
Predicting cardiac resynchronization therapy response: development and validation of a single photon emission computed tomography-based nomogram
Introduction
Cardiac resynchronization therapy (CRT) is proven to be an effective treatment for patients with symptomatic heart failure because it can improve cardiac function, quality of life, and exercise capacity, as well as reduce morbidity and mortality (1-4). However, thirty percent of patients do not respond to CRT because of suboptimal lead placement, large amounts of myocardial scar tissue, and mechanical dyssynchrony (5). Several multicenter studies have extensively explored echocardiographic measures for predicting CRT response. The PROSPECT trial (6) showed the limitations of echocardiographic dyssynchrony parameters in multicenter settings, underscoring the need for more reliable and reproducible markers. In contrast, the MARC study (7) identified apical rocking and interventricular mechanical delay (IVMD) as independent predictors of CRT response, suggesting their potential value in refining patient selection for CRT. Meanwhile, single photon emission computed tomography (SPECT) provides specific, quantifiable data on left ventricular dyssynchrony and myocardial perfusion (8,9), reducing the potential biases associated with manual assessments. Previous studies have demonstrated that systolic left ventricular mechanical dyssynchrony (LVMD) and diastolic LVMD are independent predictors of a CRT response (10-15). Compared with traditional methods, phase analysis based on gated SPECT myocardial perfusion imaging (MPI) is a well-established, highly automatic, and reproducible technique for assessing LVMD, and it has been successfully used to explore predictors of a CRT response (16-18). In addition, owing to its unique imaging methods, phase analysis based on gated SPECT MPI can optimize the placement of the left ventricle (LV) lead by combining LVMD and scar tissue, thereby improving the efficacy of CRT (19).
Clinical prediction models have been widely applied in the medical field, including in predicting disease progression and therapy outcomes (20,21). On the basis of clinical data, statistical methods, and machine learning, a prediction model can assist clinicians and researchers in predicting disease occurrence, progression, and treatment outcomes, aiding in clinical decision-making and improving the accuracy and efficiency of diagnosis and treatment (22-24). Least absolute shrinkage and selection operator (LASSO) regression (25-27) is an effective technique for constructing predictive models. It automatically selects the most relevant features and mitigates multicollinearity through a unique mechanism, thereby enhancing model performance and robustness. Previous studies (28,29) have developed CRT prediction models. However, these models perform poorly because they include a single, limited variable. Therefore, it is crucial to identify CRT responders using suitable prediction models. The aim of this study was to establish and validate two models that combine clinical features, laboratory features, imaging features, and technical features of phase analysis to predict the response to CRT. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2700/rc).
Methods
Patient population
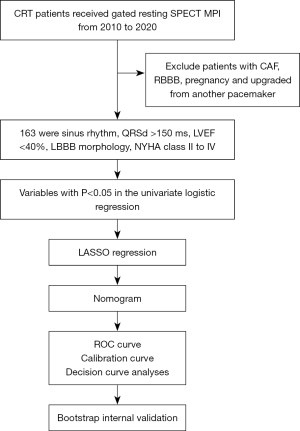
One hundred and sixty-three consecutive CRT patients were enrolled in this study between 2010 and 2020 at The First Affiliated Hospital of Nanjing Medical University. All the patients received gated resting SPECT MPI and at least 3 months of guideline-guided medical therapy (2) before surgery. The inclusion criteria were as follows: age ≥18 years, sinus rhythm, left ventricular ejection fraction (LVEF) <40%, typical left bundle branch block (LBBB) morphology, and New York Heart Association (NYHA) functional class II to IV. Patients with consistent atrial fibrillation, right bundle branch block, an upgrade from another pacemaker or pregnancy were excluded from the study. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and was granted ethical approval by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2024-SR-898). Informed consent was obtained from every participant involved. The overall research process is shown in Figure 1.
Gated myocardial perfusion SPECT
The resting electrocardiogram (ECG)-gated SPECT MPI scan was conducted approximately 60 minutes after the injection of 20 to 30 mCi of Tc-99m sestamibi. MPI images were captured with a dual-headed camera (CardioMD, Philips Medical Systems, Amsterdam, the Netherlands) following a standard protocol. The imaging parameters consisted of a 20% energy window at 140 KeV, a 180° orbit, 32 steps with 25 seconds per step, 8-bin gating, and 64 planar projections per gate. Image reconstruction and reorientation were conducted via the Emory Reconstruction Toolbox (ERToolbox; Syntermed, Atlanta, GA, USA). All SPECT planar images were reconstructed through ordered subset expectation maximization (OSEM) with three iterations and 10 subsets, followed by filtering with a Butterworth low-pass filter with a cutoff frequency of 0.3 cycles/cm and an order of 10.
To determine the LV contour parameters, the gated short-axis SPECT MPI images were processed via an interactive tool. The parameters and image data were submitted to an automatic myocardial sampling algorithm, which searches in three-dimensional (3D) for the maximal count circumferential profiles in each cardiac frame. The samples were subsequently analyzed via a multiharmonic phase analysis tool to evaluate the systolic and diastolic LVMD via Fourier approximations. The onset of mechanical contraction and relaxation for each sample was calculated via 1-harmonic and 3-harmonic Fourier approximations (30). Global LVMD was quantified via phase standard deviation (PSD) and the 95% width of the phase histogram bandwidth (PBW) (16,31).
PSD is a metric used to quantify the variability in the timing of contraction across different regions of the myocardium. It is calculated by statistically analyzing the phase angles of each myocardial pixel, reflecting the synchronization of myocardial contraction. The greater the PSD is, the more severe the LVMD. The histogram bandwidth represents the range of the phase angle distribution. It is derived from the phase histogram and shows the extent of myocardial contraction occurring across a broader or narrower time range during the cardiac cycle. A wider histogram bandwidth means that different myocardial regions contract over a broader time range during the cardiac cycle, indicating significant dyssynchrony.
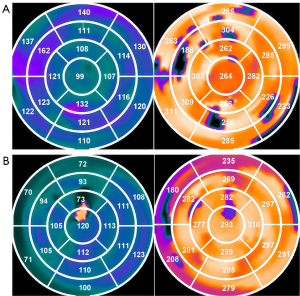
As shown in Figure 2, a phase polar map is an intuitive and effective tool in phase analysis based on gated SPECT MPI for assessing and visualizing LVMD. It employs a polar coordinate system, with the center representing the apex of the LV and the outer rings corresponding to the basal regions. In addition, the map uses color coding to depict phase angles across different regions of the LV, with each color corresponding to a specific point in the cardiac cycle. Variations in color indicate asynchronous myocardial contraction, where smoother color transitions reflect better synchrony, and abrupt or discontinuous color changes highlight significant dyssynchrony. If the LV lead is placed at the location of the three latest contraction segments, it is defined as a systolic match; if it is placed at the three latest relaxation segments, it is defined as a diastolic match.
Clinical parameters
During hospitalization, all patients received a 12-lead surface ECG. The QRS complex duration (QRSd) was measured on the basis of the widest QRS complex observed in the ECG. Twenty-four-hour Holter monitoring was used to record premature ventricular contractions (PVCs). Non-sustained ventricular tachycardia (NS-VT) was defined as three or more consecutive ventricular beats with a frequency greater than 100 beats per minute and lasting less than 30 seconds. Echocardiography was performed before and 6 months after CRT implantation to assess LV function, which was characterized by evaluating the LVEF, left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD). The phase analysis technique was applied to resting-gated SPECT MPI images to measure systolic and diastolic LVMD, as characterized by PSD and PBW. Guided by fluoroscopic venography and coronary venography, the LV lead was implanted into one of the coronary vein branches—anterolateral, lateral, or posterolateral—to ensure a satisfactory pacing threshold and no phrenic nerve capture. CRT response was defined as ≥5% improvement in LVEF under echocardiography at the 6-month follow-up.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, whereas categorical variables are expressed as count and percentage. We employed multiple imputation based on the random forest algorithm to process missing data. Variables were first assessed by univariate logistic regression, retaining variables with a significance threshold of <0.05. The selected variables were then subjected to LASSO regression to construct a predictive model, from which a nomogram was developed. Nomogram performance was evaluated by the area under the receiver operating characteristic (ROC) curve (AUC) as a measure of discriminative ability, a calibration plot as a metric of internal calibration, and the Hosmer-Lemeshow test to assess goodness of fit. Decision curve analyses (DCAs) evaluate the clinical usefulness of a model by considering the net benefits at different threshold probabilities. Internal bias correction was performed by bootstrapping for internal validation, involving 1,000 iterations of resampling with replacement from the training cohort. The analyses were performed with SPSS version 27.0 and R software (version 4.3.3).
Results
Baseline characteristics
Among the 163 CRT patients in the training cohort, 93 (57.1%) responded to CRT during follow-up. Patients who were CRT responders had a wider QRSd (164.80 vs. 154.51 ms), fewer PVCs (1,392.98 vs. 2,283.60), a lower rate of NS-VT (45.2% vs. 77.1%), and better cardiac function [based on N-terminal pro-B-type natriuretic peptide (NT-proBNP), NYHA, and LV parameters]. The characteristics of patients with and without a CRT response are presented in Table 1. The univariate logistic regression analyses revealed that QRSd, NS-VT, NT-proBNP, LVEDD, LVESD, rest scar burden, systolic PSD, systolic PBW, diastolic PSD, diastolic PBW, systolic match, diastolic match, lead in scar burden, and NYHA were significantly associated with CRT response (all P<0.05), as shown in Table 2.
Table 1
| Variables | All (n=163) | Non-responders (n=70) | Responders (n=93) | P value |
|---|---|---|---|---|
| Age (years) | 64.69±13.86 | 64.03±10.90 | 65.18±15.77 | 0.666 |
| Male | 119 (73.0) | 54 (77.1) | 65 (69.9) | 0.303 |
| Hypertension | 73 (44.8) | 34 (48.6) | 39 (41.9) | 0.400 |
| Diabetes | 40 (24.5) | 22 (31.4) | 18 (19.4) | 0.077 |
| DCM | 112 (68.7) | 48 (68.6) | 64 (68.8) | 0.973 |
| QRSd (ms) | 160.38±23.62 | 154.51±24.49 | 164.80±22.07 | 0.003 |
| NT-proBNP | 0.005 | |||
| <1,000 | 30 (18.4) | 6 (8.6) | 24 (25.8) | |
| 1,000–2,499 | 53 (32.5) | 24 (34.3) | 29 (31.2) | |
| 2,500–4,999 | 40 (24.5) | 16 (22.9) | 24 (25.8) | |
| 5,000–10,000 | 31 (19.0) | 19 (27.1) | 12 (12.9) | |
| >10,000 | 9 (5.5) | 5 (7.1) | 4 (4.3) | |
| PVC | 1,775.45±3,339.97 | 2,283.60±3,521.28 | 1,392.98±3,162.17 | 0.003 |
| NS-VT | 96 (58.9) | 54 (77.1) | 42 (45.2) | <0.001 |
| NYHA | 0.051 | |||
| II | 64 (39.3) | 20 (28.6) | 44 (47.3) | |
| III | 78 (47.9) | 41 (58.6) | 37 (39.8) | |
| IV | 21 (12.9) | 9 (12.9) | 12 (12.9) | |
| Medication | ||||
| ACEI | 83 (50.9) | 33 (47.1) | 50 (53.8) | 0.404 |
| ARB | 30 (18.4) | 9 (12.9) | 21 (22.6) | 0.114 |
| β-blocker | 155 (95.1) | 68 (97.1) | 87 (93.5) | 0.295 |
| Diuretics | 155 (95.1) | 68 (97.1) | 87 (93.5) | 0.295 |
| Spironolactone | 154 (94.5) | 68 (97.1) | 86 (92.5) | 0.198 |
| LV parameters | ||||
| EDV (mL) | 294.39±130.22 | 305.37±122.62 | 286.12±135.72 | 0.100 |
| ESV (mL) | 242.01±119.58 | 252.21±111.09 | 234.32±125.63 | 0.156 |
| LVEDD (mm) | 71.94±10.34 | 75.47±10.38 | 69.28±9.53 | <0.001 |
| LVESD (mm) | 61.55±10.91 | 65.61±10.68 | 58.49±10.11 | <0.001 |
| LVEF echo (%) | 28.89±6.83 | 29.28±6.85 | 28.59±6.83 | 0.552 |
| Rest scar burden | 29.31±13.71 | 33.11±13.83 | 26.44±12.97 | 0.002 |
| Systolic PSD (°) | 44.31±22.60 | 51.50±22.50 | 38.90±21.23 | <0.001 |
| Systolic PBW (°) | 169.36±94.41 | 198.33±93.98 | 147.55±89.16 | 0.001 |
| Diastolic PSD (°) | 55.82±23.56 | 63.55±23.27 | 50.00±22.17 | <0.001 |
| Diastolic PBW (°) | 196.76±91.71 | 224.70±89.65 | 175.73±87.98 | 0.001 |
| Systolic match | 72 (44.2) | 20 (28.6) | 52 (55.9) | 0.001 |
| Diastolic match | 120 (73.6) | 44 (62.9) | 76 (81.7) | 0.007 |
| Lead in scar burden | 27 (16.6) | 17 (24.3) | 10 (10.8) | 0.022 |
Data are shown as n (%) or mean ± standard deviation. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DCM, dilated cardiomyopathy; EDV, end-diastolic volume; ESV, end-systolic volume; LV, left ventricle; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic diameter; NS-VT, non-sustained ventricular tachycardia; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PBW, phase histogram bandwidth; PSD, phase standard deviation; PVC, premature ventricular contraction; QRSd, QRS complex duration.
Table 2
| Variables | P value | Odds ratio | 95% CI |
|---|---|---|---|
| Age | 0.599 | 1.006 | 0.983–1.030 |
| Gender (male) | 0.303 | 1.454 | 0.713–2.965 |
| Hypertension | 0.399 | 1.308 | 0.701–2.441 |
| Diabetes | 0.078 | 1.910 | 0.929–3.926 |
| DCM | 0.973 | 0.989 | 0.507–1.929 |
| QRSd | 0.007 | 1.020 | 1.005–1.035 |
| NT-proBNP | 0.030 | – | – |
| PVC | 0.117 | 1.000 | 1.000–1.000 |
| NS-VT | <0.001 | 4.098 | 2.053–8.182 |
| NYHA | 0.041 | – | – |
| ACEI | 0.403 | 0.767 | 0.412–1.428 |
| ARB | 0.117 | 0.506 | 0.216–1.186 |
| β-blocker | 0.306 | 2.345 | 0.459–11.985 |
| Diuretics | 0.306 | 2.345 | 0.459–11.985 |
| Spironolactone | 0.213 | 2.767 | 0.557–13.753 |
| EDV | 0.351 | 0.999 | 0.996–1.001 |
| ESV | 0.345 | 0.999 | 0.996–1.001 |
| LVEDD | <0.001 | 0.940 | 0.909–0.972 |
| LVESD | <0.001 | 0.937 | 0.907–0.967 |
| LVEF echo | 0.521 | 0.985 | 0.941–1.031 |
| Rest scar burden | 0.003 | 0.964 | 0.940–0.987 |
| Systolic PSD | 0.001 | 0.974 | 0.960–0.989 |
| Systolic PBW | 0.001 | 0.994 | 0.991–0.998 |
| Diastolic PSD | <0.001 | 0.974 | 0.960–0.988 |
| Diastolic PBW | 0.001 | 0.994 | 0.990–0.997 |
| Systolic match | 0.001 | 3.171 | 1.638–6.139 |
| Diastolic match | 0.008 | 2.642 | 1.292–5.401 |
| Lead in scar burden | 0.025 | 2.662 | 1.134–6.252 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; DCM, dilated cardiomyopathy; EDV, end-diastolic volume; ESV, end-systolic volume; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic diameter; NS-VT, non-sustained ventricular tachycardia; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PBW, phase histogram bandwidth; PSD, phase standard deviation; PVC, premature ventricular contraction; QRSd, QRS complex duration.
Predictor selection
All variables with P<0.05 were subjected to LASSO regression, which indicated significant correlations between QRSd, NS-VT, NT-proBNP, LVESD, rest scar burden, diastolic PSD, diastolic PBW, systolic match, diastolic match, NYHA and CRT response at the 1 − standard error (SE). value (Figure 3), after the elimination of dummy variables. The nomogram utilizes β coefficients from a regression model to assign weightings to each factor associated with the CRT response. Each factor contributes points on the basis of its weighting, with an increasing total score correlating with a higher probability of a positive CRT response. A detailed description of how to use the nomogram to predict CRT response is provided in the legend of Figure 4.

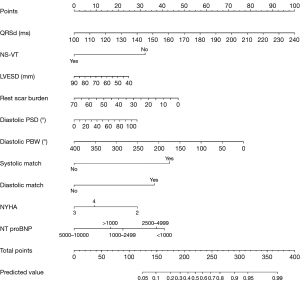
Performance of the nomogram
The AUC of nomogram, as shown in Figure 5A, was 0.845 [95% confidence interval (CI): 0.785–0.906], with a sensitivity of 0.771 and a specificity of 0.849. A higher AUC value means that the model is better at distinguishing CRT responders from non-responders. In the internal validation of bootstrapping, the nomogram maintained excellent discriminative power, with a mean AUC of 0.814 (95% CI: 0.777–0.836). Figure 5B,5C shows the summary plot of the ROC curves from 1,000 internal samples and the frequency distribution diagram of the average AUC.
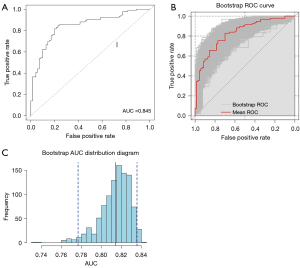
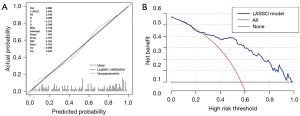
Figure 6A shows that the nomogram was well calibrated, and the mean predicted probabilities for each subgroup closely matched the observed probabilities. This was further supported by Hosmer-Lemeshow test P value of 0.129, indicating no significant difference between the predicted and observed CRT response probabilities in each subgroup. The result of the DCAs of the nomogram was shown in Figure 6B. In terms of clinical usefulness, nomogram consistently provides net benefit above the baseline, demonstrating its high practical value in clinical decision-making.
Web-based dynamic nomogram
To facilitate the use of the nomogram, a web-based dynamic tool based on the proposed prediction model was constructed (https://jzw20000624.shinyapps.io/CRTpredictionmodel/). Clinicians can easily obtain the estimated probabilities of a CRT response by inputting the ten independent factors on the website.
Discussion
In this retrospective cohort study, we established a novel prediction model based on gated SPECT MPI, which internal validation showed to have excellent predictive performance. Compared with common clinical factors, this model significantly improved the ability to predict a CRT response.
Accurately predicting the response to CRT before surgery is crucial. Our nomogram will assist clinicians in deciding whether to perform CRT, especially in the presence of SPECT. In this study, we identified independent predictive factors for CRT response and developed novel, effective, and validated nomograms to predict CRT response. QRSd, NS-VT, NT-proBNP, LVESD, rest scar burden, diastolic PSD, diastolic PBW, systolic match, diastolic match, and NYHA were included in nomogram. Although previous studies have also developed CRT prediction models, their limited accuracy has hindered widespread use in clinical practice. Liu et al. (28) retrospectively analyzed 387 CRT patients and constructed the QQ-LAE score (no prior fragmented QRS, QRSd ≥170 ms, LBBB, left atrial diameter <45 mm, and left ventricular end-diastolic dimension <75 mm) to predict a super response to CRT. In addition, Xiao et al. (29) developed an 8-variable nomogram model to predict CRT response. However, these two models showed poor discriminative abilities (AUC =0.71). The possible reason for the poor performance is that these models included a limited range of variables, primarily ECG and echocardiogram data, and failed to incorporate new variables derived using novel technologies. In recent years, advancements in echocardiography and the application of other imaging techniques have provided new insights for optimizing CRT models. In the MARC study (7), Maass et al. found that QRSAREA (derived from vectorcardiography) along with two echocardiographic markers, IVMD, and apical rocking, were significantly associated with the degree of reverse ventricular remodeling. They developed CAVIAR (CRT-Age-Vectorcardiographic QRSAREA-Interventricular Mechanical Delay-Apical Rocking) response score, which effectively predicts the extent of reverse remodeling and clinical outcome following CRT and may be used to improve patient selection. Si et al. (32) combined gated SPECT and vectorcardiography to successfully demonstrate that pacing in the recommended segments, by integrating mechanical and electrical dyssynchrony, is significantly associated with improved CRT response and better long-term prognosis. Calibration curves and DCA were used to assess the predictive accuracy and clinical utility of the nomogram. Furthermore, our model, which uses phase analysis based on gated SPECT MPI and measures LVMD through PSD, PBW, and entropy, demonstrated superior discriminative power, with an AUC value of 0.845 in the training cohort and of 0.814 in the internal validation cohort. Our model demonstrated a greater net benefit and showed greater consistency between the nomogram predictions and actual observations than previous models.
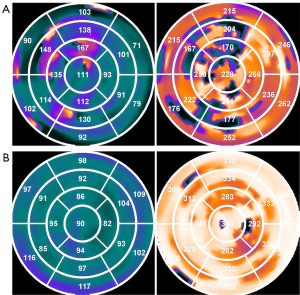
The primary therapeutic target of CRT is to correct ventricular dyssynchrony caused by abnormal cardiac electrical conduction. Thus, to solve CRT nonresponse, the primary problem is to visualize LVMD. Echocardiography is considered an effective method for observing LV dyssynchrony. However, the PROSPECT study revealed a lack of reproducibility in LVMD both within and between observers (33). Phase analysis via SPECT can display the 3D phase distribution of the entire LV, allowing for quantitative analysis of LV dyssynchrony via PSD, PBW, and entropy. Compared with echocardiography, phase analysis can more accurately and reproducibly assess LVMD because it is minimally affected by human factors during image acquisition, processing, and analysis. The phase polar map visually displays ventricular dyssynchrony through color distributions. Smoother color transitions indicate better synchrony, whereas abrupt color changes suggest significant dyssynchrony. Figure 7 presents the preoperative and postoperative polar maps of a CRT responder. By comparing these maps, clinicians can visually assess the effectiveness of CRT. Bundle branch block and contractile dysfunction lead to intraventricular conduction delay, generating regions of early and delayed activation within the LV, leading to decreased cardiac pump function, increased end-systolic volume, wall stress, and a further decline in myocardial efficiency (34-36). Pacing at the latest contraction segment initiates the contraction phase earlier, synchronizing the overall contraction of the LV. Several studies (37-39), through imaging techniques, have demonstrated that pacing at the latest LV region leads to more energetically efficient ventricular ejection, greater LV reverse remodeling, improved survival, and reduced heart failure-related hospitalization. In addition, we defined the diastolic match as the LV lead placed at the same location in the last three relaxation segments. Owing to the earlier repolarization of the part of the myocardium that depolarizes first, pacing at the latest segments causes this part of the myocardium to enter diastole earlier and may improve overall diastolic dyssynchrony. When the LV lead was implanted at both the first three late contraction segments and the first three late relaxation segments, 75% (39/52) of patients responded to CRT. In contrast, the rate of CRT response was only 17.4% (4/23) in patients whose LV lead was in the segments with neither the first three late contractions nor the first three late relaxations. Hence, pacing at the latest contraction segments while also meeting the diastolic match is expected to improve the response to CRT.
Several clinical trials (40,41) have demonstrated that patients with ischemic cardiomyopathy (ICM) exhibit a significantly lower response rate to CRT compared to those with non-ICM (NICM). One factor that might affect the response to CRT, particularly in patients with ICM, is the presence of scar tissue. Scar tissue impairs the ability of the heart to contract and conduct electrical signals properly, which can lead to decreased heart function and arrhythmias. In the context of CRT, assessing the scar burden is important for determining the potential effectiveness of therapy and planning the optimal placement of leads. Additionally, avoiding placing the LV lead in the regions of the scar results in higher response rates to CRT and a better prognosis (42,43). Yokoshiki et al. (44) reported that the presence of NS-VT in CRT patients was associated with a greater risk of heart-failure death. The irregular heartbeats caused by NS-VT may interfere with the normal function of CRT, leading to suboptimal resynchronization of the ventricles. NT-proBNP is a biomarker commonly used to assess heart failure and predict CRT response and prognosis. A high baseline level of NT-proBNP is often associated with more severe heart failure and greater ventricular dysfunction. Typically, patients with lower baseline NT-proBNP levels tend to have better responses to CRT. Therefore, optimal medication therapy to improve heart function and achieve optimal preoperative status is crucial for enhancing the response to CRT. Female sex is a widely recognized predictor of CRT response. However, our study’s findings indicate that female sex was not a significant predictor of CRT response. Arshad et al. suggested that one reason women may benefit more from CRT is that they have a higher proportion of LBBB (45). However, all enrolled patients in our study had typical LBBB morphology, which may mitigate the impact of female sex as a predictive factor.
Machine learning is a commonly used method for building prediction models because of its ability to utilize large amounts of data effectively and recognize patterns in nonlinear systems (46,47). de A Fernandes et al. (48) integrated ECG, gated SPECT MPI, and clinical variables, utilizing prediction analysis of microarrays (PAM) to create a prediction model that best fit the CRT response. However, a shortcoming of the study, as mentioned by the authors, is that it did not further investigate the value of gated SPECT MPI in guiding left ventricular lead placement. In this study, we found that achieving both a systolic match and a diastolic match, while away from scar tissue, improved the CRT response. In addition, owing to limitations such as overfitting, difficulty in interpretation, and less effective visualization compared with traditional models (49-51), the application of machine learning in clinical settings faces some obstacles. LASSO regression shrinks the coefficients of irrelevant features to zero, effectively performing automatic feature selection. Unlike complex machine learning models, LASSO creates a more concise model by clearly identifying the most relevant features, improving interpretability through its sparsity. Moreover, in situations with limited sample sizes, complex models tend to overfit and exhibit instability. LASSO incorporates an L1 regularization term that effectively reduces overfitting in small datasets. This makes LASSO more robust than models like deep neural networks, particularly in cases with small samples and high-dimensional features.
Despite the good performance of our nomogram, it has several limitations that need to be addressed. First, owing to the limited sample size, we used internal validation with bootstrap methods. The model’s performance needs to be subjected to multicenter external validation. Second, the impact of the systolic match and diastolic match on the CRT response and left ventricular lead position is based only on our hypothesis. The underlying pathophysiological mechanisms need to be further explored through scientific research. Third, we did not compare our model with those established previously. It would be beneficial to evaluate the performance of our model and identify its shortcomings by conducting a comparison with multiple other models.
Conclusions
A SPECT-based prediction model of CRT response had high predictive performance in internal validation and can be used to assist assessment of CRT indications before surgery. Additionally, pacing at the latest contraction and relaxation segments, while avoiding scarred regions and ensuring optimal preoperative status, is anticipated to improve the response to CRT.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2700/rc
Funding: This research was supported by a grant from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2700/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was granted ethical approval by the Institutional Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2024-SR-898) and informed consent was obtained from every participant involved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. Erratum in: Eur Heart J 2021;42:4901. [Crossref] [PubMed]
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427-520. Erratum in: Eur Heart J 2022;43:1651. [Crossref] [PubMed]
- Writing Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Card Fail 2022;28:e1-e167. [Crossref] [PubMed]
- Chung MK, Patton KK, Lau CP, Dal Forno ARJ, Al-Khatib SM, Arora V, et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm 2023;20:e17-e91. [Crossref] [PubMed]
- Sieniewicz BJ, Gould J, Porter B, Sidhu BS, Teall T, Webb J, Carr-White G, Rinaldi CA. Understanding non-response to cardiac resynchronisation therapy: common problems and potential solutions. Heart Fail Rev 2019;24:41-54. [Crossref] [PubMed]
- Yu CM, Abraham WT, Bax J, Chung E, Fedewa M, Ghio S, Leclercq C, León AR, Merlino J, Nihoyannopoulos P, Notabartolo D, Sun JP, Tavazzi L. Predictors of response to cardiac resynchronization therapy (PROSPECT)--study design. Am Heart J 2005;149:600-5. [Crossref] [PubMed]
- Maass AH, Vernooy K, Wijers SC, van 't Sant J, Cramer MJ, Meine M, Allaart CP, De Lange FJ, Prinzen FW, Gerritse B, Erdtsieck E, Scheerder COS, Hill MRS, Scholten M, Kloosterman M, Ter Horst IAH, Voors AA, Vos MA, Rienstra M, Van Gelder IC. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. Europace 2018;20:e1-e10. [Crossref] [PubMed]
- Cui L, Wang Y, Chen W, Huang P, Tang Z, Wang J, Li J, Tse G, Liu T, Wang Y, Chen K. Coronary microvascular dysfunction and myocardial area at risk assessed by cadmium zinc telluride single photon emission computed tomography after primary percutaneous coronary intervention in acute myocardial infarction patients. Quant Imaging Med Surg 2024;14:3816-27. [Crossref] [PubMed]
- Liu B, Yu W, Zhang F, Shi Y, Yang L, Jiang Q, Wang Y, Wang Y. Detecting obstructive coronary artery disease with machine learning: rest-only gated single photon emission computed tomography myocardial perfusion imaging combined with coronary artery calcium score and cardiovascular risk factors. Quant Imaging Med Surg 2023;13:1524-36. [Crossref] [PubMed]
- Ansalone G, Giannantoni P, Ricci R, Trambaiolo P, Laurenti A, Fedele F, Santini M. Doppler myocardial imaging in patients with heart failure receiving biventricular pacing treatment. Am Heart J 2001;142:881-96. [Crossref] [PubMed]
- Penicka M, Bartunek J, De Bruyne B, Vanderheyden M, Goethals M, De Zutter M, Brugada P, Geelen P. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation 2004;109:978-83. [Crossref] [PubMed]
- Bax JJ, Marwick TH, Molhoek SG, Bleeker GB, van Erven L, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts benefit of cardiac resynchronization therapy in patients with end-stage heart failure before pacemaker implantation. Am J Cardiol 2003;92:1238-40. [Crossref] [PubMed]
- Rouleau F, Merheb M, Geffroy S, Berthelot J, Chaleil D, Dupuis JM, Victor J, Geslin P. Echocardiographic assessment of the interventricular delay of activation and correlation to the QRS width in dilated cardiomyopathy. Pacing Clin Electrophysiol 2001;24:1500-6. [Crossref] [PubMed]
- Schuster I, Habib G, Jego C, Thuny F, Avierinos JF, Derumeaux G, Beck L, Medail C, Franceschi F, Renard S, Ferracci A, Lefevre J, Luccioni R, Deharo JC, Djiane P. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol 2005;46:2250-7. [Crossref] [PubMed]
- Morris-Thurgood JA, Turner MS, Nightingale AK, Masani N, Mumford C, Frenneaux MP. Pacing in heart failure: improved ventricular interaction in diastole rather than systolic re-synchronization. Europace 2000;2:271-5; duscussion 276.
- Zhou W, Garcia EV. Nuclear Image-Guided Approaches for Cardiac Resynchronization Therapy (CRT). Curr Cardiol Rep 2016;18:7. [Crossref] [PubMed]
- Friehling M, Chen J, Saba S, Bazaz R, Schwartzman D, Adelstein EC, Garcia E, Follansbee W, Soman P. A prospective pilot study to evaluate the relationship between acute change in left ventricular synchrony after cardiac resynchronization therapy and patient outcome using a single-injection gated SPECT protocol. Circ Cardiovasc Imaging 2011;4:532-9. [Crossref] [PubMed]
- Wang C, Shi J, Ge J, Tang H, He Z, Liu Y, Zhao Z, Li C, Gu K, Hou X, Chen M, Zou J, Zhou L, Garcia EV, Li D, Zhou W. Left ventricular systolic and diastolic dyssynchrony to improve cardiac resynchronization therapy response in heart failure patients with dilated cardiomyopathy. J Nucl Cardiol 2021;28:1023-36. [Crossref] [PubMed]
- Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol 2008;52:1402-9. [Crossref] [PubMed]
- Tang XR, Li YQ, Liang SB, Jiang W, Liu F, Ge WX, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol 2018;19:382-93. [Crossref] [PubMed]
- Maurichi A, Miceli R, Eriksson H, Newton-Bishop J, Nsengimana J, Chan M, et al. Factors Affecting Sentinel Node Metastasis in Thin (T1) Cutaneous Melanomas: Development and External Validation of a Predictive Nomogram. J Clin Oncol 2020;38:1591-601. [Crossref] [PubMed]
- Bulens P, Couwenberg A, Haustermans K, Debucquoy A, Vandecaveye V, Philippens M, Zhou M, Gevaert O, Intven M. Development and validation of an MRI-based model to predict response to chemoradiotherapy for rectal cancer. Radiother Oncol 2018;126:437-42. [Crossref] [PubMed]
- Li Y, Lu F, Yin Y. Applying logistic LASSO regression for the diagnosis of atypical Crohn's disease. Sci Rep 2022;12:11340. [Crossref] [PubMed]
- Zhou D, Liu X, Wang X, Yan F, Wang P, Yan H, Jiang Y, Yang Z. A prognostic nomogram based on LASSO Cox regression in patients with alpha-fetoprotein-negative hepatocellular carcinoma following non-surgical therapy. BMC Cancer 2021;21:246. [Crossref] [PubMed]
- Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 2010;33:1-22.
- Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol 2005;67:301-20.
- Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996;58:267-88.
- Liu X, Hu Y, Hua W, Yang S, Gu M, Niu HX, Ding LG, Wang J, Zhang S. A Predictive Model for Super-Response to Cardiac Resynchronization Therapy: The QQ-LAE Score. Cardiol Res Pract 2020;2020:3856294. [Crossref] [PubMed]
- Xiao PL, Cai C, Zhang P, Han J, Mulpuru SK, Deshmukh AJ, Yin YH, Cha YM. Better CRT Response in Patients Who Underwent Atrioventricular Node Ablation or Upgrade From Pacemaker: A Nomogram to Predict CRT Response. Front Cardiovasc Med 2021;8:760195. [Crossref] [PubMed]
- Chen J, Kalogeropoulos AP, Verdes L, Butler J, Garcia EV. Left-ventricular systolic and diastolic dyssynchrony as assessed by multi-harmonic phase analysis of gated SPECT myocardial perfusion imaging in patients with end-stage renal disease and normal LVEF. J Nucl Cardiol 2011;18:299-308. [Crossref] [PubMed]
- He Z, Garcia EV, Zhou W. Nuclear Image-Guided Methods for Cardiac Resynchronization Therapy. In: Mesquita CT, Rezende MF. editors. Nuclear Cardiology. Cham: Springer; 2021:587-608.
- Si H, He Z, Malhotra S, Zhang X, Zou F, Xue S, Qian Z, Wang Y, Hou X, Zhou W, Zou J. A novel method combining gated SPECT and vectorcardiography to guide left ventricular lead placement to improve response to cardiac resynchronization therapy: A proof of concept study. J Nucl Cardiol 2024;36:101867. [Crossref] [PubMed]
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117:2608-16. [Crossref] [PubMed]
- Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol 1990;259:H300-8. [Crossref] [PubMed]
- Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol 1999;33:1735-42. [Crossref] [PubMed]
- Wyman BT, Hunter WC, Prinzen FW, McVeigh ER. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am J Physiol 1999;276:H881-91. [Crossref] [PubMed]
- Murphy RT, Sigurdsson G, Mulamalla S, Agler D, Popovic ZB, Starling RC, Wilkoff BL, Thomas JD, Grimm RA. Tissue synchronization imaging and optimal left ventricular pacing site in cardiac resynchronization therapy. Am J Cardiol 2006;97:1615-21. [Crossref] [PubMed]
- Becker M, Franke A, Breithardt OA, Ocklenburg C, Kaminski T, Kramann R, Knackstedt C, Stellbrink C, Hanrath P, Schauerte P, Hoffmann R. Impact of left ventricular lead position on the efficacy of cardiac resynchronisation therapy: a two-dimensional strain echocardiography study. Heart 2007;93:1197-203. [Crossref] [PubMed]
- Becker M, Hoffmann R, Schmitz F, Hundemer A, Kühl H, Schauerte P, Kelm M, Franke A. Relation of optimal lead positioning as defined by three-dimensional echocardiography to long-term benefit of cardiac resynchronization. Am J Cardiol 2007;100:1671-6. [Crossref] [PubMed]
- Bilchick KC, Kuruvilla S, Hamirani YS, Ramachandran R, Clarke SA, Parker KM, Stukenborg GJ, Mason P, Ferguson JD, Moorman JR, Malhotra R, Mangrum JM, Darby AE, Dimarco J, Holmes JW, Salerno M, Kramer CM, Epstein FH. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol 2014;63:1657-66. [Crossref] [PubMed]
- Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J 2007;153:105-12. [Crossref] [PubMed]
- Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006;113:969-76. [Crossref] [PubMed]
- Adelstein EC, Tanaka H, Soman P, Miske G, Haberman SC, Saba SF, Gorcsan J 3rd. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J 2011;32:93-103. [Crossref] [PubMed]
- Yokoshiki H, Shimizu A, Mitsuhashi T, Furushima H, Sekiguchi Y, Manaka T, Nishii N, Ueyama T, Morita N, Okamura H, Nitta T, Hirao K, Okumura K. Prognostic significance of nonsustained ventricular tachycardia in patients receiving cardiac resynchronization therapy for primary prevention: Analysis of the Japan cardiac device treatment registry database. J Arrhythm 2018;34:139-47. [Crossref] [PubMed]
- Arshad A, Moss AJ, Foster E, Padeletti L, Barsheshet A, Goldenberg I, Greenberg H, Hall WJ, McNitt S, Zareba W, Solomon S, Steinberg JS; MADIT-CRT Executive Committee. Cardiac resynchronization therapy is more effective in women than in men: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol 2011;57:813-20. [Crossref] [PubMed]
- Juarez-Orozco LE, Knol RJJ, Sanchez-Catasus CA, Martinez-Manzanera O, van der Zant FM, Knuuti J. Machine learning in the integration of simple variables for identifying patients with myocardial ischemia. J Nucl Cardiol 2020;27:147-55. [Crossref] [PubMed]
- Haro Alonso D, Wernick MN, Yang Y, Germano G, Berman DS, Slomka P. Prediction of cardiac death after adenosine myocardial perfusion SPECT based on machine learning. J Nucl Cardiol 2019;26:1746-54. [Crossref] [PubMed]
- de A Fernandes F. Larsen K, He Z, Nascimento E, Peix A, Sha Q, Paez D, Garcia EV, Zhou W, Mesquita CT. A machine learning method integrating ECG and gated SPECT for cardiac resynchronization therapy decision support. Eur J Nucl Med Mol Imaging 2023;50:3022-33. [Crossref] [PubMed]
- Domingos P. A few useful things to know about machine learning. Communications of the ACM 2012;55:78-87.
- Stenwig E, Salvi G, Rossi PS, Skjærvold NK. Comparative analysis of explainable machine learning prediction models for hospital mortality. BMC Med Res Methodol 2022;22:53. [Crossref] [PubMed]
- Zhao ZW, Del Cueto M, Troisi A. Limitations of machine learning models when predicting compounds with completely new chemistries: possible improvements applied to the discovery of new non-fullerene acceptors. Digit Discov 2022;1:266-76. [Crossref] [PubMed]


