
Clinical diagnostic accuracy and non-inferiority of digital breast tomosynthesis by synthetic 2D mammography compared with full-field digital mammography for the diagnosis of breast cancer: a single-center retrospective study
Introduction
Digital mammography, also called full-field digital mammography (FFDM), has been the most widely available imaging modality for breast cancer screening. A primary limitation of FFDM is the phenomenon of summation shadowing, which has the potential to obscure certain breast lesions. Digital breast tomosynthesis (DBT) represents a quasi-three-dimensional advancement in mammography techniques. It enhances lesion detection by mitigating the masking effect caused by the overlapping fibroglandular breast tissue. Numerous studies have shown that the combination of DBT and FFDM exhibits superior performance compared to FFDM alone in both screening and diagnostic settings, contributing to the early detection of breast cancer and enhancing the diagnostic capabilities for this disease (1-6). However, using a combination of FFDM and DBT can result in an increased radiation dose, ranging from 1.5 to 2.2 times compared to the use of FFDM alone (7,8). Synthetic two-dimensional (2D) mammography (SM), which is reconstructed from DBT data, has been developed as a potential replacement for FFDM in conjunction with DBT (9). Recently numerous studies support the utilization of SM plus DBT, highlighting its comparable performance to that of FFDM plus DBT (8,10-13). However, it is important to note that certain studies have reported inconsistent results, where SM plus DBT demonstrated lower sensitivity compared to FFDM plus DBT (11,14-16).
Various DBT systems exhibit distinct technical specifications, yielding variations in DBT and SM images. Most of the published data on SM were conducted using a few DBT systems, namely C-view (Hologic, Marlborough, MA, USA) and Insight 2D (Siemens, Erlangen, Germany). Data is scarce regarding the performance of SM from other commercial DBT systems, such as S-view from Fujifilm (Tokyo, Japan), or V-view from GE Healthcare (Chicago, IL, USA).
The aim of this study was to assess diagnostic accuracy and non-inferiority of the S-view SM (Fujifilm) in combination with DBT compared with that of FFDM plus DBT in the detection of calcified and non-calcified breast cancers in a tertiary-care hospital. Additionally, we aimed to evaluate inter-observer agreement in the visibility score of suspicious calcifications on S-view SM. We present this article in concordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2498/rc).
Methods
The retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University (No. RAD-2564-08018) with a waiver of the requirement for written informed consent.
Subject
This was a retrospective observational cross-sectional study involving two certified radiologists specializing in breast imaging as observers. All patients who underwent FFDM with DBT and reconstructed S-view SM using Amulet Innovality, Fujifilm, at Maharaj Nakorn Chiang Mai Hospital, a university-affiliated tertiary care center during July 1, 2019, to June 30, 2021, were included.
A total of 177 breast cancers in 175 patients were included in the cancer cohort. Two patients had synchronous contralateral breast cancers in this group. These patients presented with various breast symptoms, such as palpable masses, breast pain, and nipple discharge, or were asymptomatic but had biopsy-confirmed ductal carcinoma in situ (DCIS) or invasive mammary carcinoma, including both ductal and lobular subtypes.
Cases with negative or benign findings [Breast Imaging Reporting and Data System (BI-RADS) 1 or 2] assessment or BI-RADS 3 with at least 365-day duration of stable mammographic and clinical follow-up were included in the negative cohort. Cases with BI-RADS 4 or 5 findings who were confirmed negative for malignancy through core needle biopsy and/or excisional biopsy were also included in the negative cohort. Three hundred and ten patients were included in the negative cohort group.
The exclusion criteria comprised patients with known breast cancer undergoing neoadjuvant chemotherapy, those with breast implantation or reconstruction, and individuals with incomplete clinical records (Figure 1).

Imaging
The electronic medical records, mammography reporting system, and Picture Archiving and Communication System (PACS) were used to access pathology reports, mammographic reports, and mammographic images. All patients underwent imaging with Amulet Innovality (Fujifilm), and the S-view SM was generated using software version 9.2.0001.
All patients underwent mammography with DBT and FFDM. Images were anonymized and uploaded to workstations (Bellus, Fujifilm). When previous studies were available, a 1-year prior mammogram was used for comparison. Forty-two patients (24%, 42/175) in the cancer cohort had no prior mammogram for comparison.
The mammographic images were assessed by two observers who had board-certified with fellowship training in breast imaging at an academic medical center. The two observers interpreted the images independently. Reader 1 and reader 2 had experience of reading DBT plus S-view SM for 5.5 and 2.5 years, respectively. Readers were blinded to clinical findings, pathologic findings, and the interpretations of the fellow observer. The DBT plus FFDM and DBT plus S-view SM image datasets were independently randomized. Reader 1 read DBT plus FFDM, whereas reader 2 read DBT plus S-view SM. There were 4-week washout periods for diminishing recall bias. Subsequently, reader 1 interpreted DBT plus S-view SM, and reader 2 interpreted DBT plus FFDM. Computer-aided detection software was not used due to the lack of availability at Maharaj Nakorn Chiang Mai Hospital.
Each reader interpreted mammographic images according to the BI-RADS 2013, 5th edition (17), and designated images under the BI-RADS category. In cases designated as BI-RADS 0, the reader indicated the location and the percentage likelihood of malignancy of the most suspicious finding.
Definition and criteria
The reference standard for a true-positive study was patients with a biopsy-proven malignancy (DCIS and invasive mammary carcinoma, including ductal and lobular origins). The reference standard for a true-negative study was a patient with BI-RADS 1 or 2, BI-RADS 3 assessments with at least 12-month stable clinical and radiologic follow-up, or BI-RADS 4–5 who were negative for malignancy after excisional biopsy and/or core needle biopsy. Test positivity cut-offs for index tests are classified into BI-RADS 4 or 5.
Statistical tests
A final BI-RADS designation was obtained from the independent readers’ responses, according to BI-RADS 2013, fifth edition, guidelines. This final BI-RADS assessment was used to evaluate and calculate sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio for positive malignancy (LR+) of DBT plus S-view SM vs. DBT plus FFDM in the detection of breast cancer. The difference in specificity and sensitivity between the two mammographic techniques was evaluated using McNemar’s test for paired proportions.
A receiver operating characteristic (ROC) curve analysis, using the likelihood of malignancy scale (0–100%, grouped by decile increments), was used to estimate the area under the ROC curve (AUC) and a corresponding 95% confidence interval (CI) for the likelihood of malignancy rate in all readers. The ROC analysis was performed individually for each reader and subsequently averaged between the two readers. The comparison in ROC between DBT plus S-view SM and DBT plus FFDM was calculated by the DeLong test.
We calculated the non-inferiority of sensitivity, specificity, and AUC using a one-sided CI. Delta was set at 5%, establishing the non-inferiority margin of sensitivity, specificity, and AUC of FFDM plus DBT. The selection of delta for this objective is obtained not only by looking at past trials of the performance of SM plus DBT vs. FFDM plus DBT (18-21) but also ideas such as choosing the largest accepted percentage of the expected difference between SM plus DBT and FFDM plus DBT. Non-inferiority would be confirmed if the lower bound of the one-sided CI of S-View SM plus DBT did not fall below the predefined margin.
The Cohen’s kappa test was used to evaluate inter-observer agreement on three aspects. First, the final BI-RADS category designation between the two observers. Second, the classification of morphology and distribution of suspicious calcification in subgroups according to the BI-RADS guidelines. Third, the Likert score for the visibility grading (1 to 5) was used for abnormal mammographic findings, where an increased numerical score indicates greater visibility ranging from poor to excellent, compared to how it is recorded in FFDM. The degree of agreement was categorized as follows: k values of 0.00–0.20 indicated poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, excellent agreement.
Subgroup analysis of suspicious calcification in the detection of breast cancer was performed to determine the diagnostic accuracy measures for DBT plus S-view SM compared to DBT plus FFDM, including sensitivity, specificity, PPV, NPV, and LR+. Suspicious calcifications represent the predominant mammographic finding in DCIS presentations.
The percentage in all subtypes of suspicious calcification detection between FFDM and SM and the LR+ rate were assessed. Statistical analyses were performed with the STATA, version 16.0 (StataCorp, TX, USA). A P value <0.05 was considered statistically significant.
In this study, sample size is calculated based on sensitivity, which is referred to from previous studies (22,23).
n, required sample size; SN, anticipated sensitivity; α, size of the critical region (1 − α is the confidence level); z1−α/2, standard normal deviate corresponding to the specified size of the critical region (α); L, absolute precision desired on either side (half-width of the CI) of sensitivity.
Results
The age of the patients in the cancer cohort ranged from 31 to 90 years (mean, 59 years), and in the negative cohort it ranged from 32 to 80 years (mean, 47 years). A total of malignant lesions was 177. There were 11 cases of DCIS and 166 cases of invasive mammary carcinoma, including ductal and lobular origin. The most common mammographic feature of breast cancer was an isolated mass. Mammographic features of cancer lesions are shown in Table 1. Very few missing data were noted.
Table 1
| Clinical data and mammographic features | Breast cancers (n=177) | Benign lesions (n=310) | P value |
|---|---|---|---|
| Age (years) | 59.6 (11.3) | 47.3 (6.4) | <0.001 |
| First degree familial history of breast cancer | 3 (1.7) | 0 | <0.05 |
| Previous history of mastectomy | 9 (5.1) | 8 (2.6) | 0.20 |
| Previous history of BCS | 1 (0.6) | 1 (0.3) | >0.999 |
| Breast composition | >0.999 | ||
| Dense breast | 152 (85.9) | 267 (86.1) | |
| Non-dense breast | 25 (14.1) | 43 (13.9) | |
| Lesion size (mm) | 22.0 (13.5) | 26.0 (11.3) | 0.70 |
| Lateralization | <0.001 | ||
| Right | 83 (46.9) | 281 (90.6) | |
| Left | 94 (53.1) | 29 (9.4) | |
| Mass | <0.001 | ||
| Absence | 27 (15.2) | 268 (86.5) | |
| Presence | 150 (84.8) | 42 (13.5) | |
| Suspicious calcification | <0.001 | ||
| Absence | 97 (54.8) | 293 (94.5) | |
| Presence | 80 (45.2) | 17 (5.5) | |
| Asymmetry | 0.17 | ||
| Absence | 176 (99.4) | 302 (97.4) | |
| Presence | 1 (0.6) | 8 (2.6) | |
| Architectural distortion | <0.001 | ||
| Absence | 157 (88.7) | 304 (98.1) | |
| Presence | 20 (11.3) | 6 (1.9) | |
| Skin thickening | <0.001 | ||
| Absence | 152 (85.9) | 309 (99.7) | |
| Presence | 25 (14.1) | 1 (0.3) | |
| Nipple retraction | <0.001 | ||
| Absence | 165 (93.2) | 310 (100.0) | |
| Presence | 12 (6.8) | 0 | |
| Axillary node lymphadenopathy | <0.001 | ||
| Absence | 150 (84.8) | 310 (100.0) | |
| Presence | 27 (15.2) | 0 | |
Data are presented as mean (SD) or n (%). BCS, breast conservative surgery; SD, standard deviation.
Of the 177 malignant lesions confirmed by pathology, DCIS was identified in 6% (n=11), invasive ductal carcinoma in 82% (n=145), invasive lobular carcinoma in 7% (n=12), mucinous carcinoma in 2.3% (n=4), papillary carcinoma in 1.7% (n=3), metaplastic carcinoma in 0.5% (n=1), and tubular carcinoma in 0.5% (n=1).
The patients’ breast densities seen in the mammogram were evaluated visually by each reader. The excellent agreement on breast composition is indicated by the Kappa’s coefficient of 0.86 (95% CI: 0.86, 0.88). The breast composition of the cancer cohort showed 2% fatty, 13% scattered fibro-glandular tissue, 80% heterogeneously dense, and 5% extremely dense breast tissue. The negative cohort presented 2% fatty, 12% scattered fibro-glandular tissue, 80% heterogeneously dense, and 6% extremely dense breast tissue. Comparing the percentage of breast composition of sample patient cases in the cancer cohort and negative cohort groups revealed no significant difference between the two cohort groups (P value >0.999). Furthermore, the distribution of breast densities in the cancer cohort did not significantly differ from the baseline background of Maharaj Nakorn Chiang Mai Hospital’s female population, which comprised 3% fatty, 15% scattered fibro-glandular tissue, 73% heterogeneously dense, and 9% extremely dense breast tissue (P value =0.49). Presumably, most of the population of Thai women had dense breast tissue.
The classification of BI-RADS categories for malignant and benign breast lesions, along with the LR, was performed on all 487 breast lesions. BI-RADS 1 accounted for 37% (n=182), BI-RADS 2 for 12% (n=58), BI-RADS 3 for 5% (n=23), BI-RADS 4 for 21% (n=104), and BI-RADS 5 for 25% (n=120). The LR for BI-RADS 4 was 2.12 (95% CI: 1.51–2.98). The LR for BI-RADS 5 could not be calculated since none of the BI-RADS 5 lesions were proven to be benign.
The diagnostic accuracy of DBT plus S-view SM and DBT plus FFDM was assessed in terms of sensitivity and specificity. The sensitivity for DBT plus S-view SM was 98.9% (95% CI: 96.0–99.9%), and for DBT plus FFDM, it was 99.4% (95% CI: 96.9–100%) (P>0.999). The specificity of DBT plus S-view SM was 85.2% (95% CI: 80.7–88.9%), and for DBT plus FFDM, it was 84.8% (95% CI: 80.8–88.6%) (P>0.999). McNemar’s test showed no statistically significant differences in sensitivity and specificity between DBT plus S-view SM and DBT plus FFDM (Table 2).
Table 2
| Diagnostic indices | S-view SM + DBT | FFDM + DBT | P value |
|---|---|---|---|
| Sensitivity (95% CI), % | 98.9 (96.0–99.9) | 99.4 (96.9–100) | >0.999 |
| Specificity (95% CI), % | 85.2 (80.7–88.9) | 84.8 (80.0–88.6) | >0.999 |
| PPV (95% CI), % | 79.2 (73.2–84.3) | 78.9 (73.0–84.1) | – |
| NPV (95% CI), % | 99.2 (97.3–99.9) | 99.6 (97.9–100) | – |
| LR+ (95% CI) | 6.7 (5.1–8.7) | 6.6 (5.0–8.5) | – |
CI, confidence interval; DBT, digital breast tomosynthesis; FFDM, full-field digital mammography; LR+, likelihood ratio for positive malignancy; NPV, negative predictive value; PPV, positive predictive value; SM, synthetic two-dimensional mammography.
We compared the AUC of the DBT plus S-view SM with that of DBT plus FFDM for each reader, as well as the overall response of all readers. Both readers exhibited outstanding discrimination ability according to the receiving operating characteristic analysis and demonstrated excellent discrimination in overall responses. There was no significant difference between the AUC of DBT plus S-view SM and DBT plus FFDM between the two readers (P=0.40 and 0.16 for readers 1 and 2, respectively). Similarly, there was no significant difference in AUC averages (P=0.68) (Table 3).
Table 3
| Reader | AUC (95% CI) | P value | |
|---|---|---|---|
| S-view SM + DBT | FFDM + DBT | ||
| 1 | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | 0.40 |
| 2 | 0.96 (0.94–0.97) | 0.96 (0.95–0.97) | 0.16 |
| Overall | 0.90 (0.87–0.92) | 0.90 (0.88–0.93) | 0.68 |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; DBT, digital breast tomosynthesis; FFDM, full-field digital mammography; SM, synthetic two-dimensional mammography.
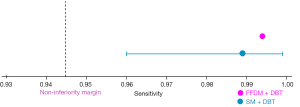
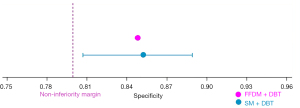
In terms of non-inferiority analysis, the non-inferiority of sensitivity for breast cancer diagnosis of S-view SM plus DBT compared to the FFDM plus DBT was confirmed, with an estimated sensitivity of 98.9% and a lower CI of 96.0% (Figure 2). Similarly, the non-inferiority analysis for specificity also demonstrated consistent results. The estimated specificity was 85.2% with a lower CI of 80.7% (Figure 3). In addition, the non-inferiority of the S-view SM plus DBT was further supported by Figure 4, which shows an estimated AUC of 0.90 with a lower CI of 0.87, confirming that the S-view SM plus DBT is not inferior to the FFDM plus DBT.

A subgroup analysis for suspicious calcification was performed to determine the diagnostic accuracy in the detection of breast cancer. The sensitivity of DBT plus S-view SM was 43.5%, and that of DBT plus FFDM was 44.6% (P=0.15). The specificity of DBT plus S-view SM was 95.2%, and that of DBT plus FFDM was 94.5% (P=0.15). McNemar’s test showed no statistically significant differences in sensitivity and specificity between DBT plus S-view SM and DBT plus FFDM for suspicious calcification in the detection of breast cancers.
Moreover, the classification of suspicious calcification in malignant and benign breast lesions and the LR were calculated. The LR for coarse heterogeneous calcification was 5.69 (95% CI: 1.88–17.20). The LR for amorphous calcification was 6.31 (95% CI: 2.38–16.70), and that of fine pleomorphic calcification was 10.30 (95% CI: 4.98–21.30). The LR for fine linear branching could not be calculated because no fine linear branching calcifications were proven to be benign.
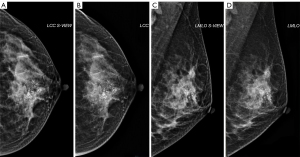
Three aspects of inter-observer agreement for the same case in each modality were evaluated using kappa statistics: the interrater agreement on the classification of the BI-RADS category, the interrater agreement on the assessment to classify the morphology and distribution of suspicious calcification, and the interrater agreement on the Likert score on S-view SM, which indicates the conspicuity of the lesion in comparison to FFDM, as shown in Table 4. According to the excellent interrater agreement on classification, the morphology, and the distribution of suspicious calcification, an example case of the patients with suspicious calcification was demonstrated in Figure 5, showing that the conspicuity of suspicious calcification of S-view SM is comparable to the FFDM.
Table 4
| Mammographic features | Cohen’s kappa (95% CI) |
|---|---|
| BI-RADS categorization | 0.98 (0.97–0.99) |
| Classification of suspicious calcification morphology | 0.96 (0.94–0.98) |
| Visibility score of suspicious calcifications | 0.59 (0.56–0.61) |
BI-RADS, Breast Imaging Reporting and Data System; CI, confidence interval.
Discussion
The early application of DBT necessitates the use of a combination of FFDM and DBT for evaluation. While the increase in diagnostic accuracy is evident, it comes at the cost of a higher radiation dose. Despite DBT’s efficacy in enhancing lesion detection, radiologists still require 2D images for the comprehensive evaluation of overall breast tissue. The ease of comparing images of the right and left breasts is notably more efficient on 2D mammography than on DBT. Additionally, the distribution of microcalcifications and asymmetry is more effectively discerned in the 2D image.
To address these considerations, SM is generated from DBT data. This approach allows for harnessing the diagnostic advantages of DBT, such as an increased cancer detection rate and a decreased recall rate, while simultaneously mitigating the need for additional radiation exposure associated with traditional FFDM. Our data suggests that there is no statistically significant difference in diagnostic accuracy (sensitivity, specificity, PPV, and NPV) of S-view SM plus DBT vs. FFDM plus DBT in both screening and diagnostic settings. In further non-inferiority analysis, the current study discovered that S-view SM plus DBT, is non-inferior to the FFDM plus DBT in terms of sensitivity, specificity, and AUC for breast cancer detection. According to a wide age range of samples. We also additionally analyzed the effect of ages on the sensitivity and specificity for breast cancer detection. We found that sensitivity is not changing regarding patients’ ages (P value =0.49). Meanwhile, specificity changes regarding ages (P value <0.001), where the younger the age, the more specificity increases.
These diagnostic accuracy results are in concordance with the findings from many recent studies performed on different DBT machines (8,10,11,22). A contrasting result was observed in a study by Gur et al. (11), where lower reader-average sensitivity was reported with SM plus DBT (77.2%) compared to FFDM plus DBT (82.6%). Nevertheless, the other findings in their study aligned with those of previous research. In our study, the distribution of breast densities in the cancer cohort did not differ significantly from that of Maharaj Nakorn Chiang Mai Hospital’s population during the study period. The dense breasts were found 85% in the cancer group and 82% in the institution’s population group, which supports the fact that Asian women tend to have denser breasts than Western women (24). Over the past few decades, both the annual incidence and mortality rate from breast cancer in Asia have increased more rapidly than in Western countries (25-27). Since dense breasts lower the sensitivity of mammography, the implementation of DBT in Asia may potentially improve early detection of breast cancer, thus leading to a better prognosis. SM plus DBT can help women with improved breast cancer detection with only a slight increase in radiation exposure. Our study confirms the comparable performance of S-view SM plus DBT to FFDM plus DBT in a dense breast population, aligning with prior studies where the majority of the population had non-dense breasts and utilized different DBT machines (8,10,11,14,22).
In this study, the inter-observer agreement on the designation of the BI-RADS category between S-view SM plus DBT and FFDM plus DBT demonstrates excellent agreement, indicating similar interpretations of mammographic findings. This result aligns with a recent study that showed comparable performance between SM and FFDM in terms of the probability of malignancy assigned to various mammographic findings (8).
While some studies have compared interpretative performance and conspicuity scores for visibility of suspicious calcification between SM and FFDM, SM was not found to be inferior to FFDM (19,28,29). The debate over whether SM can replace FFDM in combination with DBT continues (10,20,21). Nelson et al. have highlighted that SM images exhibit visual enhancements for larger microcalcifications in in vitro evaluation (30). In real-world situations, the structural background noise, a factor influencing the conspicuity of lesions, may not be as clearly produced as in the controlled in vitro environment. In contrast, Spangler et al. noted that FFDM excels in depicting calcifications, primarily attributed to its high contrast resolution and tailored processing algorithms (21). Given the above, our team performed a subgroup analysis of suspicious calcifications, revealing no statistically significant difference in measures of diagnostic accuracy in sensitivity and specificity for breast cancer detection between these two modalities. This finding aligns with a recent Dodelzon et al. study (18) that demonstrated no significant difference in sensitivity for biopsy-proven malignant microcalcifications between SM and FFDM by readers, with a consensus sensitivity of 89% (95% CI: 78–99%) for SM and 80% (95% CI: 67–93%) for FFDM (P=0.3).
From our investigation, out of a total of 97 suspicious calcifications, 66 calcifications were associated with masses, and 31 calcifications were standalone. Noticeably, there have been a few delicate factors affecting clear visibility for suspicious calcifications. First, the distribution of calcification, in-group distribution, or area less than 2 cm, the visibility of calcifications is poorer seen on S-view SM, demonstrating a mean Likert score for visibility on S-view SM of 4.13 [standard deviation (SD) =0.91], compared to that of 5.0 of FFDM. This result is concordant with the previous Chikarmane et al. study (31), which found that calcifications <2 cm were less conspicuous on SM than FFDM. Second, an associated finding of calcification, when calcification is found accompanied with a mass, visibility of morphology is more clearly seen than that of calcifications that are standalone.
Inter-observer agreement on the classification in the morphology of suspicious calcifications achieved an excellent level of agreement between our two readers for both S-view SM plus DBT and FFDM plus DBT. Furthermore, inter-observer agreement in the clear visibility of mammographic findings on S-view SM, compared with that of FFDM, demonstrated a moderate agreement, with a Likert score correlation coefficient noted at 0.59.
Our study exhibits some limitations. First, it is characterized as a retrospective single-site investigation, thereby potentially introducing inference bias. Second, although encompassing both screening and diagnostic populations, a notable portion of participants, approximately half, presented with a palpable mass, indicating a comparatively higher prevalence of diagnostic cases relative to screening cases. Furthermore, the absence of a dedicated session for screening and diagnostic cases within Maharaj Nakorn Chiang Mai Hospital is due to a limited number of breast radiologists. Third, the quantity of isolated suspicious calcifications is notably limited, potentially compromising the robustness of the subgroup analysis concerning diagnostic accuracy. Subsequent studies could mitigate this limitation by enlarging the sample size within this subgroup, thereby enhancing the representativeness of the findings.
Conclusions
In the study, S-view SM demonstrates comparable and non-inferior performance to FFDM in both non-calcified and calcified breast cancer detection, exhibiting similar sensitivity and specificity when used in combination with DBT in both diagnostic and screening cases in a group of Asian women. The integration of DBT and SM emerges as a viable option, providing the potential to reduce radiation exposure without compromising diagnostic efficacy.
Acknowledgments
We would like to thank the Clinical Surgical Research Center at Chiang Mai University, Chiang Mai, Thailand for partial support.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2498/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2498/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University (No. RAD-2564-08018) with a waiver of the requirement for written inform consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Caumo F, Bernardi D, Ciatto S, Macaskill P, Pellegrini M, Brunelli S, Tuttobene P, Bricolo P, Fantò C, Valentini M, Montemezzi S, Houssami N. Incremental effect from integrating 3D-mammography (tomosynthesis) with 2D-mammography: Increased breast cancer detection evident for screening centres in a population-based trial. Breast 2014;23:76-80. [Crossref] [PubMed]
- Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, Izadi M, Jebsen IN, Jahr G, Krager M, Niklason LT, Hofvind S, Gur D. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013;267:47-56. [Crossref] [PubMed]
- Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, Tuttobene P, Bricolo P, Fantò C, Valentini M, Montemezzi S, Macaskill P. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013;14:583-9. [Crossref] [PubMed]
- Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, Hayes MK, Copit DS, Carlson KL, Cink TM, Barke LD, Greer LN, Miller DP, Conant EF. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014;311:2499-507. [Crossref] [PubMed]
- Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fantò C, Ostillio L, Tuttobene P, Luparia A, Houssami N. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol 2016;17:1105-13. [Crossref] [PubMed]
- Conant EF, Beaber EF, Sprague BL, Herschorn SD, Weaver DL, Onega T, Tosteson AN, McCarthy AM, Poplack SP, Haas JS, Armstrong K, Schnall MD, Barlow WE. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat 2016;156:109-16. [Crossref] [PubMed]
- Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015;24:93-9. [Crossref] [PubMed]
- Zuley ML, Guo B, Catullo VJ, Chough DM, Kelly AE, Lu AH, Rathfon GY, Lee Spangler M, Sumkin JH, Wallace LP, Bandos AI. Comparison of two-dimensional synthesized mammograms versus original digital mammograms alone and in combination with tomosynthesis images. Radiology 2014;271:664-71. [Crossref] [PubMed]
- Chikarmane SA, Offit LR, Giess CS. Synthetic Mammography: Benefits, Drawbacks, and Pitfalls. Radiographics 2023;43:e230018. [Crossref] [PubMed]
- Skaane P, Bandos AI, Eben EB, Jebsen IN, Krager M, Haakenaasen U, Ekseth U, Izadi M, Hofvind S, Gullien R. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology 2014;271:655-63. [Crossref] [PubMed]
- Gur D, Zuley ML, Anello MI, Rathfon GY, Chough DM, Ganott MA, Hakim CM, Wallace L, Lu A, Bandos AI. Dose reduction in digital breast tomosynthesis (DBT) screening using synthetically reconstructed projection images: an observer performance study. Acad Radiol 2012;19:166-71. [Crossref] [PubMed]
- Hamad W, Michell MJ, Myles JP, Gilbert FJ, Chen Y, Jin H, Loveland J, Halling-Brown M, Satchithananda K, Morel J, Wasan R, Taylor C, Sharma N, Valencia A, Teh W, Majid F, De Visser RM, Iqbal A, Duffy SW. Diagnostic performance of tomosynthesis plus synthetic mammography versus full-field digital mammography with or without tomosynthesis in breast cancer screening: A systematic review and meta-analysis. Int J Cancer 2025;156:969-79. [Crossref] [PubMed]
- Dhamija E, Mohan SL, Anand R, Khan MA, Deo SVS, Hari S. Comparison of Full-Field Digital Mammography with Synthesized Mammography from Tomosynthesis in a Diagnostic population: Prospective Study. Indian J Radiol Imaging 2025;35:25-34. [Crossref] [PubMed]
- Zuckerman SP, Conant EF, Keller BM, Maidment AD, Barufaldi B, Weinstein SP, Synnestvedt M, McDonald ES. Implementation of Synthesized Two-dimensional Mammography in a Population-based Digital Breast Tomosynthesis Screening Program. Radiology 2016;281:730-6. [Crossref] [PubMed]
- Aujero MP, Gavenonis SC, Benjamin R, Zhang Z, Holt JS. Clinical Performance of Synthesized Two-dimensional Mammography Combined with Tomosynthesis in a Large Screening Population. Radiology 2017;283:70-6. [Crossref] [PubMed]
- Lee KE, Song SE, Cho KR, Bae MS, Seo BK, Kim SY, Woo OH. Performance of Digital Mammography-Based Artificial Intelligence Computer-Aided Diagnosis on Synthetic Mammography From Digital Breast Tomosynthesis. Korean J Radiol 2025;26:217-29. [Crossref] [PubMed]
- American College of Radiology. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 2013. Available online: https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Reporting-and-Data-Systems/BI-RADS
- Dodelzon K, Simon K, Dou E, Levy AD, Michaels AY, Askin G, Katzen JT. Performance of 2D Synthetic Mammography Versus Digital Mammography in the Detection of Microcalcifications at Screening. AJR Am J Roentgenol 2020;214:1436-44. [Crossref] [PubMed]
- Choi JS, Han BK, Ko EY, Ko ES, Hahn SY, Shin JH, Kim MJ. Comparison between two-dimensional synthetic mammography reconstructed from digital breast tomosynthesis and full-field digital mammography for the detection of T1 breast cancer. Eur Radiol 2016;26:2538-46. [Crossref] [PubMed]
- Gilbert FJ, Tucker L, Gillan MG, Willsher P, Cooke J, Duncan KA, Michell MJ, Dobson HM, Lim YY, Suaris T, Astley SM, Morrish O, Young KC, Duffy SW. Accuracy of Digital Breast Tomosynthesis for Depicting Breast Cancer Subgroups in a UK Retrospective Reading Study (TOMMY Trial). Radiology 2015;277:697-706. [Crossref] [PubMed]
- Spangler ML, Zuley ML, Sumkin JH, Abrams G, Ganott MA, Hakim C, Perrin R, Chough DM, Shah R, Gur D. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. AJR Am J Roentgenol 2011;196:320-4. [Crossref] [PubMed]
- Clauser P, Baltzer PAT, Kapetas P, Woitek R, Weber M, Leone F, Bernathova M, Helbich TH. Synthetic 2-Dimensional Mammography Can Replace Digital Mammography as an Adjunct to Wide-Angle Digital Breast Tomosynthesis. Invest Radiol 2019;54:83-8. [Crossref] [PubMed]
- Buderer NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 1996;3:895-900. [Crossref] [PubMed]
- Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol 2001;30:959-65. [Crossref] [PubMed]
- Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med 2014;11:101-15. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Ghoncheh M, Momenimovahed Z, Salehiniya H. Epidemiology, Incidence and Mortality of Breast Cancer in Asia. Asian Pac J Cancer Prev 2016;17:47-52. [Crossref] [PubMed]
- Mariscotti G, Durando M, Houssami N, Fasciano M, Tagliafico A, Bosco D, Casella C, Bogetti C, Bergamasco L, Fonio P, Gandini G. Comparison of synthetic mammography, reconstructed from digital breast tomosynthesis, and digital mammography: evaluation of lesion conspicuity and BI-RADS assessment categories. Breast Cancer Res Treat 2017;166:765-73. [Crossref] [PubMed]
- Choi JS, Han BK, Ko EY, Kim GR, Ko ES, Park KW. Comparison of synthetic and digital mammography with digital breast tomosynthesis or alone for the detection and classification of microcalcifications. Eur Radiol 2019;29:319-29. [Crossref] [PubMed]
- Nelson JS, Wells JR, Baker JA, Samei E. How does c-view image quality compare with conventional 2D FFDM? Med Phys 2016;43:2538. [Crossref] [PubMed]
- Chikarmane SA, Yeh ED, Wang A, Ratanaprasatporn L, Giess CS. Conspicuity of Screen-Detected Malignancies on Full Field Digital Mammography vs. Synthetic Mammography. Acad Radiol 2020;27:757-63. [Crossref] [PubMed]


