
The malignant possibility of intraductal lesions with different ultrasound manifestations under different age and nipple discharge status
Introduction
Intraductal papillary lesions of the breast are intraductal abnormalities in the lactiferous duct of the breast (1). Due to their heterogeneous nature, the diagnosis and treatment of intraductal lesions are controversial (2-4). Intraductal lesions include duct ectasia, intraductal papilloma (IP), IP with atypia or ductal carcinoma in situ (DCIS), and solid papillary carcinoma (SPC). The malignant rate of intraductal lesions ranges from 4.4% to 11.0% (5).
Ultrasound (US) is an imaging technique with excellent superficial soft tissue resolution (6-8). Breast Imaging Reporting and Data System (BI-RADS) is a standardized system developed by the American College of Radiology (ACR) to standardize the reporting and classification of breast imaging results, such as mammography, US, and magnetic resonance imaging (MRI). The BI-RADS system aims to improve the clarity and consistency of imaging reports and provide clear follow-up recommendations for clinicians. The BI-RADS system classifies breast imaging findings into categories 0–6, each corresponding to a different level of malignancy risk and management recommendations: 0: incomplete, additional imaging needed; 1: negative, no findings; 2: benign findings; 3: probably benign, short-term follow-up recommended; 4: suspicious, biopsy should be considered (this category is further subdivided into 4A, 4B, and 4C based on the likelihood of malignancy); 5: highly suggestive of malignancy, appropriate action should be taken; and 6: known biopsy-proven malignancy. The BI-RADS has refined its descriptors for US findings, including those related to mass shape, orientation, margins, and posterior acoustic features; however, it does not provide categorical guidance for intraductal lesions, as such lesions are considered “special cases” (9,10). A previous study (11) reported that based on the final assessment, BI-RADS 4A is recommended for intraductal lesions which indicates a need for biopsy. However, it is not yet clear whether biopsy or open surgery should be recommended for all affected women. Further, the relationship between patient age, nipple discharge, US findings, and the malignant rate of intraductal lesions was not examined. It is vital that radiologists and surgeons are able to differentiate between patients who can be included in the regular follow-up program and those who require further surgical management.
Thus, this study sought to identify the risk factors present in patients with intraductal lesions, which are often malignant. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-924/rc).
Methods
This was a retrospective, cross-sectional, single-center study. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Independent Ethics Committee of Shanghai Cancer Center, Fudan University (Shanghai, China) (No. 2107238-18), and the requirement of individual consent for this retrospective analysis was waived.
The data of women with positive US examination findings and postoperative pathology results confirming intraductal papillary lesions, who were treated at Fudan University Shanghai Cancer Center from January 2018 to December 2019, were collected. The inclusion criteria were complete ultrasonographic imaging and pathologic data indicating duct ectasia, IP, IP with atypia or DCIS, or SPC. The exclusion criteria were other malignant tumors after surgery, two or more lesions in one breast (including primary and secondary breast cancer), incomplete pathologic data, and/or loss to follow-up. Medical records were reviewed to collect patient data, including age, nipple discharge (yes/no), and pathological diagnosis.
Image evaluation
The US features of the intraductal masses were retrospectively reviewed in consensus, without clinical or pathologic information, by two dedicated breast imaging radiologists with 5 and 11 years of experience, respectively. The morphologic type of the intraductal lesions was classified as follows based on the US features (Figure 1): type I: simple duct ectasia; type II: duct ectasia with a mass inside the duct (papillary; a mass incompletely or completely filling the duct); type III: mixed echo-cystic solid nodules; type IV: a regular hypoechoic nodule without a definite dilated duct around it; and type V: an irregular hypoechoic nodule with or without a definite dilated duct around it.

Statistical analysis
The statistical analyses were performed using SPSS version 29.0 for Windows (IBM, Armonk, NY, USA). The Chi-squared test was used to analyze the categorical variables. The Chi-squared test was used to evaluate whether patient age, nipple discharge, and US findings were associated with malignancy. A P value <0.05 was considered statistically significant.
Results
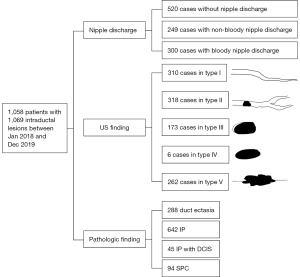
A total of 1,058 patients with 1,069 pathologically proven intraductal lesions were included in this study. The age of the patients ranged from 18 to 86 years (mean age: 46.8±11.6 years). The pathologic findings of the 1,069 lesions revealed 288 duct ectasias, 642 IP, 45 IP with DCIS, and 94 SPCs. The benign lesions usually coexisted with ischesis, calcium deposition, inflammatory cell infiltration, apocrine metaplasia, introlobular neoplasia, ductal hyperplasia, papillary hyperplasia, and/or atypical hyperplasia (Table 1).
Table 1
| Coexisting pathological changes | Duct ectasia (n=288) | Papilloma (n=642) |
|---|---|---|
| Ischesis | 110 | 71 |
| Calcium deposition | 90 | 97 |
| Inflammatory cell infiltration | 69 | 13 |
| Apocrine metaplasia | 39 | 96 |
| Introlobular neoplasia | 2 | 5 |
| Ductal hyperplasia | 112 | 388 |
| Papillary hyperplasia | 26 | 2 |
| Atypical hyperplasia | 20 | 79 |
Data are presented as number.
Among the 1,069 ductal lesions, there were 520 cases of no nipple discharge, 249 cases of non-bloody nipple discharge (yellow, clear, white, purulent, acne-like, and green), and 300 cases of bloody nipple discharge (red and brown) (Table 2). In terms of the US findings, 310, 318, 173, 6, and 262 cases were classified as types I, II, III, IV, and V, respectively (Figure 2).
Table 2
| Nipple discharge | Duct ectasia | IP | IP with DCIS | SPC | Total |
|---|---|---|---|---|---|
| None (n=520) | 162 | 306 | 37 | 15 | 520 |
| Non-bloody (n=249) | |||||
| Yellow | 37 | 113 | 0 | 5 | 155 |
| Clear | 15 | 63 | 0 | 6 | 84 |
| White | 4 | 1 | 0 | 0 | 5 |
| Purulent | 1 | 1 | 0 | 0 | 2 |
| Acne-like | 0 | 2 | 0 | 0 | 2 |
| Green | 0 | 1 | 0 | 0 | 1 |
| Bloody (n=300) | |||||
| Red | 55 | 117 | 2 | 51 | 225 |
| Brown | 14 | 38 | 6 | 17 | 75 |
DCIS, ductal carcinoma in situ; IP, intraductal papilloma; SPC, solid papillary carcinoma.
An alluvial diagram was used to show the age, nipple discharge, US findings, and pathology distribution (Figure 3). An alluvial diagram is a type of flow diagram that visualizes how data or entities transition between different categories across multiple dimensions. It uses flowing bands (called “alluvial flows”) to represent the movement or relationships between variables, making it particularly useful for showing transitions, distributions, and relationships in complex datasets. The alluvial diagram showed that the relationship between the variables was complicated and intertwined. In general terms, the lesions were more likely to be malignant in older patients and those with bloody nipple discharge. The general malignant ratio was 13.0% (139/1,069). However, when patient age, nipple discharge, or US findings were included as single influencing factors related to pathology diagnosis, different results were obtained, and the difference was statistically significant (Table 3). The linear trend Chi-Square statistical analysis showed that the malignant rate linearly increased as patient age increased (P<0.001). In terms of age stratification, a difference in the malignant rate was observed between the patients aged 18–59 years and those aged 60–86 years (P<0.001). Specifically, the patients aged between 18 and 59 years had a similar rate of malignancy (3.8–7.8%), as did those aged between 60 and 86 years (51.9–54.9%). Thus, 60 years could be the threshold for significantly increased malignancy.

Table 3
| Factors | Total, n | Benign, n | Malignant, n (%) |
|---|---|---|---|
| Age (years) | |||
| 18–29 | 52 | 50 | 2 (3.8) |
| 30–39 | 226 | 216 | 10 (4.4) |
| 40–49 | 440 | 412 | 28 (6.4) |
| 50–59 | 192 | 177 | 15 (7.8) |
| 60–69 | 108 | 52 | 56 (51.9) |
| 70–86 | 51 | 23 | 28 (54.9) |
| Nipple discharge | |||
| None | 520 | 468 | 52 (10.0) |
| Non-bloody | 249 | 238 | 11 (4.4) |
| Bloody | 300 | 224 | 76 (25.3) |
| US | |||
| I | 310 | 279 | 31 (10.0) |
| II | 318 | 288 | 30 (9.4) |
| III | 173 | 155 | 18 (10.4) |
| IV | 6 | 6 | 0 (0) |
| V | 262 | 202 | 60 (22.9) |
| Total | 1,069 | 930 | 139 (13.0) |
US, ultrasound.
A statistically significant difference was also found between nipple discharge status (none, non-bloody, and bloody) and malignant rates (10.0%, 4.4%, and 25.3%, respectively), such that the patients with bloody nipple discharge had a high malignant rate.
In terms of the US type, as only six patients had US type IV, this US type was excluded from the statistical analysis. However, US types I, II, and III had similar rates of malignancy (9.4–10.4%), while US type V had a higher rate of malignancy (22.9%), and the difference was statistically significant.
Discussion
Previous research has shown that a personal history of breast cancer, mass size, branch duct involvement, and US features could be possible predictors of malignancy; however, different studies have reported different predictors of malignancy and different malignancy rates (12-18). In our daily clinical practice, we have noted that the US features of benign and malignant IP tumors overlap; that is, we have noted that the same US features can be found in patients of different pathology results. We have also observed that the pathology results of young patients, even those with bloody nipple discharge, are mostly benign. Therefore, the classification of all intraductal lesions as malignant could be inaccurate and inappropriate, and thus more detailed category guidance is urgently needed.
In terms of the US type findings, we propose a new method that differs to the methods of previous studies (11,19). In clinical practice, we have found that in cases with simple ductal ectasia on US, the pathological result can still be IP or even SPC. Thus, simple ductal ectasia cannot simply be classified as benign, and it certainly cannot be ignored in the study of intraductal lesions. Multiple masses may be present in different locations in the same duct near or far away from the nipple areola area; thus, we classify intraductal masses as one type (type II). In this subtype, the “solid mass” in the duct may not only be papillary neoplasms, but may also be ischesis or other coexisting benign pathological changes. In type III, the cystic part may be very small; thus, the US scan should be performed carefully to find the narrow cystic echo next to the solid echo. In terms of type IV, only six patients had this type in this study, and due to the small number of patients, a formal statistical comparison could not be performed. Thus, our results must be considered observational. In type V, the signs of malignancy accord to the BI-RADS classification of solid masses, and the patients with type V US findings had a malignant rate of 22.9%.
Unlike previous studies, with the exception of type V, the US finding variable was not a special predictor of malignancy. Types I–III can be found in all kinds of patients, including those with pathologically proven ductal ectasia, whose US findings can also manifest as solid masses inside the duct. This may be because bleeding into the duct, debris, or inspissated secretions in mammary duct ectasia have similar features.
The relatively large number of patients included in our study permitted us to conduct quite an accurate analysis of the different parameters of each group; however, the present study had some limitations. First, as a single-center study, the representativeness of the results is limited. Second, breast-duct microscopy was not included in this study. Despite these factors, several measures were employed to optimize data quality. First, the breast cancer center at this hospital is famous for its professional diagnosis and treatment, and patients came from different districts around the country, and even abroad, which ensured the quality of this single-center study. Second, an image interpretation committee was established to standardize the assessment criteria used for evaluation.
Conclusions
The data in this study covered a large patient population and a wide age range; thus, the data likely reflect real clinical situations in which physicians have to make decisions about intraductal mass lesions, regardless of their pathologic nature. Further prospective, multicenter studies of intraductal masses with larger patient populations and subjective criteria need to be conducted to confirm our findings.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-924/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-924/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Independent Ethics Committee of Shanghai Cancer Center, Fudan University (Shanghai, China) (No. 2107238-18), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rakha EA, Ellis IO. Diagnostic challenges in papillary lesions of the breast. Pathology 2018;50:100-10. [Crossref] [PubMed]
- Zhang B, Guo Z, Lei Z, Liang W, Chen X. Kaiser score diagnosis of breast MRI lesions: Factors associated with false-negative and false-positive results. Eur J Radiol 2024;178:111641. [Crossref] [PubMed]
- Jin Z, Al Qaysi N, Hanna M, Moses V, Spiguel L, Shaw C, Asirvatham JR. Surgical excision versus clinical follow-up: Outcomes of benign intraductal papillomas diagnosed on core needle biopsy. Am J Surg 2024;233:114-9. [Crossref] [PubMed]
- Keating N, Cevik J, Hopkins D, Lippey J. Malignant upgrade rate and associated clinicopathologic predictors for concordant intraductal papilloma without atypia: A systematic review and meta-analysis. J Surg Oncol 2024;129:1025-33. [Crossref] [PubMed]
- Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist 2006;11:435-49. [Crossref] [PubMed]
- Hooley RJ, Scoutt LM, Philpotts LE. Breast ultrasonography: state of the art. Radiology 2013;268:642-59. [Crossref] [PubMed]
- Wang XY, Cui LG, Feng J, Chen W. Artificial intelligence for breast ultrasound: An adjunct tool to reduce excessive lesion biopsy. Eur J Radiol 2021;138:109624. [Crossref] [PubMed]
- Al Sarakbi W, Worku D, Escobar PF, Mokbel K. Breast papillomas: current management with a focus on a new diagnostic and therapeutic modality. Int Semin Surg Oncol 2006;3:1. [Crossref] [PubMed]
- Rao AA, Feneis J, Lalonde C, Ojeda-Fournier H. A Pictorial Review of Changes in the BI-RADS Fifth Edition. Radiographics 2016;36:623-39.
- Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE. BI-RADS® fifth edition: A summary of changes. Diagn Interv Imaging 2017;98:179-90.
- Hsu HH, Yu JC, Hsu GC, Chang WC, Yu CP, Tung HJ, Tzao C, Huang GS. Ultrasonographic alterations associated with the dilatation of mammary ducts: feature analysis and BI-RADS assessment. Eur Radiol 2010;20:293-302. [Crossref] [PubMed]
- Zhang H, Sun Y, Zhang J, Wang S. Breast Imaging Reporting and Data System Classification for Central-Type Intra-Ductal Papillary Masses: Current Problems and Evaluation of Modified Parameters. Ultrasound Med Biol 2021;47:960-6. [Crossref] [PubMed]
- Ueng SH, Mezzetti T, Tavassoli FA. Papillary neoplasms of the breast: a review. Arch Pathol Lab Med 2009;133:893-907. [Crossref] [PubMed]
- Hodorowicz-Zaniewska D, Szpor J, Basta P. Intraductal papilloma of the breast - management. Ginekol Pol 2019;90:100-3. [Crossref] [PubMed]
- Shouhed D, Amersi FF, Spurrier R, Dang C, Astvatsaturyan K, Bose S, Phillips E. Intraductal papillary lesions of the breast: clinical and pathological correlation. Am Surg 2012;78:1161-5.
- Ganesan S, Karthik G, Joshi M, Damodaran V. Ultrasound spectrum in intraductal papillary neoplasms of breast. Br J Radiol 2006;79:843-9. [Crossref] [PubMed]
- Shin HJ, Kim HH, Kim SM, Yang HR, Sohn JH, Kwon GY, Gong G. Papillary lesions of the breast diagnosed at percutaneous sonographically guided biopsy: comparison of sonographic features and biopsy methods. AJR Am J Roentgenol 2008;190:630-6. [Crossref] [PubMed]
- Skandarajah AR, Field L, Yuen Larn Mou A, Buchanan M, Evans J, Hart S, Mann GB. Benign papilloma on core biopsy requires surgical excision. Ann Surg Oncol 2008;15:2272-7. [Crossref] [PubMed]
- Kim WH, Chang JM, Moon WK, Cho N, Yi A, Koo HR, Kim SJ. Intraductal mass on breast ultrasound: final outcomes and predictors of malignancy. AJR Am J Roentgenol 2013;200:932-7. [Crossref] [PubMed]


