereas those with an ICC less than 0.75 were excluded.
Data balancing with synthetic minority oversampling technique (SMOTE)
Data imbalance, especially between the regrowth and non-regrowth groups, may induce model bias toward the majority class, thereby compromising prediction reliability (28). To address this, the SMOTE was utilized. This approach works by effectively expanding the sample size of minority categories, thereby mitigating the disparity in class representation.
Radiomic feature selection and dimensionality reduction
Missing values resulting from failures in radiomic feature extraction were imputed using the residual method before data normalization. The validation set was normalized using the same parameters (mean and standard deviation) derived from the training set to ensure comparability. An initial two-sample t-test was initially conducted to exclude features with low correlation or redundancy on the training set, followed by dimensionality reduction using t-distributed Stochastic Neighbor Embedding (t-SNE) to further refine the feature set. Standardizing all radiomic features with an ICC ≥0.75 using Z-scores, the least absolute shrinkage and selection operator (LASSO) was applied to identify and select the most relevant features from the training cohort, with the optimal regularization parameter (λ) determined through 10-fold cross-validation. The selected features were then used to train the model.
Model construction
Logistic regression (LR) models were constructed using radiomic features from T2WI, CE-T1WI, and a feature-level fusion of both sequences. Model performance was initially assessed on the training set and externally validated on the test set. Predictive accuracy was quantified via receiver operating characteristic (ROC) curve analysis [area under the curve (AUC)], accuracy, sensitivity, and specificity.
Model interpretation using SHAP
SHAP was implemented to interpret and understand each feature’s contribution and importance within the model. It provided a visual representation of feature importance within the complex model architecture, elucidating how each feature augmented or attenuated the probability of specific outcomes.
Statistical analysis
The Kolmogorov-Smirnov test was used to test the normality of imaging features in the training and test sets. In Table 2, normally distributed data were presented as ; non-normally distributed data were presented as the interquartile range (Q25, Q75). Quantitative data were analyzed using independent sample t-tests or Wilcoxon rank-sum tests, and qualitative data were analyzed using Chi-squared tests or Fisher’s exact tests. DeLong’s test was conducted to compare the models constructed using the same sequence features. The Bonferroni method was used for post-hoc pairwise comparisons. Statistical significance was set at P<0.05.
Table 2
| Characteristic | Total | Training set (n=92) | Test set (n=24) | |||||
|---|---|---|---|---|---|---|---|---|
| Regrowth (n=59) | Non-regrowth (n=33) | P value | Regrowth (n=14) | Non-regrowth (n=10) | P value | |||
| Patient’s age (years)† | 41.0 (35.75–44.0) | 41.0 (36.0–43.0) | 40.55±7.45 | 0.326 | 42.5±6.71 | 38.6±6.28 | 0.179 | |
| BMI (kg/m2)† | 22.1 (20.4–24.05) | 21.6 (20.5–24.25) | 22.08±2.47 | 0.823 | 23.01±2.65 | 21.55±1.81 | 0.138 | |
| Uterine fibroid volume (cm3)† | 88.05 (57.14–177.14) | 94.03 (65.22–173.95) | 88.05 (43.6–176.75) | 0.43 | 70.7 (41.68–138.8) | 172.88±122.58 | 0.095 | |
| RFV (cm3)† | 16.41 (8.65–34.87) | 28.69 (11.91–58.21) | 10.14 (5.92–17.05) | <0.001* | 17.89 (12.1–39.49) | 14.48 (6.46–18.52) | 0.254 | |
| NPV (cm3)† | 73.5 (38.19–130.11) | 68.44 (38.53–122.45) | 77.91 (38.68–153.94) | 0.666 | 65.98±52.51 | 155.61±114.24 | 0.043* | |
| NPVR (%)† | 81.03 (63.78–90.05) | 71.08 (54.2–84.5) | 88.49 (82.94–93.71) | <0.001* | 66.79±16.44 | 88.45±6.86 | 0.001* | |
| Number of fibroids† | 2.0 (1.0–6.0) | 2.0 (1.0–6.0) | 2.0 (1.0–6.0) | 0.53 | 2.5 (1.0–3.75) | 4.0 (1.25–9.5) | 0.335 | |
| Fibroids size (cm)† | 6.0 (4.0–9.0) | 6.57 (5.58–8.1) | 6.27±1.77 | 0.236 | 6.18±1.62 | 7.12±1.68 | 0.206 | |
| RF thickness (cm)† | 0.71 (0.36–1.25) | 1.07 (0.61–1.64) | 0.37 (0.28–0.62) | <0.001* | 1.13±0.79 | 0.37±0.17 | 0.004* | |
| T2WI SI types (hypo/iso/hyperintensity) (%)‡ | 5/90/21 (4.3/77.6/18.1) | 3/38/18 (5.1/64.4/30.5) | 2/30/1 (6.1/90.9/3.0) | 0.008* | 0/12/2 (0/85.7/14.3) | 0/10/0 (0/100/0) | 0.618 | |
| CE-T1WI SI enhancement (SIE/ME/SE) (%)‡ | 26/41/49 (22.4/35.3/42.2) | 5/19/35 (8.5/32.2/59.3) | 17/14/2 (51.5/42.4/6.1) | <0.001* | 1/3/10 (7.1/21.4/71.4) | 3/5/2 (30.0/50.0/20.0) | 0.042* | |
Data are expressed as mean ± standard deviation, median (interquartile range), or n (%). †, Mann-Whitney U test; ‡, Chi-squared tests (Pearson’s Chi-squared, continuous corrected Chi-squared); *, a significant difference (P<0.05). BMI, body mass index (18.5–23.9 kg/m2); CE-T1WI, contrast enhanced T1-weighted imaging; NPV(R), non-perfused volume (ratio); RF, residual uterine fibroid; RFV, residual fibroid volume; SI, signal intensity; SIE/ME/SE, slight enhancement/moderate enhancement/significant enhancement.
Results
Demographic and clinical data
A total of 116 eligible patients were included in this study based on the inclusion and exclusion criteria. Of these, 73 patients experienced RF regrowth, whereas 43 had further reductions in RF volume. Patients were randomly allocated to a training cohort (N=92) and a test cohort (N=24) for model development and validation. The patient selection process flowchart is shown in Figure 2. Clinical, radiological, and statistical characteristics of the training and test sets are summarized in Table 2. Significant differences in these characteristics, including NPVR, RF thickness, and CE-T1WI SI enhancement (P<0.05), were observed in both sets.

Data preparation for modeling
In total, 218 features were retrieved, comprising 109 features from T2WI and 109 from CE-T1WI. After applying the ICC threshold of 0.75, 81 features were retained from T2WI and 86 from CE-T1WI. Dimensionality reduction was performed using t-tests (P<0.05). To mitigate the impact of data imbalance on the ultimate model’s performance and interpretability, SMOTE was utilized to balance the dataset, generating 18 new non-regrowth RF patients, which increased the total number of patients in this group from 43 to 61 for inclusion in subsequent analyses. LASSO regression was used for feature selection and regression analysis, retaining features with non-zero coefficients. Therefore, the final T2WI and CE-T1WI models retained 13 and 3 radiomic features, respectively, whereas the fusion model retained 13 radiomic features, including 5 CE-T1WI and 8 T2WI features. The interested reader can find the model equations in a supplementary appendix online.
Performance of the radiomic models
The LR models were constructed using T2WI, CE-T1WI, and feature-level fusion, with the fusion model performing the best (Table 3). The AUCs for the T2WI, CE-T1WI, and fusion models in the training set were 0.933 [95% confidence interval (CI): 0.879–0.987], 0.757 (95% CI: 0.651–0.863), and 0.946 (95% CI: 0.897–0.995), respectively. The AUCs for the T2WI, CE-T1WI, and fusion models in the test set were 0.926 (95% CI: 0.817–1.000), 0.879 (95% CI: 0.731–1.000), and 0.972 (95% CI: 0.910–1.000), respectively. The performance of the three models was further evaluated and compared in the test set. DeLong’s test, with Bonferroni correction, revealed significant statistical differences in AUC values between the T2WI, fusion, and CE-T1WI sequences (P<0.016, P<0.006). Although the fusion model’s AUC was slightly higher than that of the T2WI sequence, the difference was not statistically significant (P<0.142).
Table 3
| Predictive models | Set | AUC (95% CI) | Accuracy | Precision | Sensitivity | Specificity | MCC |
|---|---|---|---|---|---|---|---|
| T2WI | Train | 0.933 (0.879–0.987) | 0.902 | 0.926 | 0.909 | 0.892 | 0.783 |
| Test | 0.926 (0.817–1.000) | 0.875 | 1.000 | 0.833 | 1.000 | 0.628 | |
| CE | Train | 0.757 (0.651–0.863) | 0.772 | 0.797 | 0.864 | 0.606 | 0.666 |
| Test | 0.879 (0.731–1.000) | 0.833 | 0.857 | 0.857 | 0.8 | 0.657 | |
| Fusion model | Train | 0.946 (0.897–0.995) | 0.902 | 0.897 | 0.945 | 0.838 | 0.779 |
| Test | 0.972 (0.910–1.000) | 0.833 | 1.000 | 0.778 | 1.000 | 0.745 |
AUC, area under the receiver operating characteristic curve; CE, contrast-enhanced; CI, confidence interval; LR, logistic regression; MCC, Matthews correlation coefficient; T2WI, T2-weighted imaging.
SHAP interpretation
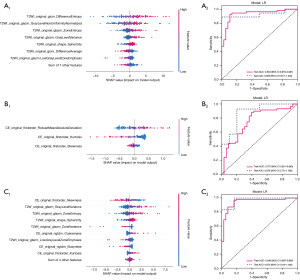
SHAP values were computed for every chosen radiomic feature within the top-performing LR model using an approximation method, namely Kernel SHAP, which combines insights from Local Interpretable Model-agnostic Explanations (LIME) and Shapley values to compute feature importance through weighted linear regression. The SHAP feature significance plot is presented in Figure 3, listing the most important features in descending order based on the mean absolute SHAP values, which reflect each feature’s average contribution to the model’s predictions across all patients. For the T2WI, CE-T1WI, and fusion models, the most influential features were T2WI original-glcm-Difference-Entropy, CE-T1WI original-firstorder-Robust Mean Absolute Deviation, and CE-T1WI original-firstorder-Skewness, respectively. These features include first-order and texture features based on the grayscale distribution of images. The SHAP summary plots in Figure 3 further illustrate the impact of radiomic features on the decision-making process of the radiomics models, as well as feature interactions. Positive SHAP values, shown in red, indicate an increased risk of adverse outcomes, whereas negative values, shown in blue, indicate a reduced risk. Higher SHAP values correlate with a higher risk of adverse outcomes. In individual sample predictions, SHAP waterfall plots were created for two randomly selected patients from the RF regrowth and non-regrowth groups (Figures 4,5), illustrating each feature’s positive and negative contributions to the outcome.


Discussion
We have demonstrated the application of the radiomics model constructed using LR, extracting 14 significant radiomic features from T2WI, 3 from CE-T1WI, and 13 important features (8 from T2WI and 5 from CE-T1WI) through feature-level fusion to predict the regrowth of RFs following HIFU treatment. These radiomics models showed superior performance in an independent test set. The SHAP approach of the models revealed that the radiomic features extracted from both sequences primarily reflected SI, which is closely associated with RF regrowth following HIFU treatment.
In our study, we similarly demonstrated that fibroids with hyperintensity on T2WI and those with significant enhancement on CE-T1WI are more prone to regrowth. T2WI is particularly accurate in displaying the histological characteristics of uterine fibroids, with SI attributed to pathological hallmarks including cellularity, vascular density, perfusion status, necrosis, edema, and calcification (29). CE-T1WI directly reflects the blood supply within residual fibroids (30). T2WI isointense and hypointense signals fibroids typically have better prognoses, whereas hyperintense fibroids are generally considered to have poorer prognoses, despite size reduction (17). Those with significant enhancement on CE-T1WI are often difficult to completely ablate and are prone to recurrence (31). However, the subjectivity inherent in interpreting T2WI and CE-T1WI, alongside challenges in detecting subtle SI changes, poses limitations for conventional MRI. Although these MR sequences encode biological information about fibroid cellular architecture and integrity, radiomics uniquely extracts an abundance of quantitative features that characterize these microstructural phenotypes. Feature-level fusion involves combining the features extracted from both sequences to generate a new feature vector, which can better represent and analyze multi-source data (32,33).
In previous studies, Zhang et al. and Zhou et al. (11,12) have demonstrated the prediction of RF regrowth 1 year after HIFU treatment using radiomics; similarly, Qin et al. and Cheng et al. (22,34) successfully predicted ablation outcomes and long-term re-intervention rates for uterine fibroids preoperatively. In radiomics, feature sets from T2WI and CE-T1WI are often combined to leverage complementary information provided by different modalities. These feature sets capture specific tissue contrast information under different imaging modalities.
We selected significant features to build an efficient supervised learning model. Our results indicate that the developed radiomics model, utilizing objective and reliable imaging biomarkers, has great potential for clinical prediction of RF regrowth 1 year post-HIFU treatment. The SHAP technology affords both global model interpretability and local sample-specific explanations for radiomics-based predictions. Radiomics data require better interpretation of how internal features influence outcomes to make medical AI systems more trustworthy for clinicians.
We found that first-order statistical features in all three models quantitatively analyzed the distribution characteristics of grayscale values, such as mean, variance, skewness, and kurtosis. These statistical features reflect the overall brightness and contrast information of tissues in different modalities. The analysis of structure and texture, including features such as GLCM, GLRLM, GLSZM, and NGTDM, describes the texture and structural information within the images. These features reveal subtle grayscale structure variations and heterogeneity in the images, which may be related to the disease.
SHAP analysis revealed that elevated SI in T2WI and CE-T1WI correlated with heightened RF regrowth risk following HIFU therapy, suggesting that regrowth may stem from microstructural and microvascular properties underlying these hyperintense regions. Uterine fibroids primarily consist of collagenous stroma and smooth muscle cells. Residual MRI signal depends on the proportion of smooth muscle cells to fibrous connective tissue. This implies that the higher the smooth muscle cell content, the more likely the RF will regrow. Conversely, RFs with a higher fibrous tissue content and fewer smooth muscle cells, coupled with insufficient blood supply, are more likely to shrink or even disappear postoperatively. Although ultrasound waves exhibit variations in focal intensity, location, and spatial distribution of intensity across fibroids with different tissue compositions, perfusion can have a substantial impact on thermal dose volumes in some cases (35). However, it has less impact than ultrasound absorption for predicting peak temperature elevation (36), which is consistent with previous studies (9,10).
Radiomics features represent specific physical and biological characteristics (37,38). Additionally, we found that difference entropy, robust mean absolute deviation, and skewness were the most important features in the three models. Difference entropy reflects the heterogeneity and complexity of fibroid internal tissue, which may be related to pathological changes or regrowth speed; robust mean absolute deviation measures the variability in tissue density, with higher values potentially indicating unevenness or complexity in internal structure; and skewness describes the symmetry of tissue composition distribution, with positive and negative skewness indicating the proportions of low-signal and high-signal regions within the fibroid (39).
SHAP technology provides insights into the mechanisms by which radiomic features drive global prediction outcomes. Meanwhile, for case-specific interpretability, SHAP waterfall plots deliver rapid and intuitive explanations, outperforming complex nomogram-based methods. Clearly, the importance of the same feature differs between the two groups of cases, and for different patients, highly important features may have less influence in some cases. Thus, SHAP also demonstrates good specificity in individual prediction.
Our study has some limitations. First, our sample size was relatively small, and further studies with larger samples are needed to support the robustness of the model. Second, the retrospective single-center design necessitates validation of the model’s generalizability using multicenter and prospective datasets. Finally, to simplify model interpretation, we only used traditional radiomics, but future studies could incorporate habitat technology and deep learning features, which may yield different results in radiomics analysis. Nonetheless, our study establishes a solid foundation for applying interpretable radiomics models in clinical practice.
Conclusions
In summary, the radiomics prediction model developed using features selected from T2WI and CE-T1WI sequences provides a novel feature-level fusion model to assist in the clinical assessment of HIFU treatment uterine fibroid prognosis. The application of the SHAP technique may enhance the understanding of the internal prediction processes for both physicians and patients, thereby increasing the credibility of the radiomics models, aiding in the individualized management of patients undergoing HIFU treatment.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1844/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1844/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was conducted with approval from the Chongqing Haifu Hospital (No. 2023-011), and the requirement for informed consent was waived for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bulun SE. Uterine fibroids. N Engl J Med 2013;369:1344-55. [Crossref] [PubMed]
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. [Crossref] [PubMed]
- Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501-12. [Crossref] [PubMed]
- Liu X, Tang J, Luo Y, Wang Y, Song L, Wang W. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG 2020;127:1422-8. [Crossref] [PubMed]
- Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology 2003;226:897-905. [Crossref] [PubMed]
- Mohr-Sasson A, Machtinger R, Mashiach R, Nir O, Inbar Y, Maliyanker N, Goldenberg M, Rabinovici J. Long-term outcome of MR-guided focused ultrasound treatment and laparoscopic myomectomy for symptomatic uterine fibroid tumors. Am J Obstet Gynecol 2018;219:375.e1-7. [Crossref] [PubMed]
- Lee JY, Chung HH, Kang SY, Park EJ, Park DH, Son K, Han JK. Portable ultrasound-guided high-intensity focused ultrasound with functions for safe and rapid ablation: prospective clinical trial for uterine fibroids-short-term and long-term results. Eur Radiol 2020;30:1554-63. [Crossref] [PubMed]
- Wang L, Liu Y, Lin J, Pan Y, Liu Y, Lv F. The Predictive Effect of Quantitative Analysis of Signal Intensity Heterogeneity on T2-Weighted MR Images for High-intensity Focused Ultrasound Treatment of Uterine Fibroids. Acad Radiol 2024;31:2848-58. [Crossref] [PubMed]
- Yuan Y, Xu W, Shen H, Lin Z, Xu F, Shi Q, Zhan P, Liu M, Shu J, Chen J, Xing HR. Long-term outcomes of ultrasound guided high intensity focused ultrasound ablation for patients with uterine fibroids classified by T2WI: a multicenter retrospective study. Int J Hyperthermia 2023;40:2212887. [Crossref] [PubMed]
- Liu Y, Wu X, Wu A, Gong C, Wang Z, Zhang L. Ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids: long-term outcomes and factors affecting local recurrence. Int J Hyperthermia 2021;38:1341-8. [Crossref] [PubMed]
- Zhou Y, Zhang J, Chen J, Yang C, Gong C, Li C, Li F. Prediction using T2-weighted magnetic resonance imaging-based radiomics of residual uterine myoma regrowth after high-intensity focused ultrasound ablation. Ultrasound Obstet Gynecol 2022;60:681-92. [Crossref] [PubMed]
- Zhang J, Yang C, Gong C, Zhou Y, Li C, Li F. Magnetic resonance imaging parameter-based machine learning for prognosis prediction of high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia 2022;39:835-46. [Crossref] [PubMed]
- Zhao WP, Chen JY, Chen WZ. Dynamic contrast-enhanced MRI serves as a predictor of HIFU treatment outcome for uterine fibroids with hyperintensity in T2-weighted images. Exp Ther Med 2016;11:328-34. [Crossref] [PubMed]
- Wang Y, Gong C, He M, Lin Z, Xu F, Peng S, Zhang L. Therapeutic dose and long-term efficacy of high-intensity focused ultrasound ablation for different types of uterine fibroids based on signal intensity on T2-weighted MR images. Int J Hyperthermia 2023;40:2194594. [Crossref] [PubMed]
- Zhao WP, Chen JY, Chen WZ. Effect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol 2015;41:423-31. [Crossref] [PubMed]
- Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol 2007;196:184.e1-6. [Crossref] [PubMed]
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009;34:584-9. [Crossref] [PubMed]
- Kim YS, Lee JW, Choi CH, Kim BG, Bae DS, Rhim H, Lim HK. Uterine Fibroids: Correlation of T2 Signal Intensity with Semiquantitative Perfusion MR Parameters in Patients Screened for MR-guided High-Intensity Focused Ultrasound Ablation. Radiology 2016;278:925-35. [Crossref] [PubMed]
- Sainio T, Saunavaara J, Komar G, Mattila S, Otonkoski S, Joronen K, Perheentupa A, Blanco Sequeiros R. Feasibility of apparent diffusion coefficient in predicting the technical outcome of MR-guided high-intensity focused ultrasound treatment of uterine fibroids - a comparison with the Funaki classification. Int J Hyperthermia 2021;38:85-94. [Crossref] [PubMed]
- Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:4006. [Crossref] [PubMed]
- Qin S, Jiang Y, Wang F, Tang L, Huang X. Development and validation of a combined model based on dual-sequence MRI radiomics for predicting the efficacy of high-intensity focused ultrasound ablation for hysteromyoma. Int J Hyperthermia 2023;40:2149862. [Crossref] [PubMed]
- Qin S, Lin Z, Liu N, Zheng Y, Jia Q, Huang X. Prediction of postoperative reintervention risk for uterine fibroids using clinical-imaging features and T2WI radiomics before high-intensity focused ultrasound ablation. Int J Hyperthermia 2023;40:2226847. [Crossref] [PubMed]
- Coppola F, Faggioni L, Gabelloni M, De Vietro F, Mendola V, Cattabriga A, Cocozza MA, Vara G, Piccinino A, Lo Monaco S, Pastore LV, Mottola M, Malavasi S, Bevilacqua A, Neri E, Golfieri R. Human, All Too Human? An All-Around Appraisal of the "Artificial Intelligence Revolution" in Medical Imaging. Front Psychol 2021;12:710982. [Crossref] [PubMed]
- Lundberg S, Lee SI. A Unified Approach to Interpreting Model Predictions [Internet]. arXiv; 2017 [cited 2024 Aug 20]. Available online: http://arxiv.org/abs/1705.07874
- Kirpalani A, Chong J, Yang N, Jenkins SJ, Nisenbaum R, Prabhudesai V, Anthwal S, Colak E. Diffusion-weighted imaging properties of uterine fibroids pre- and post-uterine fibroid embolisation. Eur J Radiol 2014;83:1620-5. [Crossref] [PubMed]
- Liu Y, Xiao Z, Luo Y, Qiu X, Wang L, Deng J, Yang M, Lv F. Predictive value of contrast-enhanced MRI for the regrowth of residual uterine fibroids after high-intensity focused ultrasound treatment. Insights Imaging 2024;15:274. [Crossref] [PubMed]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010;29:1310-20. [Crossref] [PubMed]
- Fernandez A, Garcia S, Herrera F, Chawla NV. SMOTE for Learning from Imbalanced Data: Progress and Challenges, Marking the 15-year Anniversary. J Artif Int Res 2018;61:863-905.
- Zhao WP, Zhang J, Han ZY, Yao JP, Zhou X, Liang P. A clinical investigation treating different types of fibroids identified by MRI-T2WI imaging with ultrasound guided high intensity focused ultrasound. Sci Rep 2017;7:10812. [Crossref] [PubMed]
- Petralia G, Summers PE, Agostini A, Ambrosini R, Cianci R, Cristel G, Calistri L, Colagrande S. Dynamic contrast-enhanced MRI in oncology: how we do it. Radiol Med 2020;125:1288-300. [Crossref] [PubMed]
- Keserci B, Duc NM. The role of T1 perfusion-based classification in magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur Radiol 2017;27:5299-308. [Crossref] [PubMed]
- Lv W, Ashrafinia S, Ma J, Lu L, Rahmim A. Multi-Level Multi-Modality Fusion Radiomics: Application to PET and CT Imaging for Prognostication of Head and Neck Cancer. IEEE J Biomed Health Inform 2020;24:2268-77. [Crossref] [PubMed]
- Xv Y, Wei Z, Lv F, Jiang Q, Guo H, Zheng Y, Zhang X, Xiao M. Multiparameter computed tomography (CT) radiomics signature fusion-based model for the preoperative prediction of clear cell renal cell carcinoma nuclear grade: a multicenter development and external validation study. Quant Imaging Med Surg 2024;14:7031-45. [Crossref] [PubMed]
- Cheng Y, Yang L, Wang Y, Kuang L, Pan X, Chen L, Cao X, Xu Y. Development and validation of a radiomics model based on T2-weighted imaging for predicting the efficacy of high intensity focused ultrasound ablation in uterine fibroids. Quant Imaging Med Surg 2024;14:1803-19. [Crossref] [PubMed]
- deSouza NM, Gedroyc W, Rivens I, Ter Haar G. Tissue specific considerations in implementing high intensity focussed ultrasound under magnetic resonance imaging guidance. Front Oncol 2022;12:1037959. [Crossref] [PubMed]
- Hyvärinen M, Huang Y, David E, Hynynen K. Comparison of computer simulations and clinical treatment results of magnetic resonance-guided focused ultrasound surgery (MRgFUS) of uterine fibroids. Med Phys 2022;49:2101-19. [Crossref] [PubMed]
- van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77:e104-7. [Crossref] [PubMed]
- Mu W, Schabath MB, Gillies RJ. Images Are Data: Challenges and Opportunities in the Clinical Translation of Radiomics. Cancer Res 2022;82:2066-8. [Crossref] [PubMed]
- Tomaszewski MR, Gillies RJ. The Biological Meaning of Radiomic Features. Radiology 2021;298:505-16. [Crossref] [PubMed]


