
Long-term white matter changes in patients who develop severe depression after multiple COVID-19 infections: a 3–6-month study
Introduction
Long coronavirus disease 2019 (COVID-19) refers to the long-term symptoms that last for 2 to 3 months after recovery from COVID-19. It poses a new clinical challenge in the management of COVID-19 infection. Psychological issues like depression, anxiety, sleep disorders, and cognitive impairment are common symptoms in long COVID-19 (1). Of these symptoms, depression is the most common psychological issue worldwide. In the COVID-19 pandemic, psychological issues have been a heavy burden, and an additional 53.2 million cases of major depressive disorder have been reported globally (2). Psychological symptoms can occur 4 weeks after other symptoms (3) and can persist up to 20 months after COVID-19 infection (4,5).
A dynamic change has been observed in the white matter (WM) of patients who recover from COVID-19. Huang et al. showed that compared to healthy controls, COVID-19 patients had a lower volume fraction of intracellular water in the corona radiata, corpus callosum, and superior longitudinal fasciculus (SLF) (6). After 2 years, recovered COVID-19 patients showed longitudinal recovery trends for WM, but some showed persistent WM abnormalities at 2 years after discharge (7). Benedetti et al. showed that the severity of psychopathology is closely associated with WM microstructure change. The Beck Depression Inventory was found to be negatively associated with axial diffusivity (AD) in a small cluster, including the left superior corona radiata, SLF, and posterior corona radiata (8). Impact of Event Scale-Revised scores are negatively associated with AD in several WM tracts in bilateral superior and posterior corona radiata and SLF, and also involve the inferior longitudinal fasciculus, external capsule, and anterior thalamic radiations (ATRs) (8). These studies revealed that COVID-19 is closely related to the severity of depression and WM damage. Previous studies have primarily focused on WM changes following initial COVID-19 infection. However, multiple COVID-19 infections are common, and can lead to prolonged periods of low-grade inflammation. Despite this, alterations in cerebral function and structure in individuals experiencing multiple COVID-19 infections remain unclear, and their correlation with the psychological burden has not been thoroughly investigated. Therefore, this study aimed to evaluate long-term changes in WM among patients who experienced mild to moderate depression following their initial COVID-19 infection and then developed severe depression after a subsequent infection. By doing so, we sought to identify the potential structural changes associated with psychological issues arising from multiple COVID-19 infections and gather objective evidence for the early prevention and treatment of these patients. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2168/rc).
Methods
Patients
The study design is illustrated in Figure 1. In total, 1,453 COVID-19 patients at the Shanghai Mobile Field Hospital from April to June 2022 who underwent psychological assessment for depression, and evaluation of the degree of anxiety and insomnia on the day of out-hospitalization were identified. In total, 103 patients had mild to moderate depression. Subsequently, these patients were followed-up for 3–6 months via cell phone. During the follow-up period, 13 patients were lost to follow up, 21 were not reinfected with COVID-19, and 17 did not have their infection confirmed by nucleic acid testing at tertiary hospitals, while 52 were reinfected with COVID-19 from December 2022 to January 2023. These 52 patients underwent comprehensive face-to-face psychological assessments for depression, anxiety, and insomnia within 3–6 months of their second infection. Among them, 32 patients with severe depression were allocated to the patient group and subsequently underwent magnetic resonance (MR) scans 1 week after the finial psychological assessment. Consecutive psychological outpatients at our hospital who were infected with COVID-19 between December 2022 and January 2023 were prospectively recruited for this study. These patients underwent psychological assessment for depression, anxiety, and insomnia within 3–6 months of the infection. The patients with mild to moderate depression were allocated to the control group and underwent MR scans the next day. Age-, sex-, and education-matched patients were recruited for the final analysis.
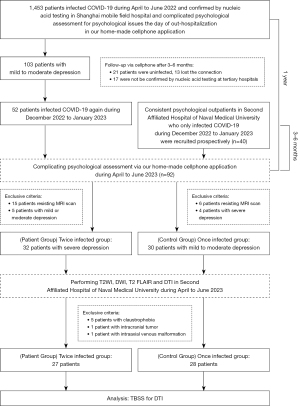
Patients were excluded from the study if they met any of the following exclusion criteria: (I) aged under 18 years old or were left handed; (II) had a previous history of neoplasm or other intracranial diseases; (III) had psychiatric complaints before the COVID-19 infection; (IV) had an organic disease or other serious substance addiction (e.g., tobacco, alcohol, or drug addiction); and/or (V) had contraindications to magnetic resonance imaging (MRI) (e.g., claustrophobia or metal implants).
Data on the age, gender, and educational background of the patients were collected. Educational background was classified as follows: below senior high school, senior high school, graduate, or post graduate. All the COVID-19 infections were confirmed by nucleic acid testing at tertiary hospitals. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Review Board of the Second Affiliated Hospital of Naval Medical University (No. 2023-L032). Verbal and written informed consent were obtained from all the patients.
Psychological assessment
The psychological assessment was conducted using Wen Juan Xing (Ranxing Information Technology Co., Ltd., Changsha, China), an online research tool that converts traditional paper scales into electronic formats. The assessments were carried out under the supervision of a psychiatrist with more than 5 years of experience to ensure the reliability and consistency of the evaluation process.
Depression symptoms were measured using the 9-item Patient Health Questionnaire (PHQ-9) from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). The PHQ-9 evaluates the severity of depression symptoms over the past 2 weeks. Each item is rated from 0 (never) to 3 (nearly every day). The total score of the PHQ-9 ranges from 0 to 27, with higher scores indicating more severe depression symptoms. Total scores of 5, 10, 15, and 20 represent the cut-off values for mild, moderate, moderate-severe, and severe depression, respectively. The PHQ-9 has excellent internal reliability, as evidenced by a Cronbach’s alpha of 0.89 in the PHQ-9 Primary Care Study and 0.86 in the PHQ-9 Ob-Gyn Study (9). Further, the test-retest reliability of the PHQ-9 is also excellent (9).
The Generalized Anxiety Disorder-7 (GAD-7) scale from the DSM-5 was used to measure anxiety symptoms. The GAD-7 scale uses 7 items to assess the frequency of anxiety symptoms over the past 2 weeks based on a 4-point Likert scale, on which 0 represents “never” and 3 represents “nearly every day”. Higher scores indicate more severe functional impairment due to anxiety. Total scores of 5, 10, and 15 represent cut-off values for mild, moderate, and severe anxiety, respectively. The internal consistency of the GAD-7 is excellent (Cronbach’s alpha: 0.92), and its test-retest reliability is good (10).
The Athens Insomnia Scale (AIS) was used to measure insomnia symptoms. This 8-item scale assesses the frequency of insomnia symptoms over the past month based on a 4-point Likert scale, on which 0 represents “never” and 3 represents “severe”. Higher scores indicate more severe functional impairment as a result of insomnia. Total scores of 4 and 6 represent cut-off values for mild and obvious insomnia, respectively. The internal consistency of the AIS is excellent (Cronbach’s alpha: 0.90), and its test-retest reliability is good (11).
MRI acquisition
Axial T2-weighted imaging (T2WI), T2-fluid attenuated inversion recovery (T2-FLAIR) imaging, and axial diffusion weighted imaging (DWI) were performed to exclude patients with tumors or other intracranial diseases. The T2WI parameters were as follows: repetition time (TR) =6505 ms, echo time (TE) =103.4 ms, slice thickness =5.0 mm, slice gaP =0 mm, number of slices =30, field of view (FOV) =24×24 cm2, matrix =512×512, and number of excitation (NEX) =1. The T2-FLAIR parameters were as follows: TR =9,000 ms, TE =122.4 ms, slice thickness =5.0 mm, slice gaP =0 mm, number of slices =30, FOV =24×24 cm2, matrix =512×512, and NEX =1. The DWI parameters were as follows: TR =3,200 ms, TE =58.2 ms, slice thickness =5.0 mm, slice gaP=0 mm, number of slices =30, matrix =256×256, and NEX =1. The diffusion tensor imaging (DTI) parameters were as follows: TR =5,500 ms, TE =57.5 ms, FOV =240×240 mm2, matrix =160×160, slice thickness =3.0 mm, number of slices =50, b value =1,000, NEX =2, and number of diffusion weighting directions =30. The DTI was performed using the multiplexed sensitivity encoding technique, which applies multi-shot along the phase encoding direction to achieve a high signal-to-noise ratio, and which is less susceptible to distorted images.
Image and statistical analyses
TBSS
Before the data analysis, two experienced neuroradiologists visually inspected the DTI images to screen for noise artifacts. The raw digital imaging and communications in medicine images were converted to neuroimaging informatics technology initiative images using dcm2niix (https://github.com/rordenlab/dcm2niix). The image data were analyzed using Tract-Based Spatial Statistics (TBSS) (12) and the Functional Magnetic Resonance Imaging of the Brain (FMRIB) software library (FSL, version 4.1.8; http://www.fmrib.ox.ac.uk/fsl). First, susceptibility distortions were corrected using the top-up tool. Second, motion and eddy current distortions were corrected using the eddy_correct tool. Third, brain masks from the b0 image of each patient were created using the FSL brain extraction tool. Fourth, the DTIFIT was used to generate the fractional anisotropy (FA), mean diffusivity (MD), AD, and radial diffusivity (RD) maps. Next, FMRIB’s nonlinear registration tool was used to align the FA, MD, AD, and RD maps of each patient to the FMRIB58_FA template in the Montreal Neurological Institute space. Using a FA threshold value of 0.2, a mean FA image was calculated from all the patients’ maps and a mean FA skeleton was subsequently generated. Next, each patient’s FA map was projected onto the skeleton. The script “tbss_non_FA” was used to obtain individual skeletonized MD, AD, and RD values. The voxel-wise differences in the skeletonized DTI metrics between the two groups were compared by two-sample t-tests. Threshold-free cluster enhancement analysis with 5,000 permutations via FSL randomize tool (version 2.1) was performed. Age and gender were added as covariates to minimize their potential effects. Statistical maps were set at P<0.05 [two-sided, family-wise error (FEW) corrected]. The brain regions showing significant differences in the DTI metrics were overlaid onto the Johns Hopkins University WM Tractography Atlas.
Statistical analysis
A chi-square test was performed for sex and education. Unpaired two-sample t-tests and Kruskal-Wallis tests (of the non-normally distributed samples) were carried out to evaluate age differences. Similarly, unpaired two-sample t-tests and Kruskal-Wallis tests (of the non-normally distributed samples) were conducted to compare the neuropsychological tests. To produce aggregate results at the subject level, post-hoc analyses were performed. For each patient, the mean levels of each diffusion metric were computed in regions that were tested for significance with TBSS. Bivariate correlation analyses, using Pearson correlation for normally distributed samples and Spearman correlation for non-normally distributed samples, were performed to assess the relationships between the DTI parameters and neuropsychological test scores among all the COVID-19 patients. Correlation coefficients of 0.2–0.4 indicated a mild relationship, 0.4–0.7 indicated a moderate relationship, and >0.7 indicated a strong relationship. All analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
Demographic and psychological characteristics
Of the initial 62 patients in both groups, 7 were excluded due to claustrophobia (n=5), intracranial tumor (n=1), and intra-axial venous malformations (n=1). Thus, 27 patients were included in the twice-infected (patient) group, and an additional 28 patients were included in the once-infected (control) group. No significant difference was observed between the two groups in terms of age (51.857±2.770 vs. 48.148±2.0678 years, P=0.222), gender (female: 75.0% vs. 55.6%, P=0.130), and educational background (P=0.317) (Table 1). No significant difference was found in the baseline PHQ-9 scores assessed after the first COVID-19 infection between the two groups (8.643±2.599 vs. 8.667±2.842, P=0.974). However, the twice-infected group had significantly higher PHQ-9, GAD-7, and AIS scores than the once-infected group (all P<0.001) (Table 1).
Table 1
| Clinical characteristics | Twice-infected group (n=27) | Once-infected group (n=28) | P value |
|---|---|---|---|
| Age (years) | 51.857±2.770 | 48.148±2.0678 | 0.222 |
| Gender | 0.130 | ||
| Female | 15 | 21 | |
| Male | 12 | 7 | |
| Educational background | 0.317 | ||
| Below senior high school | 1 | 1 | |
| Senior high school | 19 | 16 | |
| Graduate | 6 | 9 | |
| Above graduate | 1 | 2 | |
| PHQ | 17.29±2.600 | 8.64±2.599 | <0.001 |
| GAD | 15.44±3.490 | 7.64±2.376 | <0.001 |
| AIS | 15.852±3.219 | 10.179±2.763 | <0.001 |
Data are presented as mean ± SD or number. AIS, Athens Insomnia Scale; GAD, Generalized Anxiety Disorder; PHQ, Patient Health Questionnaire; SD, standard deviation.
WM
Compared to the once-infected group, the twice-infected group had decreased FA values in several white matter tracts (WMTs) (PFWE<0.05), including the bilateral corticospinal tracts (CSTs), ATRs, and right SLF (Figure 2) (Table 2) (PFWE<0.05). However, no significant differences were observed between the two groups in terms of MD, RD, and AD.
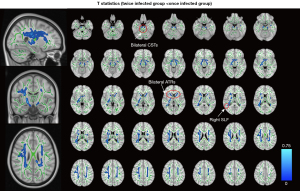
Table 2
| Cluster index | Side | Brain regions (JHU) | Voxel size | MNI coordinate | P value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 1 | R | Corticospinal tract | 2,454 | 35 | –47 | 10 | 0.023 |
| 2 | L | Corticospinal tract | 1,300 | –26 | 14 | 28 | 0.025 |
| 3 | R | Anterior thalamic radiation | 1,264 | 18 | –11 | –6 | 0.038 |
| 4 | R | Anterior thalamic radiation | 225 | 21 | 17 | 7 | 0.048 |
| 5 | L | Corticospinal tract | 221 | –6 | –19 | –24 | 0.048 |
| 6 | L | Anterior thalamic radiation | 172 | –12 | 9 | 2 | 0.046 |
| 7 | R | Superior longitudinal fasciculus | 129 | 36 | –11 | 27 | 0.049 |
| 8 | R | Anterior thalamic radiation | 21 | 10 | 20 | 22 | 0.050 |
| 9 | R | Anterior thalamic radiation | 11 | 22 | 16 | 16 | 0.050 |
| 10 | R | Corticospinal tract | 6 | 13 | –11 | –13 | 0.050 |
FA, fractional anisotropy; JHU, Johns Hopkins University White-Matter Tractography Atlas; L, left; MNI, Montreal Neurological Institute space; R, right.
Correlation between FA and psychological scores
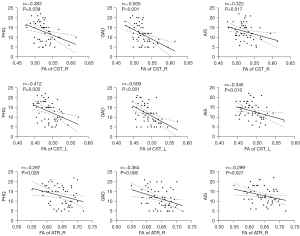
The twice-infected group had significantly lower FA values than the once-infected group in the whole region (0.534±0.015 vs. 0.561±0.015, P<0.001). All the psychological scores were mildly to moderately negatively correlated with FA (r=−0.491, r=−0.570, and r=−0.355, respectively, with P<0.001, P<0.001, and P=0.008, respectively). Further, the FA values of the CST_L, CST_R, and ATR_R were notably lower in the twice-infected group than the once-infected group (Figure 3). Additionally, the psychological scores were mildly to moderately negatively correlated with FA in CST_L, CST_R, and ATR_R (Figure 4). However, no significant correlations were found when the analysis was conducted separately within each group.

Discussion
The present study found a discernible impairment in the integrity of WM 3–6 months after the mild depression patients developed severe depression following another COVID-19 infection. Specifically, decreased FA was evident in the bilateral CSTs, ATRs, and right SLF. The identified abnormalities in these brain regions may provide valuable insights into potential mechanisms underlying the progression from mild or moderate to severe depression after a second COVID-19 infection. Given these findings, depression complaints from individuals recovering from COVID-19 should be taken seriously, as such patients show long-term WM changes. Timely psychological intervention is necessary to address and mitigate potential psychological sequelae in this patient population.
The patients who developed severe depression in the present study had decreased FA values for the bilateral CSTs, ATRs, and right SLF. A decreased FA indicates the deterioration of axonal myelin sheaths, which impairs the integrity of WM. Previous research has shown WM involvement in COVID-19 infection (13), with new lesions detected by follow-up MRI in recovered patients (14). Huang et al. reported a lower intracellular volume fraction in specific WM regions, including the bilateral corona radiata (anterior and superior parts), genu of the corpus callosum, and SLF, even one year after recovery. Interestingly, WM tends to display fewer abnormalities in cases with shorter hospital stays and longer follow-up times. Huang et al. reported a correlation between the severity of the condition and greater microstructural changes in WM (6), which is consistent with the findings of our study. Our results also highlighted the enduring effect of COVID-19 on WM integrity. After the second infection, the patients who developed depression had more pronounced impairment in WM integrity. Previous findings on individuals with major depressive disorder have reported damage in the left SLF and right ATR (15). The effect of COVID-19-related depression on these specific brain regions suggests a potential structural basis for the occurrence and progression of COVID-19-related depression. Notably, the bilateral CSTs had decreased FA in the patient group, which is not common in patients solely experiencing depression or anxiety. A study on COVID-19 also reported the involvement of the CST, and documented a decrease in the CST 10 months post-recovery (16). CST is the major neural tract in the human brain for motor function, and notably facilitates the movement of the distal extremities such as the hands (17). Our results showed that multiple COVID-19 infections may induce damage in the CST. However, we did not analyze motor function deficits; thus, further research needs to be conducted.
Unlike the localized impairment of GM typically observed near the frontal lobe or the limb system, such as in the insular, olfactory cortices, hippocampi, Heschl’s gyrus, and cingulate (18), the range of impaired WM appears to be more extensive. The exact mechanism underlying WM damage following COVID-19 infection remains unclear. Huang et al. proposed hypoxic-ischemic changes as the potential mechanism for WM damage, rather than inflammatory storms, based on the lesion locations (7). However, according to previous studies, systemic immune dysregulation contributes to DTI signs of widespread WM microstructure abnormalities in major depressive disorder (19,20). The interaction between innate and adaptive immune systems, as well as neurotransmitters, has emerged as a mechanism underlying mood disorders, psychosis, and anxiety disorders. Interlukin-1β, a potent pro-inflammatory cytokine crucial for host defense against infections and injuries, has been found to have a negative relationship with FA in major depressive disorder (21,22). Additionally, chronic and low-grade inflammation has been linked to a decrease in WM integrity in depressed patients (20). The persistent and chronic inflammation of the physical condition of patients with multiple COVID-19 infections over several months may cause severe and broader damage of WMTs in certain individuals.
To the best of our knowledge, this research represents the first attempt to explore the relationship between the frequency of COVID-19 infection and WM change. The study found long-term objective alterations or damage in cerebral function and microstructure in patients with severe depression following multiple COVID-19 infections. These findings emphasize the need for timely psychological intervention for individuals reporting depression after a COVID-19 infection and those at risk of multiple infections. Multiple infections accompanied by depression should be treated with the utmost seriousness. However, the study had some limitations. First, it was not a longitudinal study, and another group was used to reveal the baseline cerebral function and structure change in patients with severe depression following additional COVID-19 infection. Second, the sample size was small, emphasizing the need for investigation in a larger clinical cohort with a longitudinal approach. Third, due to the widespread nature of COVID-19 infections in the general population, it was challenging to prospectively recruit patients without COVID-19 infection as a “true” control group. However, as mentioned above, previous studies had revealed that compared to healthy control patients, COVID-19 patients show WM damage in several regions, such as the corona radiata, corpus callosum, SLF (6), and ATR (8). This damage persists 1 to 2 years after discharge from hospital (7,12,13). Our results showed that the bilateral CSTs, ATRs, and right SLF were more severely impaired 3 to 6 months after patients progressed from mild to severe depression following another COVID-19 infection. However, if retrospective patient datasets collected before the COVID-19 infection are available, further investigation is encouraged to provide a more comprehensive understanding of the observed changes.
Conclusions
The present study showed that individuals with severe depression following multiple COVID-19 infections show widespread and profound WM damage, and this damage is negatively correlated with the severity of depression. The findings provide evidence of objective damage in cerebral microstructure, highlighting the necessity of timely psychological intervention for individuals at risk of multiple COVID-19 infections to prevent worsening psychological issues and cerebral microstructure changes.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2168/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2168/coif). Y.C. received funding for this study from the National Natural Science Foundation of China (grant No. 92259203). Y.X. received funding for this study from the National Natural Science Foundation of China (grant No. 82271994) and the National Health Commission Radiological Imaging Database Construction Project (grant No. YXFSC2022JJSJ010). S.L. received funding for this study from the National Key R&D Program of China (grant No. 2022YFC2010000), the Key Program of National Natural Science Foundation of China (grant No. 81930049), and the Shanghai Science and Technology Innovation Action Plan Program (grant No. 19411951300). J.D. is an employee of GE Healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Review Board of the Second Affiliated Hospital of Naval Medical University (No. 2023-L032). Verbal and written informed consent were obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davido B, Seang S, Tubiana R, de Truchis P. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect 2020;26:1448-9. [Crossref] [PubMed]
- Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021;398:1700-12. [Crossref] [PubMed]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601-15. [Crossref] [PubMed]
- Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604-15. [Crossref] [PubMed]
- Zhao Y, Shi L, Jiang Z, Zeng N, Mei H, Lu Y, et al. The phenotype and prediction of long-term physical, mental and cognitive COVID-19 sequelae 20 months after recovery, a community-based cohort study in China. Mol Psychiatry 2023;28:1793-801. [Crossref] [PubMed]
- Huang S, Zhou Z, Yang D, Zhao W, Zeng M, Xie X, Du Y, Jiang Y, Zhou X, Yang W, Guo H, Sun H, Liu P, Liu J, Luo H, Liu J. Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain 2022;145:1830-8. [Crossref] [PubMed]
- Huang S, Zhou X, Zhao W, Du Y, Yang D, Huang Y, Chen Y, Zhang H, Yang G, Liu J, Luo H. Dynamic white matter changes in recovered COVID-19 patients: a two-year follow-up study. Theranostics 2023;13:724-35. [Crossref] [PubMed]
- Benedetti F, Palladini M, Paolini M, Melloni E, Vai B, De Lorenzo R, Furlan R, Rovere-Querini P, Falini A, Mazza MG. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: A multimodal magnetic resonance imaging study. Brain Behav Immun Health 2021;18:100387. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Journal of General Internal Medicine 2001;16:606-13. [Crossref] [PubMed]
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. [Crossref] [PubMed]
- Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 2000;48:555-60. [Crossref] [PubMed]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487-505. [Crossref] [PubMed]
- Ibrahim I, Škoch A, Dezortová M, Adla T, Flusserová V, Nagy M, Douchová I, Fialová M, Filová V, Pajuelo D, Ibrahimová M, Tintěra J. Evaluation of microstructural brain changes in post-coronavirus disease 2019 (COVID-19) patients with neurological symptoms: a cross-sectional study. Quant Imaging Med Surg 2024;14:5499-512. [Crossref] [PubMed]
- Hellgren L, Birberg Thornberg U, Samuelsson K, Levi R, Divanoglou A, Blystad I. Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open 2021;11:e055164. [Crossref] [PubMed]
- Lai CH, Wu YT. Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychol Med 2014;44:2825-32. [Crossref] [PubMed]
- Tian T, Wu J, Chen T, Li J, Yan S, Zhou Y, Peng X, Li Y, Zheng N, Cai A, Ning Q, Xiang H, Xu F, Qin Y, Zhu W, Wang J. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight 2022;7:e155827. [Crossref] [PubMed]
- Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med 2014;46:193-9. [Crossref] [PubMed]
- Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020;25:100484. [Crossref] [PubMed]
- Aronica R, Enrico P, Squarcina L, Brambilla P, Delvecchio G. Association between Diffusion Tensor Imaging, inflammation and immunological alterations in unipolar and bipolar depression: A review. Neurosci Biobehav Rev 2022;143:104922. [Crossref] [PubMed]
- Green C, Shen X, Stevenson AJ, Conole ELS, Harris MA, Barbu MC, et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav Immun 2021;92:39-48. [Crossref] [PubMed]
- Sugimoto K, Kakeda S, Watanabe K, Katsuki A, Ueda I, Igata N, Igata R, Abe O, Yoshimura R, Korogi Y. Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Transl Psychiatry 2018;8:141. [Crossref] [PubMed]
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018;281:8-27. [Crossref] [PubMed]


