
Complete regression of a huge fetal hepatic hemangioma with cardiac complication after atrioventricular septal repair: a case description and literature analysis
Introduction
Hepatic hemangioma (HH) is the most common benign mesenchymal tumor of the liver in fetus and infants (1,2). According to International Society for the Study of Vascular Anomalies (ISSVA), HH was classified as congenital hepatic hemangioma (CHH) and infantile hepatic hemangioma (IHH) (3,4). Most CHH could regress partially or completely without therapies (5), but non-involuting CHH or progressive IHH may lead to serious complications, such as high-output congestive heart failure, cardiomegaly, non-immune hydrops, intratumoral bleeding, thrombocytopenia, and so on (6-8). Thus, positive therapies for non-involuting CHH and progressive IHH are needed. The most common therapy is oral propranolol with or without dexamethasone or rapamycin (9-13). Selective catheterization and coil embolization of the right and left hepatic and right phrenic arteries could be performed when the drug resistance appeared (13). If the patients have liver failure, surgery or liver transplantation needs to be considered (10,11,13-15). However, medical therapy has side effects, resistance, and no response of tumor (16). Embolization, surgery or liver transplantation is invasive. So we performed a literature analysis on the basis of the case, a rapidly regressed HH with cardiac surgery, to explore new strategies for treating similar cases in the future.
Case presentation
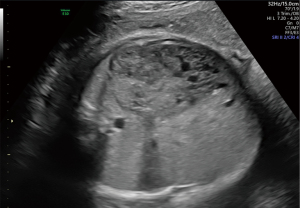
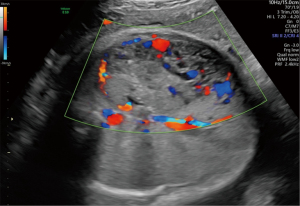
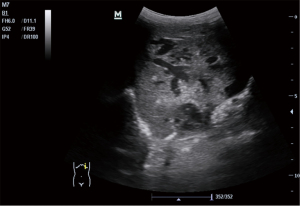
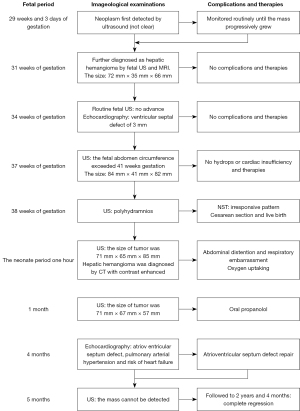
A 30-year-old pregnant woman was referred to West China Second Hospital for further diagnosis at 31 weeks of gestation because of a large fetal abdominal mass detected during a routine third-trimester scan. The first detection of the neoplasm was 29 weeks and 3 days of gestation by ultrasound examination in other hospital. In our hospital, ultrasound examination of the fetus revealed a well-delineated, hypoechoic, heterogeneous solid mass measuring 72 mm × 35 mm × 66 mm in close relation to the left hepatic lobe at 35 gestational weeks. The internal echo was cellular and there were irregular, echoless areas in center (Figure 1). The color Doppler showed that there exists abundant blood flow in and surrounding the mass with arteriovenous shunts (Figures 2,3). The drained veins were enlarged. Fetal MRI showed an extensive heterogeneous upper abdominal mass that compromised left hepatic lobe with flow-voids within it (Figure 4). According to the imaging features, the diagnosis of fetal HH was firstly considered. During the follow-up, fetal echocardiography at 34 weeks of gestation showed the possibility of ventricular septal defect (measuring 3 mm) without other complications. At 37 weeks of gestation, ultrasound showed that the fetal abdominal circumference exceeded 41 gestational week and the mass enlarged to 84 mm × 41 mm × 82 mm. However, no signs of hydrops, cardiac insufficiency, or fetal anemia were noted. At 38 weeks of gestation, non-stress test (NST) demonstrated an irresponsive pattern and polyhydramnios, an elective cesarean section was performed delivering a female infant in breech presentation weighing 3,680 g. Apgar score was 10-10-10 at 1, 5 and 10 minutes, respectively. The infant was referred to division of neonatology because of abdominal distention and respiratory embarrassment. Her respiratory rate was 62–65 times per minute, and saturation of peripheral oxygen (SpO2) was 88–90%. The bedside ultrasound revealed a 71 mm × 65 mm × 85 mm heterogeneous left hepatic mass with tubular or reticular hypoechoic areas (Figure 5), supplied by affluent blood flow and drained by left hepatic vein. A postnatal computed tomography (CT) scan with contrast enhancement demonstrated a heterogeneous hepatic mass, behaving centripetal enhancement with central necrosis. There exists many vessels in the mass and drained into the inferior vein. Echocardiography showed atrioventricular septal defect, mitral, tricuspid regurgitation and pulmonary arterial hypertension. The blood test of white blood cell (WBC), hemoglobin (HGB), hematocrit (HCT), platelet (PLT) was normal, but alpha-fetoprotein (AFP) was 64,271.7 ng/mL and NSE was 33.00 ng/mL. The diagnosis of HH was strongly considered, but hemangioendothelioma, hepatoblastoma, germ cell tumour, neuroblastoma and mesenchymal hamartoma should be differentiated. Then the neonate received oral propranolol because of the cardiac complication and was monitored by ultrasound examination routinely. As the infant grew, the hepatic mass gradually shrank, however, the pulmonary artery pressure gradually increased and the left ventricular ejection fraction gradually decreased. When the infant was 4 months old, the size of the hepatic mass reduced to 37 mm × 41 mm × 50 mm. At the same time, echocardiography revealed severe pulmonary arterial hypertension and reduced left ventricular systolic function. Therefore, the infant received atrioventricular septal repair surgery. During the hospitalization following cardiac surgery, the HH was almost undetectable by bedside ultrasound (Figure 6 was the postoperative imaging of 1 month). The interval since the last ultrasound examination was only 1 week. The baby was followed up by ultrasound until the age of 2 years and 4 months. The mass regressed completely and all the examinations were normal (the course was list in Figure 7).

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
There exists some research about the clinical manifestation, diagnosis, therapy and prognosis of fetal HH (9,17-19). Based on the guidance of the ISSVA, HHs are classified into CHH and IHH (3,4). CHH was common in fetus, as IHH in infancy. CHH was fully formed and present at birth, while IHH was detected incidentally (5). According to the “Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring”, the diagnosis of hemangioma is often clear after a review of patient’s history, physical examination, and imaging (20-23), histologic confirmation is rarely required (19). If HH occurred after infancy, the imaging features were not classic or if the clinical history did not support a hemangioma diagnosis, magnetic resonance imaging (MRI) of the liver with dynamic acquisition pattern for clarification was recommended and biopsy should be considered (19). Most majority of CHH usually regress spontaneously after delivery without severe complications (5). However, some huge congenital hemangiomas or progressive IHH may have poor prognosis if they appear serious complications (6-8). The common therapy methods include intrauterine and neonate treatments (continue monitoring, medical, interventional treatments and surgery) (10,11,14,16). After systematic monitoring and therapies, many hemangiomas could gradually reduce in size, even regress completely or partially, but some diffuse IHH without therapies may lead to death (24).
In this report, we presented a case of a giant fetal HH diagnosed prenatally using two-dimensional, color Doppler ultrasound and fetal MRI between 29 and 34 weeks’ gestation. Although biopsy and immunohistochemical staining for glucose transporter protein-1 (GLUT1) was not performed to confirm the pathological type (25,26), according to the progress of genesis and development of lesion and typical features of ultrasound and MRI (27-30), the diagnosis of HH was not complex (RICH most possibly). Meanwhile, ventricular septal defect was also diagnosed by fetal echocardiography. The fetus was monitored closely without any complication. After the live birth of the fetus, the mass gradually shrank as the infant grew and the size of tumor was 37 mm × 41 mm × 50 mm. However, the pulmonary artery pressure gradually increased and the left ventricular systolic function decreased with atrioventricular septal defect, mitral and tricuspid regurgitation. To avoid the complication of cardiac failure, at 4 months, atrioventricular septal repair was selected and awesomely, the HH regressed completely during the hospitalization following cardiac surgery. The interval between the last ultrasound examination and the involution of the lesion was only 1 week.
Is it an occasional case or there exists correlation between atrioventricular septal defect and HH? If there were possible positive impacts on HH with heart complication through cardiac surgery? In order to explore probable mechanisms and potential added therapies for clinical experience, we need to compare the diagnosis, clinical symptoms, unique therapeutic method and prognosis of this case with other studies. For these purposes, we conducted an updated review of the literature for cases of HH. According to the ISSVA’s classification of vascular tumors (International Society of Study of Vascular Anomalies), a computerized review of the MEDLINE-PubMed database and China National Knowledge Infrastructure was performed using the terms “vascular anomalies”, “hemangioma”, “hemangioendothelioma” (as the classification may not be normative before 2014, this item was included for studies before that year), “hepatic and/or liver”, “tumor and/or neoplasms”, and “congenital and/or infantile hepatic hemangioma” in combination with, but not limited to “fetal”, “fetus”, “prenatal diagnosis”, “in utero”, “infant”, “infancy” and “neonate”. Table 1 presents the cases with diagnosis of HH (including CHH, IHH and infantile hepatic hemangioendotheliomas). Cases that were not followed from the fetal to neonatal period, those with a final diagnosis of hamartoma, hepatoblastoma, or vascular malformations, cases with unclear imaging manifestations or without therapies during the prenatal and neonatal periods, lesions without follow-up or endpoint, or cases involving induction of the fetus, as well as review studies, were not included in our review.
Table 1
| Study | No. of cases | First detection | Largest axis (cm) | Echocardiography in utero | Complications in utero | Treatments in utero | Echocardiography after birth | Medication after birth | Interventional therapy after birth | Endpoint |
|---|---|---|---|---|---|---|---|---|---|---|
| Zenzen 2009 (13) | 1 | 3 days neonate | 5.2 | NC | NC | NC | 3 days: patent ductus arteriosus, biventricular hypertrophy; 20 days: right ventricular dilatation and atrial septal defect | Conservative treatment with prednisolone | Selective catheterization and coil embolization of the right and left hepatic and right phrenic arteries | Liver transplantation, RICH was confirmed |
| Liu 2023 (31) | 1 | 32gw | 5.8 | NC | NO | NO | NC | NO | NO | Resection with laparotomy |
| Prokurat 2002 (32) | 17 | Neonate to 16 yr | 4 to 20 | NC | NC | NC | NC | Steroids | Embolization | 7 were arteriovenous malformations or hamartomatoid component; of the HH, 4 lobectomy, 6 segmental hepatectomy; all survival |
| Boon 1996 (33) | 39 | Neonate to 6 months | NC | NC | NC | NC | NC | Corticosteroid, interferon alfa-2a | Embolization | 7 died, 23 alive and well, 9 without follow-up or with NC outcome |
| Zhang 2010 (34) | 12 | Fetus to 5 yr | 5 to 13.8 | NC | NC | NC | NC | NO | NO | 10 well alive and 2 fetal demise |
| Schmitz 2009 (11) | 1 | 28+1 gw | 6.3 | Cardiomegaly | NC | Oral dexamethasone | Cardiomegaly | Platelets transfusion; corticosteroids | Partly hepatic arterials occlusion | Survival (with tumor or not is unclear) |
| Cabrita 2009 (7) | 1 | 26 gw | 7.3 | NO | None | None | NO | Prednisolone; α-2a-interferon | NO | Survival (with asymptomatic tumor) |
| Morris 1999 (35) | 1 | 17 gw | 4.5 | Dilated right ventricle and depressed function | Polyhydramnios | Oral dexamethasone | Dilated right ventricle, slightly depressed function, low-normal left ventricular function | Prednisone | NO | Undetectable on sonography |
| Chou 2005 (30) | 1 | 33 gw | 3.72 | NO | Normal | None | NO | Interferon | NO | NC |
| Morimura 2003 (14) | 1 | 36+5 gw | 9.6 | NO | Reassuring pattern (NST) | None | NO | α-interferon, prednisolone | NO | Died for DIC |
| Sepulveda 2021 (17) | 3 | 33+3 gw | 8.9 | NO | Normal | None | NO | NA | NA | Fetal demise |
| 30 gw | 8.1 | NO | Normal | None | NO | Vasoactive drugs, corticosteroids, blood transfusions | Transcatheter embolization | Complete regression | ||
| 34 gw | 6.0 | NO | Normal | None | NO | NO | NO | Normal | ||
| Tenkumo 2017 (28) | 1 | 29+3 gw | 4.5 | NO | NC | NC | NO | NC | NC | Spontaneous regression |
| Ling 2018 (9) | 6 | 36 gw | 8.6 | NC | Slowing down fetal heart rate | None | NC | Oral propranolol, dexamethasone | NO | Tumor shrinkage and calcification plaques |
| 31+6 gw | 9.2 | NC | Cardiothoracic ratio (<0.33) | None | NC | Oral propranolol, dexamethasone | NO | Tumor shrinkage and calcification plaques | ||
| 36 gw | 4.5 | NC | None | None | NC | Oral propranolol, dexamethasone | NO | Tumor shrinkage and calcification plaques | ||
| 34+2 gw | 5.6 | NC | None | None | NC | Oral propranolol, dexamethasone | NO | Tumor shrinkage and calcification plaques | ||
| 36+3 gw | 5.0 | NC | None | None | NC | Oral propranolol, dexamethasone | NO | Tumor shrinkage and calcification plaques | ||
| 37+1 gw | 5.5 | NC | None | None | NC | NO | Interventional sclerotherapy | Tumor shrinkage and calcification plaques | ||
| Zhang 2019 (10) | 6 | 38 gw | 4.3 | NC | None | None | NC | NO | NO | Survival with tumor |
| 37 gw | 4.3 | NC | None | None | NC | Oral propranolol | NO | Tumor shrinkage | ||
| 31 gw | 3.8 | NC | None | None | NC | NO | NO | No growth of tumor | ||
| 34 gw | 5.7 | NC | None | None | NC | Oral propranolol | NO | Complete regression | ||
| 35 gw | 0.9 | NC | None | None | NC | NO | NO | Survival with tumor | ||
| 38 gw | 2.4 | NC | None | None | NC | NO | NO | Little reduction in size | ||
| Chuileannain 1999 (18) | 1 | 33 gw | 12 | NC | Abnormal fetal heart rate | None | NC | NO | Intravenous alpha-interferon | Died for hemorrhage |
| Shen 2018 (27) | 1 | 33 gw | 4.4 | NC | NC | NC | NC | NO | NO | Surgery and diagnosed as IHH |
| Chen 2021 (29) | 15 | 29.5±3.6 gw | 8.4 | NC | None | None | NC | 1 glucocorticoid | 3 intravenous alpha-interferon | 1 surgery, 3 regression, 11 tumor shrinkage |
| Yang 2022 (12) | 14 | 28–39 gw | 3.2–5.8 | 2 has cardiac insufficiency | NC | None | 2 cardiac insufficiencies | Propranolol, glucocorticoid | NO | 4 complete regression, 2 unchanged, 8 tumor shrinkage |
| Tang 2023 (36) | 1 | 23 gw | 6.4 | NC | Cardiothoracic ratio 0.5, pericardial and pleural effusion | Corticosteroids and β-blockers through placenta | NC | NO | NO | Normal (with tumor or not is unclear) |
| Xie 2022 (8) | 22 | 30–40 gw | 4.2–9.9 | NC | 7 cases of intrauterine distress | None | NC | Propranolol, dexamethasone or rapamycin | Interventional therapy of tumor | 3 complete regression, 19 partial regression |
| Long 2020 (23) | 4 | 33 gw to 2 days | 1.9–7.8 | NC | None | None | NC | NO | NO | Reductions in size with calcification |
| Li 2023 (37) | 12 | 22–39 gw | 2.9–6.9 | NC | 2 cardiomegaly and 1 pleural effusion | None | NC | Oral medicine | NO | 10 complete Regression,1 partial regression, 1 stable |
| Wang 2020 (38) | 2 | 27 gw | NC | ASD, patent ductus arteriosus, pulmonary arterial hypertension | High cardiothoracic ratio | None | ASD, patent ductus arteriosus and pulmonary arterial hypertension | Methylprednisolone, hydrochlorothiazide, milrinone | NO | Surgery |
| 15 days neonate | NC | ASD, pulmonary arterial hypertension | NC | NC | ASD, pulmonary arterial hypertension | Milrinone, hydragogue | Hepatic artery embolization | Died for respiratory and cardiac failure | ||
| Shen 2017 (39) | 3 | 7–34 days | NC | PDA, ASD, pulmonary arterial hypertension | NC | NC | PDA, ASD, pulmonary arterial hypertension | Propranolol, milrinone and sildenafil | Hepatic artery embolization | 1 died for cardiac failure and 2 tumors with size decreasing |
ASD, atrial septal defect; DIC, disseminated intravascular coagulation; gw, gestation week; HH, hepatic hemangioma; IHH, infant hepatic hemangioma; NA, not applicable; NC, not clear; NO, did not receive this examination or therapy; NST, non-stress test; PDA, patent ductus arteriosus; PE, pleural effusion; RICH, rapidly involuting congenital hemangioma; yr, year.
Twenty-four studies of 168 cases were included in the comparative summary (Tables S1,S2), and we found that the most common fetal complications were as previously mentioned (6-8). Other rare complications in utero were polyhydramnios (35), NST demonstrating a reassuring or non-reassuring pattern (14) and gradually slowing down of fetal heart rate (9). Aiming to ease the risk of fetal complications, some studies introduced treatments in utero, including injection of corticosteroids and beta blockers through placenta or oral dexamethasone by pregnant woman (11,35,36).
The complications of neonates and infancy included anemia, thrombocytopenia, high-output congestive heart failure, pulmonary arterial hypertension, patent ductus arteriosus (PDA), atrial septal defect (ASD), respiratory failure (11,13,14,31,32,34,39,40) and so on. Monitoring, medical treatment, occlusion of the supplied arteries, and excision of neoplasm were the therapeutic methods (31,34,37-41). Schmitz et al. (11) selected the occlusion of parts of the hepatic arteries and the infant survived to 2 years old (tumor regressing or not was unclear). Sepulveda et al. (17) and one case of Ling (9), the tumor completely regressed using transcatheter embolization or interventional sclerotherapy with pingyangmycin. Three neonates with cardiac complications received hepatic artery embolization and the size of mass reduced without complete regression (39). Although, for most rapidly involuting congenital hemangioma (RICH), there was no need for medical or surgery therapies (41). Selective catheterization and coil embolization of the right and left hepatic and right phrenic arteries was performed by Zenzen et al. (13), but finally liver transplantation was chosen even for RICH because of liver failure. Other studies presented the medical therapy for inhibiting the growth of neonates and infancy liver tumors, including propranolol with or without dexamethasone or rapamycin (9-13), interferon (7,14,25,30), prednisolone (7,13), methylprednisolone (38), hydrochlorothiazide, milrinone, vasoactive drugs, and corticosteroids (11,17,25). However, only 22 cases (22/168) underwent the complete regression of mass with medical treatments (10,12,35,37), while 24 cases received surgery of liver (13,27,29,31,32,38) and 11 cases died for DIC, hemorrhage, respiratory and cardiac failure (14,33,35,37-39,41). Partial regression or survived with tumor was the most common endpoint-. Among these studies included, there were no reports about the correlation between cardiac surgery and complete regression of HH. Our case report presented an unexpected disappearance of a HH after cardiac atrioventricular septal defect repair surgery. This may provide new ideas for treating similar cases in the future. However, the correlation between atrioventricular septal defect and hemangioma, as well as the mechanism underlying haemodynamic changes after atrioventricular septal repair, remain unclear and require further research.
Acknowledgments
None.
Footnote
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-444/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kochin IN, Miloh TA, Arnon R, Iyer KR, Suchy FJ, Kerkar N. Benign liver masses and lesions in children: 53 cases over 12 years. Isr Med Assoc J 2011;13:542-7.
- Stanley RH, Lauri AA. World Health Organization classification of tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2010:241-2.
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJ, Mitchel S, Powell J, Prendiville J, Vikkula MISSVA Board and Scientific Committee. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015;136:e203-14. [Crossref] [PubMed]
- Hsi Dickie B, Fishman SJ, Azizkhan RG. Hepatic vascular tumors. Semin Pediatr Surg 2014;23:168-72. [Crossref] [PubMed]
- Gong X, Li Y, Yang K, Chen S, Ji Y. Infantile hepatic hemangiomas: looking backwards and forwards. Precis Clin Med 2022;5:pbac006. [Crossref] [PubMed]
- Franchi-Abella S, Gorincour G, Avni F, Guibaud L, Chevret L, Pariente D. Hepatic haemangioma-prenatal imaging findings, complications and perinatal outcome in a case series. Pediatr Radiol 2012;42:298-307. [Crossref] [PubMed]
- Cabrita SV, Gonçalves S, Rodrigues H, Guerra N, Moura P. Antenatal diagnosis of congenital hepatic hemangioma: a case report. Cases J 2009;2:6829. [Crossref] [PubMed]
- Xie LL, Huang YB, Dong KR, Yang SB, Shen C, Ma YY. Clinical outcome of giant fetal hepatic hemangioma: analysis of 22 cases. Chin J Perinat Med 2022;4:278-83.
- Jiao-Ling L, Xiu-Ping G, Kun-Shan C, Qiu-Ming H, Xiao-Fen L, Bo-Yang Y, Qian F. Huge fetal hepatic Hemangioma: prenatal diagnosis on ultrasound and prognosis. BMC Pregnancy Childbirth 2018;18:2. [Crossref] [PubMed]
- Zhang D, Wang J. Prenatal diagnosis and management of fetal hepatic hemangioma. Zhejiang Da Xue Xue Bao Yi Xue Ban 2019;48:439-45. [Crossref] [PubMed]
- Schmitz R, Heinig J, Klockenbusch W, Kiesel L, Steinhard J. Antenatal diagnosis of a giant fetal hepatic hemangioma and treatment with maternal corticosteroid. Ultraschall Med 2009;30:223-6. [Crossref] [PubMed]
- Yang S, Huang Y, Dong K, Sun L, Xiong Y, Shen C. Postnatal outcomes and prognosis of fetal intra-abdominal solid masses diagnosed by prenatal ultrasound. Chin J Perinat Med 2022;5:355-9.
- Zenzen W, Perez-Atayde AR, Elisofon SA, Kim HB, Alomari AI. Hepatic failure in a rapidly involuting congenital hemangioma of the liver: failure of embolotherapy. Pediatr Radiol 2009;39:1118-23. [Crossref] [PubMed]
- Morimura Y, Fujimori K, Ishida T, Ito A, Nomura Y, Sato A. Fetal hepatic hemangioma representing non-reassuring pattern in fetal heart rate monitoring. J Obstet Gynaecol Res 2003;29:347-50. [Crossref] [PubMed]
- Deb G, Donfrancesco A, Ilari I, De Sio L, Milano GM, Ghitti C, Fontana G, Sandri A, Helson L. Hemangioendothelioma: successful therapy with interferon-alpha: a study in Association with the Italian Pediatric Haematology/Oncology Society (AIEOP). Med Pediatr Oncol 2002;38:118-9. [Crossref] [PubMed]
- Ji Y, Chen S, Xiang B, Xu Z, Jiang X, Liu X, Wang Q, Lu G, Yang L. Clinical features and management of multifocal hepatic hemangiomas in children: a retrospective study. Sci Rep 2016;6:31744. [Crossref] [PubMed]
- Sepulveda W, Sepulveda F, Corral E, Gutierrez J. Giant hepatic hemangioma in the fetus: case reports and updated review of the literature. J Matern Fetal Neonatal Med 2021;34:2554-66. [Crossref] [PubMed]
- Chuileannain FN, Rowlands S, Sampson A. Ultrasonographic appearances of fetal hepatic hemangioma. J Ultrasound Med 1999;18:379-81. [Crossref] [PubMed]
- Iacobas I, Phung TL, Adams DM, Trenor CC 3rd, Blei F, Fishman DS, Hammill A, Masand PM, Fishman SJ. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J Pediatr 2018;203:294-300.e2. [Crossref] [PubMed]
- Dong SZ, Zhu M, Zhong YM, Yin MZ. Use of foetal MRI in diagnosing hepatic hemangioendotheliomas: a report of four cases. Eur J Radiol 2010;75:301-5. [Crossref] [PubMed]
- Sepulveda W, Wong AE, Sepulveda F, Martinez-Ten P, Ximenes R. Fetal magnetic resonance imaging and three-dimensional ultrasound in clinical practice: general aspects. Best Pract Res Clin Obstet Gynaecol 2012;26:575-91. [Crossref] [PubMed]
- Chaturvedi A, Klionsky NB, Saul D. Ultrasound with Doppler evaluation of congenital hepatic vascular shunts. Pediatr Radiol 2018;48:1658-71. [Crossref] [PubMed]
- Long Y, Kuang H, Luo Y, Jiang M, Wu X. Prenatal sonographic characteristics of fetal hepatic hemangioma and analysis on its misdiagnosis and missed diagnosis. Chin J Ultrasonogr 2020;8:679-83.
- Yang K, Feng L, Chen S, Ji Y. Progressive infantile hepatic hemangioma not responding to propranolol. J Dermatol 2019;46:e275-6. [Crossref] [PubMed]
- Berenguer B, Mulliken JB, Enjolras O, Boon LM, Wassef M, Josset P, Burrows PE, Perez-Atayde AR, Kozakewich HP. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol 2003;6:495-510. [Crossref] [PubMed]
- North PE, Waner M, Mizeracki A, Mihm MC Jr. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol 2000;31:11-22. [Crossref] [PubMed]
- Shen Y, Cai A, Wang X, Wang B. Analysis of Prenatal and Postpartum Ultrasound Diagnosis of Fetal Abdominal Mass in 19 Cases. Journal of Practical Obstetrics and Gynecology 2018;10:769-73.
- Tenkumo C, Hanaoka U, AboEllail MA, Ishimura M, Morine M, Maeda K, Hata T. HDlive Flow with HDlive silhouette mode in diagnosis of fetal hepatic hemangioma. Ultrasound Obstet Gynecol 2017;49:541-2. [Crossref] [PubMed]
- Chen Y, Tu Y, Yang C, Shang N, Huang Y, Zhong W. Prenatal Ultrasound Combined with MRI in the Diagnosis of Fetal Haptic Hemangioma. Chinese Journal of Medical Imaging 2021;5:503-6.
- Chou SY, Chiang HK, Chow PK, Wu CF, Liang SJ, Hsu CS. Fetal hepatic hemangioma diagnosed prenatally with ultrasonography. Acta Obstet Gynecol Scand 2005;84:301-2. [Crossref] [PubMed]
- Liu D, Yu J, Yang Y, Ouyang M, Zhang M, Zeng S, Xu G. Unusual presentation of a case of fetal hepatic mass: a case report. BMC Pregnancy Childbirth 2023;23:290. [Crossref] [PubMed]
- Prokurat A, Kluge P, Chrupek M, Kościesza A, Rajszys P. Hemangioma of the liver in children: proliferating vascular tumor or congenital vascular malformation? Med Pediatr Oncol 2002;39:524-9. [Crossref] [PubMed]
- Boon LM, Burrows PE, Paltiel HJ, Lund DP, Ezekowitz RA, Folkman J, Mulliken JB. Hepatic vascular anomalies in infancy: a twenty-seven-year experience. J Pediatr 1996;129:346-54. [Crossref] [PubMed]
- Zhang Z, Chen HJ, Yang WJ, Bu H, Wei B, Long XY, Fu J, Zhang R, Ni YB, Zhang HY. Infantile hepatic hemangioendothelioma: a clinicopathologic study in a Chinese population. World J Gastroenterol 2010;16:4549-57. [Crossref] [PubMed]
- Morris J, Abbott J, Burrows P, Levine D. Antenatal diagnosis of fetal hepatic hemangioma treated with maternal corticosteroids. Obstet Gynecol 1999;94:813-5. [Crossref] [PubMed]
- Tang H, Yang C, Li J, Wang W, Chen D, Wu Q. Prenatal diagnosis and intrauterine treatment of a giant fetal hepatic hemangioma:a case report. Chin J Perinat Med 2023;4:331-4.
- Li Y, Yan Y, Yang Z, Xue X, Zhang C, Wang Y, Liu G, Pei Q. The Analysis of Ultrasonographic Features and Prognosis of Fetal Congenital Hepatic Hemangioma. Chinese J Ultrasound Med 2023;4:468-71.
- Wang P, Zhu X, Rao W, Xie W. Infantile hepatic hemangioendothelioma with heart failure in 2 cases. Chin J Pediatr 2020;7:508-11.
- Shen X, Lin L, Shi L. Three cases of infantile hepatic hemangioendothelioma complicated with persistent pulmonary hypertension in neonates. Chin J Pediatr 2017;7:547-8. [Crossref] [PubMed]
- Xie LL, Huang YB, Dong KR, Yang SB, Shen C, Ma YY. Postnatal treatment and evolution patterns of giant fetal hepatic hemangioma: a case series of 29 patients. BMC Pediatr 2024;24:8. [Crossref] [PubMed]
- Roebuck D, Sebire N, Lehmann E, Barnacle A. Rapidly involuting congenital haemangioma (RICH) of the liver. Pediatr Radiol 2012;42:308-14. [Crossref] [PubMed]




