
Free-breathing pediatric cardiac dark-blood imaging with reverse double inversion-recovery and single-shot deep learning reconstruction
Introduction
Magnetic resonance imaging (MRI) is crucial for providing non-invasive, high-resolution diagnostic imaging without ionizing radiation, making it particularly valuable for pediatric patients, who are more sensitive to radiation and often require multiple imaging studies. However, pediatric imaging presents unique challenges compared to adult imaging, including differences in anatomy, physiology, and the need for sedation to manage motion artifacts (1). Recent advancements in pediatric MRI have focused on developing faster imaging techniques and integrating artificial intelligence (AI) to enhance image quality and reduce the need for sedation (2-6).
Dark-blood T2-weighted fast spin-echo (DB-FSE) is a routinely used sequence for assessing myocardial edema (7-10). This sequence usually acquires k-space data from multi-shot acquisitions across multiple cardiac cycles with breath-holding (11). However, it is well-known that the DB-FSE sequence is very sensitive to arrhythmia, cardiac motion, and respiratory motion (12,13). For pediatric imaging, commonly with high heart rates and free-breathing, DB-FSE is challenging and often yields suboptimal image quality due to the presence of both cardiac and respiratory motion. Therefore, improving the image quality of free-breathing DB-FSE is important for edema assessment in pediatric imaging.
Cardiac motion is a major cause of the signal dropout artifacts in DB-FSE, due to two different reasons. One key reason is the slice misregistration between the double inversion recovery (DIR) preparation pulse and the FSE echo train, which falsely nulls the out-of-slice myocardial spins and causes signal reduction (14). Reverse DIR (RDIR) is a relatively new technique that changes the slice-selective inversion pulse to mid-diastole in the previous cardiac cycle, thereby reducing the signal dropout artifacts (15). Although a previous study (15) has validated the effects of RDIR in adults, how RDIR performs in pediatric patients remains unclear. The second reason for signal dropout is the motion sensitivity of the FSE echo train (16). As the heart is moving, spins in a long echo train can gradually move out of slice and cause loss of refocusing, eventually leading to signal reduction.
Respiratory motion causes ghosting artifacts in the presence of multi-shot acquisition. Single-shot acquisition cannot only reduce the scanning time but also greatly reduce these ghosting artifacts (11). However, traditional single-shot DB-FSE, such as half-Fourier acquisition single-shot turbo spin-echo (HASTE) (17-19), often has a suboptimal image resolution that is required for imaging of young patients. Recently, AI-assisted compressed sensing (ACS) has been proposed to accelerate MRI (20). Specifically, this method introduces AI as a regularization term into the compressed sensing (CS) reconstruction model (21-23) to maintain high image quality at a high acceleration factor. ACS has been validated in several applications, including lumbar spine imaging (24), knee imaging (25,26), kidney imaging (27), anal fistula imaging (28), liver imaging (29,30), and breath-hold dark blood T2-weighted imaging (11). However, the effect of ACS in free-breathing pediatric imaging remains underexplored.
In this work, we aimed to achieve high-resolution and motion-robust edema assessment in free-breathing pediatric cardiac dark-blood imaging by combining RDIR and single-shot DB-FSE enhanced by ACS reconstruction. To validate the proposed method, we compared its image quality with that of multi-shot RDIR DB-FSE and routine multi-shot DIR DB-FSE in 20 healthy children and 47 pediatric patients. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1933/rc).
Methods
Pulse sequence
Multi-shot DB-FSE based on DIR (MS-DIR)
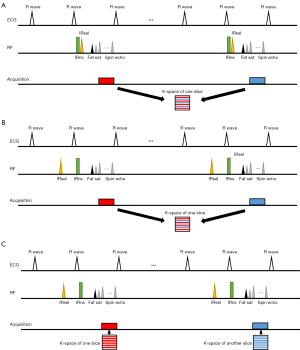
Figure 1A shows the sequence diagram of routine MS-DIR. In standard DIR FSE, the nonselective IR (IRns) and the slice-selective IR (IRsel) are sequentially performed in early systole, whereas the fat sat module and FSE readout sequentially lie in the late-diastole. In multi-shot acquisition, a total of 4–5 shots are acquired over multiple heartbeats. Acquisition is performed every two heartbeats to allow for signal recovery.
Multi-shot DB-FSE based on RDIR (MS-RDIR)
Figure 1B shows the sequence diagram of MS-RDIR. In RDIR, the IRsel is performed in the cardiac cycle before the IRns to eliminate the timing mismatch between IRsel and the FSE echo train, thereby minimizing the risk of slice misregistration. The total acquisition time of MS-RDIR is the same as that of MS-DIR.
Single-shot DB-FSE based on RDIR (SS-RDIR)
Figure 1C shows the sequence diagram of SS-RDIR. A total of 46–52 lines are acquired for each slice in the single-shot acquisition, reducing the total acquisition shot number to 1 shot.
To preserve the image quality at a highly accelerated condition, ACS is applied to reconstruct the image for SS-RDIR. Specifically speaking, the ACS method firstly trained a convolutional neural network (CNN) via a substantial dataset comprising two million fully sampled images to reduce artifacts and noise in an image reconstructed from highly undersampled k-space. A routine CS algorithm is then run with the network output as an additional constraint for the image estimate. The result of the CS algorithm is then used as the final reconstruction. Validation from a retrospective MRI study with 6,066 cases demonstrated its capability to handle 3–10-fold undersampled k-space reconstruction while maintaining high image quality. A detailed description of the ACS method can be found in reference (20).
Participant population
This prospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments and approved by the institutional ethics board of the Children’s Hospital of Fudan University (approval No. 2024164). Written informed consent was provided by all participants’ legal guardians. From January 2024 to September 2024, 20 healthy children (age range, 1–16 years) and 47 pediatric patients (age range, 0.5–15 years) were consecutively included in this study. To ensure the adequacy of our sample size for the patient study, we performed a sample size computation using G*Power (version 3.1.9.7; Heinrich-Heine-University, Düsseldorf, Germany) based on results obtained from the healthy children study.
Image acquisition
All patients were imaged on a 3T scanner (uMR790, United Imaging Healthcare, Shanghai, China) with a 24-channel body coil and a 24-channel spine coil in Children’s Hospital of Fudan University. Three sequences (MS-DIR, MS-RDIR, and SS-RDIR) were performed during free-breathing in all children. Anywhere from five to 11 slices were acquired in different children for each protocol to cover the entire left ventricle (LV). Three averages were used to reduce motion artifacts for all methods. The combined acceleration factor and echo train length (ETL) were increased to achieve high-resolution single-shot imaging in SS-RDIR. Meanwhile, the bandwidth of SS-RDIR was set to 700 Hz/pixel to balance the echo spacing and image SNR. The parameters of the three sequences are detailed in Table 1.
Table 1
| Parameters | MS-DIR | MS-RDIR | SS-RDIR |
|---|---|---|---|
| FOV, mm2 | 360×260/320 | 360×260/320 | 360×260 |
| Voxel size, mm2 | 1.4×1.76 | 1.4×1.76 | 1.4×1.76 |
| Slice thickness, mm | 6.0 | 6.0 | 6.0 |
| Bandwidth, Hz/pixel | 400 | 400 | 700 |
| Echo train length | 25 | 25 | 46–52 |
| Echo spacing, ms | 5.62 | 5.62 | 3.84 |
| TE, ms | 67.4 | 67.4 | 84.5–96 |
| Flip angle, ° | 120 | 120 | 120 |
| Number of average | 3 | 3 | 3 |
| Combined acceleration factor | 1.8 | 1.8 | 3 |
| Shot number (range) | 4–5 | 4–5 | 1 |
| Acquisition slices (range) | 5–11 | 5–11 | 5–11 |
| Scan time/slice, heartbeats (range) | 24–30 | 24–30 | 6 |
| Total scan time, heartbeats (range) | 120–330 | 120–330 | 30–66 |
DB-FSE, dark-blood T2-weighted fast spin-echo; DIR, double inversion recovery; FOV, field of view; MS-DIR, multi-shot DB-FSE based on DIR; MS-RDIR, multi-shot DB-FSE based on RDIR; RDIR, reverse double inversion recovery; SS-RDIR, single-shot DB-FSE based on RDIR; TE, echo time.
Image analysis
Several quantitative indexes were evaluated to compare MS-DIR, MS-RDIR, and SS-RDIR, including myocardial signal-to-noise ratio (SNR), myocardium-to-blood contrast-to-noise ratio (CNR), and the contrast ratio (CR) between myocardium and blood. The myocardium and blood pool regions of interest (ROIs) were carefully contoured by two readers [Y.E. and Z.C., with 3 and 4 years, respectively, of cardiac magnetic resonance (MR) experience]. SNR was calculated based on the average signal of the LV wall relative to the standard deviation (SD) of the blood pool ROI, which was placed in the middle of the pool to exclude the slow-flow-induced bright-rim area. Myocardium-to-blood CNR was calculated by (the average signal of the LV wall − the average signal of the blood pool)/SD of the blood pool. CR was calculated by the average signal of the LV wall relative to the average signal of the blood pool. The quantitative analysis from two readers was averaged to generate the final results.
Qualitative scoring was also performed to evaluate the performance of each imaging method. The patient images were blindly and independently evaluated by an MR physicist and a radiologist (C.H. and X.H.) both with more than 10 years of experience in cardiovascular MRI, respectively. Three criteria, namely the myocardial visibility, ghosting artifacts, and overall image quality were graded with a 5-point scale (1: non-diagnostic; 2: poor; 3: fair; 4: good; 5: excellent). The scores from the two readers were averaged to obtain the final score.
Statistical analysis
Statistical analysis was performed with MATLAB (version 2022b; MathWorks, Natick, MA, USA) and SPSS (version 26.0; IBM Corp., Armonk, NY, USA). All measurement data were described as the mean ± SD and categorical data were presented as the percentage. The normality was assessed using the Shapiro-Wilk test, and the homogeneity of variance was assessed using Levene test. For quantitative parameters, one-way analysis of variance (ANOVA) and post hoc test were used to assess the differences among the three imaging methods. For qualitative comparisons, the Kruskal-Wallis test was used due to non-normality of the data. The Bonferroni correction was applied as a P value adjustment when multiple comparisons were made. The inter-observer agreement was evaluated by the intraclass correlation coefficient (ICC). Statistical significance was considered when P<0.05.
Results
The three sequences were successfully performed in 20 healthy individuals and 47 patients. The demographic information and the characteristics of patients are listed in Table 2.
Table 2
| Clinical characteristics | Healthy children (n=20) | Pediatric patients (n=47) |
|---|---|---|
| Sex | ||
| Female | 10 | 17 |
| Male | 10 | 30 |
| Age, years | 8±4 | 8±3 |
| Height, cm | 127.3±32.2 | 129.1±30.0 |
| Weight, kg | 34±17 | 33±16 |
| R-R interval, ms | 810±146 | 755±131 |
| Clinical diagnosis | ||
| Congenital heart disease | – | 12 |
| Arrhythmia | – | 12 |
| Kawasaki disease | – | 11 |
| Myocarditis | – | 7 |
| Cardiomyopathy | – | 3 |
| Anemia | – | 1 |
| Heart failure | – | 1 |
The data are described as the number or mean ± standard deviation.
Healthy children study
Figure 2 shows the example of the MS-DIR, MS-RDIR, and SS-RDIR images acquired in a 16-year-old healthy girl. The MS-DIR images were contaminated by strong signal dropout (yellow arrowheads). Meanwhile, the MS-DIR and MS-RDIR images were contaminated by motion-related ghosting artifacts (green arrowheads). These artifacts were considerably suppressed in SS-RDIR.

Table 3 shows the quantitative evaluation and qualitative comparison among MS-DIR, MS-RDIR, and SS-RDIR in 20 healthy children. The total acquisition time (seconds) of SS-RDIR (64.8±21.8) was significantly reduced compared to MS-DIR (222.5±69.3, P<0.001) and MS-RDIR (234.0±64.1, P<0.001). The SNR and CNR were comparable across all three techniques (P=0.094 for SNR and P=0.054 for CNR). However, the CR of MS-RDIR (11.6±3.6) was significantly lower than MS-DIR (14.5±5.7, P=0.002) and SS-RDIR (16.4±5.0, P<0.001). There was no significant difference between MS-DIR and SS-RDIR in terms of CR. Qualitative comparison showed a significant reduction of ghosting artifacts in SS-RDIR (4.70±0.18) compared to MS-DIR (3.95±0.28, P<0.001) and MS-RDIR (3.97±0.31, P<0.001), and a significant improvement of overall quality in SS-RDIR (4.42±0.19) compared to MS-DIR (3.87±0.27, P=0.004) and MS-RDIR (3.95±0.34, P=0.010).
Table 3
| Variables | MS-DIR | MS-RDIR | SS-RDIR | P value |
|---|---|---|---|---|
| Total acquisition time, s | 222.5±69.3 | 234.0±64.1 | 64.8±21.8†,‡ | <0.001 |
| SNR | 82.1±27.1 | 75.7±25.3 | 86.9±21.4 | 0.094 |
| CNR | 76.1±26.9 | 69.1±24.9 | 81.6±26.9 | 0.054 |
| CR | 14.5±5.7 | 11.6±3.6† | 16.4±5.0‡ | <0.001 |
| Myocardial visibility | 4.40±0.31 | 4.62±0.23 | 4.40±0.30 | 0.219 |
| Ghosting artifacts | 3.95±0.28 | 3.97±0.31 | 4.70±0.18†,‡ | <0.001 |
| Overall quality | 3.87±0.27 | 3.95±0.34 | 4.42±0.19†,‡ | <0.001 |
Data are presented as the mean ± standard deviation. †, P<0.05 of the MS-RDIR or SS-RDIR methods, as compared to the MS-DIR method; ‡, P<0.05 between the MS-RDIR and SS-RDIR methods. CNR, contrast-to-noise ratio; CR, contrast ratio; DB-FSE, dark-blood T2-weighted fast spin-echo; DIR, double inversion recovery; MS-DIR, multi-shot DB-FSE based on DIR; MS-RDIR, multi-shot DB-FSE based on RDIR; RDIR, reverse double inversion recovery; SNR, signal-to-noise ratio; SS-RDIR, single-shot DB-FSE based on RDIR.
Patient study
Since the main objective of the study was to show a higher overall quality from the proposed approach, we calculated the minimum sample size for the patient study using the overall quality results obtained from the healthy children. With a type-I error set at 0.05 and a power of 0.80, the minimum sample size was 16. Thus, we had a sufficient sample size in our study for the patient analysis conducted.
Figure 3 shows the example of the MS-DIR, MS-RDIR, and SS-RDIR images acquired from a 12-year-old boy with congenital heart disease. The MS-DIR and MS-RDIR images also exhibited signal dropout (yellow arrowheads) or ghosting artifacts (green arrowheads). In contrast, the SS-RDIR images showed significantly fewer artifacts, highlighting superior image quality. More examples can be found in Figures S1,S2.

Figure 4 shows the quantitative evaluation among MS-DIR, MS-RDIR, and SS-RDIR in 47 patients. The total acquisition time (seconds) of SS-RDIR (62.1±24.0) was significantly shorter than that of MS-DIR (216.6±74.2; P<0.001) and MS-RDIR (229.8±79.2; P<0.001). There was no significant difference among MS-DIR, MS-RDIR, and SS-RDIR in terms of SNR (P=0.065) and CNR (P=0.089); however, the CR of MS-RDIR (11.3±3.8) trended lower than that of MS-DIR (13.7±5.1, P=0.070) and significantly lower than that of SS-RDIR (13.9±5.9, P=0.041). There was no significant difference between MS-DIR and SS-RDIR in terms of CR.

Figure 5 shows the score distribution comparison and qualitative comparison among MS-DIR, MS-RDIR, and SS-RDIR images of 47 patients, on a scale of 1 (worst) to 5 (best). For myocardial visibility, MS-RDIR obtained the highest score, but there was no significant difference among MS-DIR, MS-RDIR, and SS-RDIR (3.98±0.42 vs. 4.38±0.35 vs. 3.96±0.44, P=0.053). For ghosting artifacts, three techniques had significant differences (P<0.001), and paired analysis showed that SS-RDIR scored significantly higher than MS-DIR (4.94±0.11 vs. 3.77±0.29, P<0.001) and MS-RDIR (3.78±0.34, P<0.001). There was no significant difference between MS-DIR and MS-RDIR in terms of ghosting artifacts. For overall quality, three techniques had significant differences (P=0.026), and MS-DIR scored similar with MS-RDIR (3.61±0.40 vs. 3.88±0.41, P=0.251) and significantly lower than SS-RDIR (3.96±0.52, P=0.023). The overall quality score was not significantly different between MS-RDIR and SS-RDIR.

Table 4 shows the ICCs for different comparisons. Both qualitative and quantitative comparisons had good agreement among the two readers (ICC 0.00–0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60; moderate agreement; 0.61–0.80, good agreement; greater than 0.81, excellent agreement) (27).
Table 4
| Variables | MS-DIR (95% CI) | MS-RDIR (95% CI) | SS-RDIR (95% CI) |
|---|---|---|---|
| Myocardial visibility | 0.83 (0.69, 0.90) | 0.75 (0.55, 0.86) | 0.89 (0.81, 0.94) |
| Ghosting artifacts | 0.71 (0.47, 0.84) | 0.85 (0.73, 0.92) | 0.71 (0.49, 0.84) |
| Overall quality | 0.83 (0.65, 0.91) | 0.87 (0.75, 0.93) | 0.89 (0.81, 0.94) |
| SNR | 0.62 (0.31, 0.79) | 0.65 (0.37, 0.81) | 0.72 (0.49, 0.84) |
| CNR | 0.66 (0.37, 0.81) | 0.68 (0.41, 0.82) | 0.73 (0.53, 0.85) |
| CR | 0.81 (0.63, 0.90) | 0.84 (0.71, 0.91) | 0.87 (0.76, 0.93) |
CI, confidence interval; CNR, contrast-to-noise ratio; CR, contrast ratio; DB-FSE, dark-blood T2-weighted fast spin-echo; DIR, double inversion recovery; ICC, intraclass correlation coefficient; MS-DIR, multi-shot DB-FSE based on DIR; MS-RDIR, multi-shot DB-FSE based on RDIR; RDIR, reverse double inversion recovery; SNR, signal-to-noise ratio; SS-RDIR, single-shot DB-FSE based on RDIR.
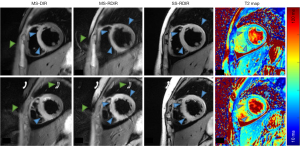
Figure 6 shows two slices of the MS-DIR, MS-RDIR, SS-RDIR, and T2 maps acquired in a 14-year-old girl with an indication of myocarditis. The MS-DIR indicated that the edema was mainly in the septum. However, the MS-RDIR and SS-RDIR showed that the edema was in the anterior wall, septal wall, and inferior wall (blue arrowheads). The locations of edema seen in the MS-RDIR and SS-RDIR images were better matched with the elevated T2 in the T2 maps. Green arrowheads point to the ghosting artifacts that were absent in the SS-RDIR images. The average T2 values in the diseased (red contour) and remote myocardium (white contour) were 48 and 43 ms for the first slice, and 56 and 44 ms for the second slice, respectively.
Discussion
In this study, we propose a new framework based on a combination of RDIR dark-blood preparation and deep learning-assisted reconstruction for improved free-breathing DB-FSE in pediatric imaging. Compared with routine DIR multi-shot DB-FSE, the use of RDIR and single-shot acquisition with ACS reconstruction significantly reduces the signal dropout and respiratory ghosting artifacts, thereby improving the overall image quality. The study shows the potential of the SS-RDIR method to achieve high-resolution and motion-robust edema assessment during free-breathing.
The heart rates of children are generally higher than those of adults, and to some extent, children are unable to control their breathing. Therefore, free-breathing with multiple averages is often used in practice for pediatric imaging. However, although routinely used, DB-FSE is very sensitive to cardiac and respiratory motion, leading to severe signal dropout artifacts and ghosting artifacts during free-breathing. From the results, our method offers several advantages. Firstly, the use of the RDIR technique in pediatric imaging can reduce the risk of signal dropout compared with DIR. This is evident from the comparison of myocardial visibility scores between MS-DIR and MS-RDIR. Reduction of signal dropout is critical to the diagnosis of edema, as regional dropout may render the normal-intensity region seemingly hyper-enhanced, potentially causing false positives if judgement is solely based on the image features (31-33). Secondly, the single-shot acquisition can greatly reduce ghosting artifacts caused by inter-shot respiratory motion. This is evident from the large improvement of ghosting score for SS-RDIR relative to the other two methods. The reduction of signal dropout and ghosting artifacts also improved the overall image quality of the proposed methods relative to MS-DIR. Thirdly, SS-RDIR has the shortest scanning time (30–60 seconds) compared to MS-DIR and MS-RDIR (3–5 minutes). This large reduction of scan time substantially improves patient comfort and imaging efficiency.
This is the first study to validate the performance of ACS reconstruction in pediatric imaging during free-breathing. Previous studies had reported that the ACS reconstruction significantly shortened the imaging time by reducing the number of breath-holds (11) or decreasing the total breath-hold time (11,29,30). Our study shows a new application of the ACS reconstruction to fulfill single-shot acquisition in a free-breathing setting. Despite the 3-fold acceleration, we did not find any noticeable aliasing artifacts from the ACS-accelerated SS-RDIR imaging. The method can also be combined with other imaging sequences, such as late gadolinium enhancement (LGE), to improve their motion robustness during free-breathing.
Compared with previous DB-FSE studies (11,15), our study has the following innovations. First, we combined sequence optimization and the reconstruction method to optimize dark-blood imaging effect while reducing imaging time. Our results demonstrated that this optimization scheme maintains high image quality, even in children with high heart rates. Second, this is the first AI-accelerated dark-blood imaging study to be conducted under free breathing. Our results showed that the DB-FSE can achieve high-quality images with minimal motion artifacts, even in the free-breathing state.
Limitations
Firstly, the method was only validated in 20 healthy individuals and 47 patients. Further larger studies involving more patients with myocardial edema are needed to fully verify the value of the method in clinical pediatric imaging. Secondly, a main limitation of the proposed technique is that the echo train for the single-shot FSE acquisition, even after the use of ACS, remains quite long (46–52 echoes, 176.64–199.68 ms). This longer echo train increases the risk of the latter echoes on loss of spin refocusing, since they may move out of the excitation slice due to cardiac motion. The high heart rate of children exacerbates the problem, which explains why the myocardial visibility of SS-RDIR is lower than that of MS-RDIR. Future research should focus on how to leverage deep learning or other techniques for even higher accelerations of DB-FSE, so that the ETL can remain similar to that of multi-shot imaging. To that end, a variety of acceleration methods, based on, for example, super resolution (34-36) or unsupervised learning (37,38), await to be investigated. Another limitation is that we did not explore registration of the three source images before averaging. Performing registration before averaging may improve the image quality, especially for the single-shot acquisition, yet this was not explored. Finally, the technique was only validated in children, although the technique should be also applicable for adult patients with its current setting. Investigation of this topic is highly warranted in future studies.
Conclusions
RDIR reduces signal dropout artifacts in free-breathing DB-FSE imaging, and the single-shot acquisition with ACS reconstruction reduces ghosting artifacts. The proposed framework that combines RDIR and single-shot ACS-accelerated acquisition shows an improvement of overall image quality despite high heart rate and free-breathing. These improvements could directly enhance diagnostic confidence in cardiac dark-blood pediatric imaging, making high-quality and motion-robust imaging more accessible in clinical settings. Further technical development of acceleration methods is needed to shorten the ETL. Employment of these techniques is promising to enhance image quality and reduce the need for sedation in pediatric patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1933/rc
Funding: This work was funded in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1933/coif). J.H. is a current employee of Shanghai United Imaging Healthcare Co. Ltd. C.H. reports that this work was partially supported by the National Key R&D Program of China (No. 2024YFF0509200), National Natural Science Foundation of China (No. 62001288), and the Shanghai Science and Technology Commission (No. 22TS1400200). X.H. reports this work was partially supported by the Children’s Hospital of Fudan University Med-X Program (No. EKYY202425). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics board of Children’s Hospital of Fudan University (approval No. 2024164). Written informed consent was provided by all participants’ legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kibrom BT, Manyazewal T, Demma BD, Feleke TH, Kabtimer AS, Ayele ND, Korsa EW, Hailu SS. Emerging technologies in pediatric radiology: current developments and future prospects. Pediatr Radiol 2024;54:1428-36. [Crossref] [PubMed]
- Banka P, Geva T. Advances in pediatric cardiac MRI. Curr Opin Pediatr 2016;28:575-83. [Crossref] [PubMed]
- Dong SZ, Zhu M, Bulas D. Techniques for minimizing sedation in pediatric MRI. J Magn Reson Imaging 2019;50:1047-54. [Crossref] [PubMed]
- Kozak BM, Jaimes C, Kirsch J, Gee MS. MRI Techniques to Decrease Imaging Times in Children. Radiographics 2020;40:485-502. [Crossref] [PubMed]
- Hirsch FW, Frahm J, Sorge I, Klee D, Prenzel F, Krause M, Lacher M, Voit D, Gräfe D. Real-time MRI: a new tool of radiologic imaging in small children. Eur J Pediatr 2023;182:3405-17. [Crossref] [PubMed]
- Anupindi SA, Dillman JR. Body MRI in pediatrics: where we are and what the future holds. Pediatr Radiol 2025;55:8-11. [Crossref] [PubMed]
- Simonetti OP, Finn JP, White RD, Laub G, Henry DA. "Black blood" T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996;199:49-57. [Crossref] [PubMed]
- Cocker MS, Shea SM, Strohm O, Green J, Abdel-Aty H, Friedrich MG. A new approach towards improved visualization of myocardial edema using T2-weighted imaging: a cardiovascular magnetic resonance (CMR) study. J Magn Reson Imaging 2011;34:286-92. [Crossref] [PubMed]
- Guan X, Chen Y, Yang HJ, Zhang X, Ren D, Sykes J, Butler J, Han H, Zeng M, Prato FS, Dharmakumar R. Assessment of intramyocardial hemorrhage with dark-blood T2*-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2021;23:88. [Crossref] [PubMed]
- Henningsson M, Malik S, Botnar R, Castellanos D, Hussain T, Leiner T. Black-Blood Contrast in Cardiovascular MRI. J Magn Reson Imaging 2022;55:61-80. [Crossref] [PubMed]
- Yan X, Ran L, Zou L, Luo Y, Yang Z, Zhang S, Zhang S, Xu J, Huang L, Xia L. Dark blood T2-weighted imaging of the human heart with AI-assisted compressed sensing: a patient cohort study. Quant Imaging Med Surg 2023;13:1699-710. [Crossref] [PubMed]
- Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med 2007;57:891-7. [Crossref] [PubMed]
- Edelman RR, Botelho M, Pursnani A, Giri S, Koktzoglou I. Improved dark blood imaging of the heart using radial balanced steady-state free precession. J Cardiovasc Magn Reson 2016;18:69. [Crossref] [PubMed]
- Keegan J, Gatehouse PD, Prasad SK, Firmin DN. Improved turbo spin-echo imaging of the heart with motion-tracking. J Magn Reson Imaging 2006;24:563-70. [Crossref] [PubMed]
- Hu C, Huber S, Latif SR, Santacana-Laffitte G, Mojibian HR, Baldassarre LA, Peters DC. Reverse double inversion-recovery: Improving motion robustness of cardiac T(2) -weighted dark-blood turbo spin-echo sequence. J Magn Reson Imaging 2018;47:1498-508. [Crossref] [PubMed]
- Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson 2013;15:41. [Crossref] [PubMed]
- Wary P, Hossu G, Ambarki K, Nickel D, Arberet S, Oster J, Orry X, Laurent V. Deep learning HASTE sequence compared with T2-weighted BLADE sequence for liver MRI at 3 Tesla: a qualitative and quantitative prospective study. Eur Radiol 2023;33:6817-27. [Crossref] [PubMed]
- Sugahara T, Korogi Y, Hirai T, Hamatake S, Ikushima I, Shigematu Y, Takahashi M. Comparison of HASTE and segmented-HASTE sequences with a T2-weighted fast spin-echo sequence in the screening evaluation of the brain. AJR Am J Roentgenol 1997;169:1401-10. [Crossref] [PubMed]
- Zhang L, Kholmovski EG, Guo J, Choi SE, Morrell GR, Parker DL. HASTE sequence with parallel acquisition and T2 decay compensation: application to carotid artery imaging. Magn Reson Imaging 2009;27:13-22. [Crossref] [PubMed]
- Liao S, Mo Z, Zeng M, Wu J, Gu Y, Li G, et al. Fast and low-dose medical imaging generation empowered by hybrid deep-learning and iterative reconstruction. Cell Rep Med 2023;4:101119. [Crossref] [PubMed]
- Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. J Magn Reson Imaging 2017;45:966-87. [Crossref] [PubMed]
- Mann LW, Higgins DM, Peters CN, Cassidy S, Hodson KK, Coombs A, Taylor R, Hollingsworth KG, Accelerating MR. Imaging Liver Steatosis Measurement Using Combined Compressed Sensing and Parallel Imaging: A Quantitative Evaluation. Radiology 2016;278:247-56. [Crossref] [PubMed]
- Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med 2010;64:767-76. [Crossref] [PubMed]
- Sui H, Gong Y, Liu L, Lv Z, Zhang Y, Dai Y, Mo Z. Comparison of Artificial Intelligence-Assisted Compressed Sensing (ACS) and Routine Two-Dimensional Sequences on Lumbar Spine Imaging. J Pain Res 2023;16:257-67. [Crossref] [PubMed]
- Ni M, He M, Yang Y, Wen X, Zhao Y, Gao L, Yan R, Xu J, Zhang Y, Chen W, Jiang C, Li Y, Zhao Q, Wu P, Li C, Qu J, Yuan H. Application research of AI-assisted compressed sensing technology in MRI scanning of the knee joint: 3D-MRI perspective. Eur Radiol 2024;34:3046-58. [Crossref] [PubMed]
- Wang Q, Zhao W, Xing X, Wang Y, Xin P, Chen Y, Zhu Y, Xu J, Zhao Q, Yuan H, Lang N. Feasibility of AI-assisted compressed sensing protocols in knee MR imaging: a prospective multi-reader study. Eur Radiol 2023;33:8585-96. [Crossref] [PubMed]
- Zhao Y, Peng C, Wang S, Liang X, Meng X. The feasibility investigation of AI -assisted compressed sensing in kidney MR imaging: an ultra-fast T2WI imaging technology. BMC Med Imaging 2022;22:119. [Crossref] [PubMed]
- Tang H, Peng C, Zhao Y, Hu C, Dai Y, Lin C, Cai L, Wang Q, Wang S. An applicability study of rapid artificial intelligence-assisted compressed sensing (ACS) in anal fistula magnetic resonance imaging. Heliyon 2024;10:e22817. [Crossref] [PubMed]
- Li H, Hu C, Yang Y, Zhao Y, Lin C, Li Z, Liu Q. Single-breath-hold T2WI MRI with artificial intelligence-assisted technique in liver imaging: As compared with conventional respiratory-triggered T2WI. Magn Reson Imaging 2022;93:175-80. [Crossref] [PubMed]
- Sheng RF, Zheng LY, Jin KP, Sun W, Liao S, Zeng MS, Dai YM. Single-breath-hold T2WI liver MRI with deep learning-based reconstruction: A clinical feasibility study in comparison to conventional multi-breath-hold T2WI liver MRI. Magn Reson Imaging 2021;81:75-81. [Crossref] [PubMed]
- Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging 2007;26:452-9. [Crossref] [PubMed]
- Kim HW, Van Assche L, Jennings RB, Wince WB, Jensen CJ, Rehwald WG, Wendell DC, Bhatti L, Spatz DM, Parker MA, Jenista ER, Klem I, Crowley AL, Chen EL, Judd RM, Kim RJ. Relationship of T2-Weighted MRI Myocardial Hyperintensity and the Ischemic Area-At-Risk. Circ Res 2015;117:254-65. [Crossref] [PubMed]
- Zhu Y, Yang D, Zou L, Chen Y, Liu X, Chung YC T. (2)STIR preparation for single-shot cardiovascular magnetic resonance myocardial edema imaging. J Cardiovasc Magn Reson 2019;21:72. [Crossref] [PubMed]
- Kargar S, Borisch EA, Froemming AT, Grimm RC, Kawashima A, King BF, Stinson EG, Riederer SJ. Modified acquisition strategy for reduced motion artifact in super resolution T2 FSE multislice MRI: Application to prostate. Magn Reson Med 2020;84:2537-50. [Crossref] [PubMed]
- Lin J, Miao QI, Surawech C, Raman SS, Zhao K, Wu HH, Sung K. High-Resolution 3D MRI With Deep Generative Networks via Novel Slice-Profile Transformation Super-Resolution. IEEE Access 2023;11:95022-36.
- Bischoff LM, Peeters JM, Weinhold L, Krausewitz P, Ellinger J, Katemann C, Isaak A, Weber OM, Kuetting D, Attenberger U, Pieper CC, Sprinkart AM, Luetkens JA. Deep Learning Super-Resolution Reconstruction for Fast and Motion-Robust T2-weighted Prostate MRI. Radiology 2023;308:e230427. [Crossref] [PubMed]
- Wu Z, Li X. Adaptive Knowledge Distillation for High-Quality Unsupervised MRI Reconstruction With Model-Driven Priors. IEEE J Biomed Health Inform 2024;28:3571-82. [Crossref] [PubMed]
- Chen S, Duan J, Ren X, Wang J, Liu Y. DFUSNN: zero-shot dual-domain fusion unsupervised neural network for parallel MRI reconstruction. Phys Med Biol 2024; [Crossref]


