
Current practice and perceptions of multiparametric magnetic resonance imaging and multiparametric ultrasound and prostatic biopsies for prostate cancer diagnosis in China: a nationwide survey
Introduction
The standard procedure for prostate cancer (PCa) diagnosis is transrectal ultrasound-guided systematic biopsy, as recommended in the guidelines (1-3). However, this procedure is not without controversies. Significant concerns have been raised regarding the overtreatment of clinically insignificant prostate cancer (cisPCa) and the missed diagnosis of clinically significant prostate cancer (csPCa) (4-6). Additionally, the increasing number of puncture needles during systematic biopsy carries risks of complications, such as hematuria, erectile dysfunction, and post-biopsy infections (7,8). Therefore, investigating alternative strategy to reduce overtreatment and improve diagnostic accuracy is necessary.
In recent years, advances in medical imaging techniques lead to the widespread investigation of multiparametric magnetic resonance imaging (mpMRI) for risk assessment and PCa localization prior to biopsy (9-11). Additionally, multiparametric ultrasound (mpUS), which includes B-mode, color Doppler, elastography, and contrast-enhanced ultrasound, has been developed to improve PCa detection (12-14). Moreover, several studies from tertiary referral centers have confirmed that mpMRI-guided targeted biopsy or ultrasound-nuclear magnetic fusion image-guided targeted biopsy significantly improves the detection rate of PCa, reduces the number of biopsy cores, and lowers the risk of infection morbidity (5,15-17). These findings are be likely to have a significant impact on PCa clinical practice, promoting diagnostic accuracy and reducing overtreatment.
Currently, the incidence of PCa in Asia is gradually increasing, especially in China (18-21). However, little is known regarding the use of mpMRI and mpUS for PCa detection and biopsy in current practice in China, to the best of our knowledge. Therefore, a national survey from China was conducted among clinicians in this study to evaluate the perceived attitudes and practice patterns regarding mpMRI and mpUS for PCa diagnosis, as well as the relative use of prostatic systematic and targeted biopsies. We present this article in accordance with the CROSS reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1834/rc).
Methods
This study used a cross-sectional design and was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee for the Sichuan Cancer Hospital and Institute (No. SCCHEC-02-2023-155) and individual consent for this study was waived due to the retrospective nature.
A questionnaire regarding mpMRI and mpUS on PCa detection and biopsy was designed to collect demographic information from clinicians, including urologists, radiologists, and sonographers. However, the decision-making regarding the execution of a prostate biopsy was entrusted to the urologist, who evaluated a broader range of clinical parameters prior to the biopsy. The survey questionnaire contained single- and multiple-choice items and was electronically delivered online with invitation links to a random sample of specialists from the Chinese Doctors Association of Ultrasound (CUDA), Chinese Anti-Cancer Association of Imaging (CACA-I), and Chinese Anti-Cancer Association of Genital Urology (CACA-GU). To recruit specialists in our survey sample, clinicians were selected who designated their primary specialty as ultrasonography, radiology, or urology. The recruitment period was from December 2023 to January 2024. Survey anonymity and confidentiality were ensured by not collecting personally identifiable information such as identification card numbers, names, telephone numbers, and physical addresses. Characteristics of all participants were obtained, including gender, ages, hospital practice types, and regions. Clinic information was collected, including specialist perceptions of mpMRI and mpUS in the diagnosis of PCa, routine practice of prostate systematic biopsy and targeted biopsy, waiting time for appointment of these examinations, as well as their advantages and limitations.
After forming the initial questionnaire, three representative specialists (one urologist, one radiologist, and one ultrasonographer) with 8–10 years of experience in their primary specialty qualitatively reviewed and discussed the pilot survey to reach consensus. Next, a single e-mail included a cover letter and pilot survey was delivered to a random sample of 40 urologists, 40 radiologists, and 40 ultrasonographers for external validation. Lastly, the pilot questionnaire was finalized upon reviewing the responses and comments from the electronic survey.
Statistical analyses were performed using SPSS 26.0. Categorical variables are shown as numbers and percentages and analyzed using Pearson Chi-squared test. Univariable and multivariable logistic regression models were performed to identify characteristic variables of participants associated with response outcomes of survey items. Significance differences were defined as P<0.05.
Results
A total of 354 responses were received from 144 hospitals. The characteristics of participants are shown in Table 1. Specialties of participants were 29.7% in urologist, 34.2% in radiologist and 36.1% in sonographer; 53.9% of practice types were in academic hospitals. Except for gender, no significant differences were found in characteristics of participants. The distribution of questionnaire surveys across provinces throughout China are shown in Figure 1. The majority of them were located in western (41.8%) and eastern (46.0%) parts of China.
Table 1
| Variables | Urologists (n=105) | Radiologist (n=121) | Ultrasonographer (n=128) | P value |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Male | 98 (93.3) | 91 (75.2) | 47 (36.7) | |
| Female | 7 (6.7) | 30 (24.8) | 81 (63.3) | |
| Age (years) | 0.336 | |||
| 25–40 | 60 (57.1) | 65 (53.7) | 61 (47.7) | |
| >40 | 45 (42.9) | 56 (46.3) | 67 (52.3) | |
| Practice type | 0.948† | |||
| Academic | 58 (55.2) | 65 (53.7) | 68 (53.1) | |
| Community | 43 (41.0) | 50 (41.3) | 51 (39.8) | |
| Private | 4 (3.8) | 6 (5.0) | 9 (7.0) | |
| Region | 0.840 | |||
| Central-west | 59 (56.2) | 65 (53.7) | 67 (52.3) | |
| East | 46 (43.8) | 56 (46.3) | 61 (47.7) |
Data are presented as n (%). †, 2×3 Pearson Chi-squared test was performed between academic and community-private in row.
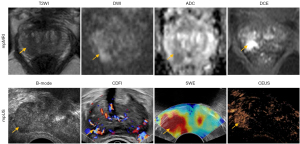
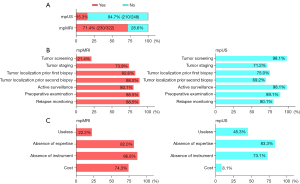
The overviews of participants’ responses on the investigation questionnaire are shown in Table S1. Representative images of mpUS and mpMRI for PCa diagnosis are shown in Figure 2. Two hundred and thirty out of 322 (71.4%) participants used mpMRI to diagnose PCa, and 15.3% (38/248) of the participants used mpUS to diagnose PCa (Figure 3A). Regarding the purpose of using mpMRI and mpUS to examine the prostate, both were widely used for tumor staging, biopsy localization, active surveillance, and detection before and after treatment (Figure 3B). The use of mpUS was a preferable option in tumor screening. The deficiency of instruments and related expertise was the main cause for not conducting mpMRI and mpUS (Figure 3C). The secondary causes were cost for mpMRI and perceived useless for mpUS, respectively.
A large proportion of participants supposed that systematic biopsy (264/354, 74.6%) was preferable in the clinic practice with transperineal ultrasound guidance (167/354, 47.2%), as shown in Figure 4A. Cognitive fusion imaging (252/354, 71.2%) and MRI-US fusion imaging (220/354, 62.1%) were suggested as the primary modalities for targeted biopsy (Figure 4B). The majority of participants believed that MRI targeted biopsy is recommended (274/354, 77.4%) and is preferable to systematic biopsy (218/354, 61.6%) for the detection of prostate lesions (Figure 4C). Meanwhile, the equilibrium results were found in the mpUS targeted biopsy (Figure 4C). Simultaneously, the deficiency of instruments and related expertise was the main cause for not using MRI and mpUS-targeted biopsy, respectively (Figure 4D).
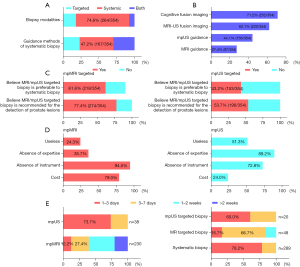
A large proportion of appointment was executed in less than 3 days, including 73.7% of mpUS examination, 78.2% of systematic biopsy, and 60.0% of mpUS-targeted biopsy (Figure 4E). However, a long waiting period for mpMRI and MRI-targeted biopsy was found, which was 39.6% and 83.4% in a week, respectively.
After using multivariable logistic regression analysis (Table 2), specialists had comparable consistent perceptions on PCa diagnosis and biopsy (all P>0.05). Clinic practice in the east region of China was more inclined to the utilization of mpMRI imaging [odds ratio (OR) =0.19; P<0.001] and recommendation of MRI-targeted biopsy (OR =0.15; P<0.001) for PCa detection. Higher ORs in agreeing mpMRI (OR =2.08; P=0.01) and MRI-targeted biopsy (OR =1.50; P=0.16) were obtained in participants from academic hospitals, compared to community and private practices. Furthermore, both practice in east region of China (OR =0.33; P=0.01) and participants from academic hospitals (OR =0.04; P<0.001) were independently associated with utilizing mpUS for PCa diagnosis. Conversely, no independent factors were found in univariable analysis regarding recommendation of mpUS-targeted prostate biopsy.
Table 2
| Covariates | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Do you use mpMRI for PCa diagnosis? (yes or no) | |||||
| Gender (male vs. female) | 0.80 (0.46–1.39) | 0.43 | – | – | |
| Age (≤40 vs. >40 years) | 1.07 (0.66–1.75) | 0.77 | – | – | |
| Specialty (urologist vs. radiologist vs. sonographer) | |||||
| Urologist | 1.56 (0.83–2.93) | 0.16 | – | – | |
| Radiologist | 1.22 (0.70–2.14) | 0.48 | – | – | |
| Sonographer | 1 | – | – | – | |
| Hospital (academic vs. community & private) | 1.78 (1.09–2.90) | 0.02 | 2.08 (1.23–3.53) | 0.01 | |
| Region (east vs. central-west) | 0.20 (0.12–0.34) | <0.001 | 0.19 (0.11–0.32) | <0.001 | |
| Believe MRI targeted biopsy is recommended for the detection of prostate lesions (yes or no) | |||||
| Gender (male vs. female) | 3.08 (1.84–5.15) | 0.00 | 1.64 (0.92–2.92) | 0.09 | |
| Age (≤40 vs. >40 years) | 1.14 (0.69–1.88) | 0.65 | – | – | |
| Specialty (urologist vs. radiologist vs. sonographer) | |||||
| Urologist | 1.36 (0.74–2.49) | 0.32 | – | – | |
| Radiologist | 0.97 (0.53–1.80) | 0.93 | – | – | |
| Sonographer | 1 | – | – | – | |
| Hospital (academic vs. community & private) | 1.79 (1.08–2.96) | 0.02 | 1.50 (0.85–2.63) | 0.16 | |
| Region (east vs. central-west) | 0.13 (0.06–0.25) | <0.001 | 0.15 (0.07–0.30) | <0.001 | |
| Do you use mpUS for PCa diagnosis? (yes or no) | |||||
| Gender (male vs. female) | – | 0.99 | – | – | |
| Age (≤40 vs. >40 years) | 0.59 (0.29–1.19) | 0.14 | – | – | |
| Specialty (urologist vs. radiologist vs. sonographer) | |||||
| Urologist | 0.65 (0.26–1.65) | 0.36 | – | – | |
| Radiologist | 1.35 (0.61–3.01) | 0.46 | – | – | |
| Sonographer | 1 | – | – | – | |
| Hospital (academic vs. community & private) | 0.05 (0.02–0.12) | <0.001 | 0.04 (0.02–0.11) | <0.001 | |
| Region (east vs. central-west) | 0.45 (0.22–0.92) | 0.29 | 0.33 (0.14–0.79) | 0.01 | |
| Believe mpUS targeted biopsy is recommended for the detection of prostate lesions (yes or no) | |||||
| Gender (male vs. female) | 0.65 (0.41–1.03) | 0.07 | – | – | |
| Age (≤40 vs. >40 years) | 0.80 (0.51–1.24) | 0.32 | – | – | |
| Specialty (urologist vs. radiologist vs. sonographer) | |||||
| Urologist | 0.80 (0.48–1.35) | 0.41 | – | – | |
| Radiologist | 0.89 (0.54–1.46) | 0.64 | – | – | |
| Sonographer | 1 | – | – | – | |
| Hospital (academic vs. community & private) | 0.78 (0.50–1.22) | 0.28 | – | – | |
| Region (east vs. central-west) | 0.81 (0.52–1.27) | 0.35 | – | – | |
CI, confidence interval; MRI, magnetic resonance imaging; mpMRI, multiparametric magnetic resonance imaging; mpUS, multiparametric ultrasound; OR, odds ratio; PCa, prostate cancer.
Discussion
In this survey, a positive perception (73.9–88.5%) of prostatic mpMRI are detected for tumor staging, biopsy localization, active surveillance, and detection before and after treatment, which aligns with the recommendations of guidelines from American College of Radiology (ACR) (22), United Kingdom National Institute for Health and Care Excellence (NICE) at NICE web, and European Association of Urology (EAU)-European Association of Nuclear Medicine (EANM)-European Society for Radiotherapy and Oncology (ESTRO)-European Society of Urogenital Radiology (ESUR)-International Society of Geriatric Oncology (SIOG) (3). However, a large proportion (84.7%) of specialists do not use of mpUS, although a broad range of usage purposes of mpUS are available, especially for tumor screening (98.1%), according to the perceived attitudes from those who used mpUS in the practice. Several studies (23,24), predominantly in the Europe and North American, have reported extensive utilization (85.7–95.9%) of prostatic mpMRI in their routine practice, which is higher than the percentage (71.4%) we reported in China. Compared to mpMRI, mpUS proposed subsequently has been considered useful in the detection of PCa in practice, such as contrast-enhanced ultrasound and elastography (25-27). The diagnostic efficacy of mpUS in detecting localized PCa appears to be on par with that of mpMRI. This is evidenced by comparable sensitivity rates (97.4% for mpUS versus 94.7% for mpMRI), negative predictive values (96.9% for mpUS versus 92.3% for mpMRI), and overall accuracy (87.2% for mpUS versus 76.9% for mpMRI) (13). Furthermore, mpUS has been associated with a lower cost-effectiveness profile compared to mpMRI in clinical practice (28). However, few national surveys have been published on perceptions of the use of prostatic mpUS in clinical practice. Our study is the first to report the practice patterns of prostatic mpUS in China. Nevertheless, more investigations are needed to explore the role of mpUS for PCa diagnosis.
In the current landscape of diagnostic imaging, prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) has demonstrated a good accuracy (92%) for diagnosis and staging of high-risk PCa (29). Besides that, micro-ultrasound (micro-US), particularly when employed with a five-point scoring system known as prostate risk identification using micro-ultrasound (PRI-MUS), has been posited as an alternative to mpMRI for the detection of csPCa (30-32). Although this modality is not included in the scope of our survey, the advantages of micro-US are manifold: firstly, it serves to exclude the presence of csPCa in patients who exhibit persistent clinical suspicion despite negative mpMRI findings (33); secondly, a combined targeted biopsy approach utilizing both mpMRI and micro-US guidance has been shown to surpass systematic biopsy in terms of PCa and csPCa detection efficacy, while simultaneously reducing the incidence of unnecessary biopsies (34). Conversely, micro-US is not without its limitations: it may be constrained by large prostate volumes and the specific localization of tumors, such as those in the anterior or transitional zones (35). Additionally, when juxtaposed with mpMRI or mpUS, multifunctional imaging techniques (e.g., contrast-enhanced imaging, diffusion-weighted imaging, and elastography) are deficient for the discrimination of malignant lesions from benign anomalies (36). Given the dearth of comparative studies between micro-US and mpMRI/mpUS in the existing literature, there is a pressing need for more prospective, blinded studies to ascertain the role of micro-US and mpUS in the diagnostic algorithm for PCa detection.
The study exhibits a high recognition of the application of systematic biopsy (74.6%) with a transperineal approach and MRI-targeted biopsy with cognitive (71.2%) or US fusion (62.1%) approach in China, which accords with the international guideline-recommended transperineal approach to reduce the risk of sepsis and improve the detection rate for csPCa located in the anterior zone of gland (3,37). However, the majority (61.6–77.4%) of specialists propose that MRI-targeted biopsy is preferable to systematic biopsy for the recommendation of PCa detection, indicating a beneficial perspective on the technique over time. These results are consistent with those previous reported by Bukavina et al. and Saar et al. (23,38). In comparison, no obvious trend of mpUS-targeted biopsy is observed in the study, which might be attributed to merely a few proportions of participants using prostatic mpUS as a result of the deficiency of specialized instruments and related expertise in clinical practice (39,40). Pepe et al. have reported that mpUS did not enhance the accuracy of cognitive fusion biopsy (41). In contrast, other studies have validated the utility of this approach, demonstrating its efficacy in improving lesion visibility and facilitating the localization of csPCa (12,42,43). In addition, our study discloses a highly efficient performance on both mpUS examination and mpUS-targeted biopsy, in comparison with the long period of MRI appointment. Considering some aspects including low costs, real-time imaging, reliable applicability for patients with MRI contraindications are distinct merits of mpUS, this technique might exhibit good perspective in the future, but more randomized controlled trials directly comparing mpUS and mpMRI are warranted.
Regarding the primary impediments to the utilization of mpMRI and mpUS in the detection of PCa and targeted biopsy procedures, which include financial constraints and a dearth of expertise, potential remedial measures could encompass the development of specialized training programs, governmental financial incentives for the equipment procurement, and the fostering of public-private partnerships aimed at enhancing the accessibility of these resources. Furthermore, to augment the availability of advanced imaging modalities, such as mpUS and mpMRI, in regions with limited access to healthcare, it is imperative to propose actionable recommendations for policymakers. These recommendations should encompass strategies like the integration of telemedicine services and the deployment of mobile diagnostic units to bridge the gap in healthcare delivery.
Several factors, including clinic practice in east region of China and from academic hospitals, show significant impacts on the use of mpMRI and mpUS as well as MRI-targeted biopsy for PCa diagnosis. Not only does clinic practice in east region of China tend to use mpMRI and mpUS, but such a setting is also predictive for an increased recommendation of MRI-targeted biopsy. In the meantime, greater use of mpMRI and mpUS as well as MRI-targeted biopsy is also confirmed from specialists in academic hospitals than those in communities and private organizations. This might contribute to greater availability and accessibility of high medical standards in economically prosperous regions (e.g., eastern region) and academically medical practices (e.g., affiliated hospital of university), demonstrating the technical interest and academic influence of participants from such areas. These findings are certainly supported to some degree by other studies, in which the use of prostatic mpMRI and MRI-targeted biopsy has been concentrated at academic medical centers and urban locations (38,44,45).
Limitations should be mentioned in the study. Firstly, based on the domestic scenarios of clinical practice, the electronic survey design has not been used in prior studies. However, the questionnaire was tested by a qualitative review and external validation to improve the reliability and validity of the findings. Secondly, since the electronic questionnaire was addressed online, a component of bias existed in specialists who might appeal to the technological topic of the survey. To reduce the reporting bias, we sent the questionnaire to more relevant specialists (urologists, radiologists, and sonographers) across various Chinese medical associations, in comparison with a solo organization opted in the previous reports (23,46,47). Thirdly, a small sample size with a relative low response rate of 23.6% may limit the generalizability of the study. To decrease sample bias, the specialist samples were randomized from the Chinese medical associations, and a slightly higher response rate was obtained compared to other publications (with most surveys having response rates <10%) (47,48). In addition, the study primarily focused on the mpMRI and mpUS in China, with limited comparisons to international practices and insufficient details on non-participants. Therefore, the generalization of these results is limited to the overall prostate diagnosis represented by these communities in China. Specifically, the cross-sectional survey results merely reflect the present overview. Longitudinal data should be studied to provide insights into trends and changes in the use of mpMRI and mpUS over time. Despite these limitations, this study provides the first perceptions of Chinese specialists on the practice patterns of prostatic mpMRI and mpUS as well as biopsies. It would be useful to recognize the status in quo of PCa detection in China and observe the evolvement of these perceptions and practice patterns over time.
Conclusions
This survey indicates that mpMRI and transperineal ultrasound-guided systematic biopsy are relatively commonly used for PCa diagnosis in clinical practice in China. Meanwhile, MRI-targeted biopsy is also recommended by most participants. However, prostatic mpUS is used only by a fraction of participants. Except for mpUS targeted biopsy, these techniques are most commonly used in practice settings in east region of China and academic hospitals.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CROSS reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1834/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1834/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of the Sichuan Cancer Hospital and Institute (No. SCCHEC-02-2023-155) and individual consent for this study was waived due to the retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moses KA, Sprenkle PC, Bahler C, Box G, Carlsson SV, Catalona WJ, et al. NCCN Guidelines® Insights: Prostate Cancer Early Detection, Version 1.2023. J Natl Compr Canc Netw 2023;21:236-46. [Crossref] [PubMed]
- Eastham JA, Auffenberg GB, Barocas DA, Chou R, Crispino T, Davis JW, Eggener S, Horwitz EM, Kane CJ, Kirkby E, Lin DW, McBride SM, Morgans AK, Pierorazio PM, Rodrigues G, Wong WW, Boorjian SA. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J Urol 2022;208:10-8. [Crossref] [PubMed]
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2024;86:148-63. [Crossref] [PubMed]
- Eklund M, Jäderling F, Discacciati A, Bergman M, Annerstedt M, Aly M, Glaessgen A, Carlsson S, Grönberg H, Nordström TSTHLM3 consortium. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N Engl J Med 2021;385:908-20. [Crossref] [PubMed]
- Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, Bloom J, Gurram S, Siddiqui M, Pinsky P, Parnes H, Linehan WM, Merino M, Choyke PL, Shih JH, Turkbey B, Wood BJ, Pinto PA. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 2020;382:917-28. [Crossref] [PubMed]
- Hugosson J, Månsson M, Wallström J, Axcrona U, Carlsson SV, Egevad L, Geterud K, Khatami A, Kohestani K, Pihl CG, Socratous A, Stranne J, Godtman RA, Hellström MGÖTEBORG-2 Trial Investigators. Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only. N Engl J Med 2022;387:2126-37. [Crossref] [PubMed]
- Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, Weidner W, Loeb S. Complications After Systematic, Random, and Image-guided Prostate Biopsy. Eur Urol 2017;71:353-65. [Crossref] [PubMed]
- Gershman B, Van Houten HK, Herrin J, Moreira DM, Kim SP, Shah ND, Karnes RJ. Impact of Prostate-specific Antigen (PSA) Screening Trials and Revised PSA Screening Guidelines on Rates of Prostate Biopsy and Postbiopsy Complications. Eur Urol 2017;71:55-65. [Crossref] [PubMed]
- Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, Decaussin-Petrucci M, Dubreuil-Chambardel M, Magaud L, Remontet L, Ruffion A, Colombel M, Crouzet S, Schott AM, Lemaitre L, Rabilloud M, Grenier N. MRI-FIRST Investigators. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019;20:100-9. [Crossref] [PubMed]
- Birosh A, Salinas-Miranda E, Breau RH, McInnes MDF, Morash C, Schieda N. Multiparametric Versus Biparametric Prostate MRI: Comparison of NPV for Clinically Significant Prostate Cancer. AJR Am J Roentgenol 2024;222:e2330496. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton MPROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Mannaerts CK, Wildeboer RR, Remmers S, van Kollenburg RAA, Kajtazovic A, Hagemann J, Postema AW, van Sloun RJG. J Roobol M, Tilki D, Mischi M, Wijkstra H, Salomon G. Multiparametric Ultrasound for Prostate Cancer Detection and Localization: Correlation of B-mode, Shear Wave Elastography and Contrast Enhanced Ultrasound with Radical Prostatectomy Specimens. J Urol 2019;202:1166-73. [Crossref] [PubMed]
- Zhang M, Tang J, Luo Y, Wang Y, Wu M, Memmott B, Gao J. Diagnostic Performance of Multiparametric Transrectal Ultrasound in Localized Prostate Cancer: A Comparative Study With Magnetic Resonance Imaging. J Ultrasound Med 2019;38:1823-30. [Crossref] [PubMed]
- Grey ADR, Scott R, Shah B, Acher P, Liyanage S, Pavlou M, et al. Multiparametric ultrasound versus multiparametric MRI to diagnose prostate cancer (CADMUS): a prospective, multicentre, paired-cohort, confirmatory study. Lancet Oncol 2022;23:428-38. [Crossref] [PubMed]
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 2018;378:1767-77. [Crossref] [PubMed]
- Burk KS, Naik S, Lacson R, Tuncali K, Lee LK, Tempany C, Cole AP, Trinh QD, Kibel AS, Khorasani R. MRI-Targeted, Systematic, or Combined Biopsy for Detecting Clinically Significant Prostate Cancer. J Am Coll Radiol 2023;20:687-95. [Crossref] [PubMed]
- Okabe Y, Patel HD, Rac G, Gupta GN. Multifocality of Prostate Cancer and Candidacy for Focal Therapy Based on Magnetic Resonance Imaging. Urology 2022;169:141-9. [Crossref] [PubMed]
- Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, Zheng Y, Ye D. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol 2021;18:282-301. [Crossref] [PubMed]
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020;77:38-52. [Crossref] [PubMed]
- Padhani AR, Barentsz J, Villeirs G, Rosenkrantz AB, Margolis DJ, Turkbey B, Thoeny HC, Cornud F, Haider MA, Macura KJ, Tempany CM, Verma S, Weinreb JCPI-RADS Steering Committee. The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway. Radiology 2019;292:464-74. [Crossref] [PubMed]
- Saar M, Linxweiler J, Borkowetz A, Fussek S, Urbanova K, Bellut L, Kristiansen G, Wullich BGerman Prostate Cancer Consortium (DPKK). Current Role of Multiparametric MRI and MRI Targeted Biopsies for Prostate Cancer Diagnosis in Germany: A Nationwide Survey. Urol Int 2020;104:731-40. [Crossref] [PubMed]
- Muthigi A, Sidana A, George AK, Kongnyuy M, Maruf M, Valayil S, Wood BJ, Pinto PA. Current beliefs and practice patterns among urologists regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol 2017;35:32.e1-7. [Crossref] [PubMed]
- Barr RG, Cosgrove D, Brock M, Cantisani V, Correas JM, Postema AW, Salomon G, Tsutsumi M, Xu HX, Dietrich CF. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 5. Prostate. Ultrasound Med Biol 2017;43:27-48. [Crossref] [PubMed]
- Sparchez Z. Contrast enhanced ultrasound of the prostate. New role in the evaluation of loco-regional therapy of prostate tumors. Med Ultrason 2018;20:125-6. [Crossref] [PubMed]
- Wildeboer RR, Postema AW, Demi L, Kuenen MPJ, Wijkstra H, Mischi M. Multiparametric dynamic contrast-enhanced ultrasound imaging of prostate cancer. Eur Radiol 2017;27:3226-34. [Crossref] [PubMed]
- Shiva M, Wei C, Molana H, Nabi G. Cost-Effectiveness of Prostate Cancer Detection in Biopsy-Naïve Men: Ultrasound Shear Wave Elastography vs. Multiparametric Diagnostic Magnetic Resonance Imaging. Healthcare (Basel) 2022;10:254. [Crossref] [PubMed]
- Pepe P, Pennisi M. Targeted Biopsy in Men High Risk for Prostate Cancer: (68)Ga-PSMA PET/CT Versus mpMRI. Clin Genitourin Cancer 2023;21:639-42. [Crossref] [PubMed]
- Rodríguez Socarrás ME, Gomez Rivas J, Cuadros Rivera V, Reinoso Elbers J, Llanes González L, Michel Mercado I, Fernandez Del Alamo J, Juarez Del Dago P, Sancha FG. Prostate Mapping for Cancer Diagnosis: The Madrid Protocol. Transperineal Prostate Biopsies Using Multiparametric Magnetic Resonance Imaging Fusion and Micro-Ultrasound Guided Biopsies. J Urol 2020;204:726-33. [Crossref] [PubMed]
- Ghai S, Perlis N, Atallah C, Jokhu S, Corr K, Lajkosz K, Incze PF, Zlotta AR, Jain U, Fleming H, Finelli A, van der Kwast TH, Haider MA. Comparison of Micro-US and Multiparametric MRI for Prostate Cancer Detection in Biopsy-Naive Men. Radiology 2022;305:390-8. [Crossref] [PubMed]
- Ghai S, Eure G, Fradet V, Hyndman ME, McGrath T, Wodlinger B, Pavlovich CP. Assessing Cancer Risk on Novel 29 MHz Micro-Ultrasound Images of the Prostate: Creation of the Micro-Ultrasound Protocol for Prostate Risk Identification. J Urol 2016;196:562-9. [Crossref] [PubMed]
- Avolio PP, Lughezzani G, Fasulo V, Maffei D, Sanchez-Salas R, Paciotti M, Saitta C, De Carne F, Saita A, Hurle R, Lazzeri M, Guazzoni G, Buffi NM, Casale P. Assessing the Role of High-resolution Microultrasound Among Naïve Patients with Negative Multiparametric Magnetic Resonance Imaging and a Persistently High Suspicion of Prostate Cancer. Eur Urol Open Sci 2023;47:73-9. [Crossref] [PubMed]
- Avolio PP, Lughezzani G, Anidjar M, Hassan T, Rompré-Brodeur A, Buffi NM, Lazzeri M, Sanchez-Salas R. The diagnostic accuracy of micro-ultrasound for prostate cancer diagnosis: a review. World J Urol 2023;41:3267-76. [Crossref] [PubMed]
- Avolio PP, Lazzeri M, Maffei D, Fasulo V, Frego N, Saitta C, de Carne F, Paciotti M, Saita A, Hurle R, Guazzoni G, Casale P, Buffi NM, Lughezzani G. Is multiparametric MRI always needed in biopsy-naïve patients with abnormal digital rectal examination? A single-institutional experience combining clinical and micro-ultrasonography-based factors to optimize prostate cancer detection. World J Urol 2024;42:9. [Crossref] [PubMed]
- Correas JM, Halpern EJ, Barr RG, Ghai S, Walz J, Bodard S, Dariane C, de la Rosette J. Advanced ultrasound in the diagnosis of prostate cancer. World J Urol 2021;39:661-76. [Crossref] [PubMed]
- Pepe P, Pennisi M. Morbidity following transperineal prostate biopsy: Our experience in 8.500 men. Arch Ital Urol Androl 2022;94:155-9. [Crossref] [PubMed]
- Bukavina L, Tilburt JC, Konety B, Shah ND, Gross CP, Yu JB, Schumacher F, Kutikov A, Smaldone MC, Kim SP. Perceptions of Prostate MRI and Fusion Biopsy of Radiation Oncologists and Urologists for Patients Diagnosed with Prostate Cancer: Results from a National Survey. Eur Urol Focus 2020;6:273-9. [Crossref] [PubMed]
- Grey A, Ahmed HU. Multiparametric ultrasound in the diagnosis of prostate cancer. Curr Opin Urol 2016;26:114-9. [Crossref] [PubMed]
- Ditonno F, Franco A, Manfredi C, Veccia A, Valerio M, Bukavina L, Zukowski LB, Vourganti S, Stenzl A, Andriole GL, Antonelli A, De Nunzio C, Autorino R. Novel non-MRI imaging techniques for primary diagnosis of prostate cancer: micro-ultrasound, contrast-enhanced ultrasound, elastography, multiparametric ultrasound, and PSMA PET/CT. Prostate Cancer Prostatic Dis 2024;27:29-36. [Crossref] [PubMed]
- Pepe P, Pepe L, Panella P, Pennisi M. Can multiparametric ultrasound improve cognitive MRI/TRUS fusion prostate biopsy. Arch Ital Urol Androl 2020;92: [Crossref] [PubMed]
- Morris DC, Chan DY, Lye TH, Chen H, Palmeri ML, Polascik TJ, Foo WC, Huang J, Mamou J, Nightingale KR. Multiparametric Ultrasound for Targeting Prostate Cancer: Combining ARFI, SWEI, QUS and B-Mode. Ultrasound Med Biol 2020;46:3426-39. [Crossref] [PubMed]
- Fulgham PF. Multiparametric ultrasound-targeted biopsy compares favorably to multiparametric MRI-transrectal ultrasound fusion-targeted biopsy on initial biopsy of men at risk for prostate cancer. World J Urol 2018;36:713-8. [Crossref] [PubMed]
- Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, Taneja SS, Thoeny H, Villeirs G, Villers A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol 2015;68:1045-53. [Crossref] [PubMed]
- Liu W, Patil D, Howard DH, Moore RH, Wang H, Sanda MG, Filson CP. Adoption of Prebiopsy Magnetic Resonance Imaging for Men Undergoing Prostate Biopsy in the United States. Urology 2018;117:57-63. [Crossref] [PubMed]
- Davies C, Castle JT, Stalbow K, Haslam PJ. Prostate mpMRI in the UK: the state of the nation. Clin Radiol 2019;74:894.e11-8. [Crossref] [PubMed]
- Stanzione A, Creta M, Imbriaco M, La Rocca R, Capece M, Esposito F, Imbimbo C, Fusco F, Celentano G, Napolitano L, Mangiapia F, Mirone V, Longo N. Attitudes and perceptions towards multiparametric magnetic resonance imaging of the prostate: A national survey among Italian urologists. Arch Ital Urol Androl 2020;92: [Crossref] [PubMed]
- Manley BJ, Brockman JA, Raup VT, Fowler KJ, Andriole GL. Prostate MRI: a national survey of Urologist’s attitudes and perceptions. Int Braz J Urol 2016;42:464-71. [Crossref] [PubMed]



