
Comparative analysis of different biopsy techniques for pancreatic lesions in diagnostic value, safety, and cost-effectiveness
Introduction
Pancreatic cancer is a common malignancy of the digestive system, with both incidence and mortality rates rising annually. Globally, it has become the seventh leading cause of cancer-related death (1,2). The disease is characterized by an insidious onset, with early symptoms typically being non-specific. Commonly, patients experience symptoms such as upper abdominal discomfort, back pain, indigestion, jaundice, and weight loss only when the disease has progressed to an advanced stage. Consequently, many patients are diagnosed at an advanced stage, often with metastasis, making them ineligible for surgery (3). In such cases, accurate pathological assessment is essential for guiding nonsurgical treatment; it is also necessary for defining the disease before neoadjuvant therapy in potentially resectable cases (4). Furthermore, conditions such as focal chronic pancreatitis can mimic malignancy, also requiring biopsy for accurate diagnosis (5).
Currently, pancreatic lesion biopsies can be guided by various imaging modalities, including ultrasound (US), computed tomography (CT), and endoscopic ultrasound (EUS) (6). Biopsies may also be performed through laparoscopic or open surgical approaches. Although surgical biopsy remains the most conclusive and reliable diagnostic approach, its high cost, invasiveness, and greater risk of complications make it a last resort. In contrast, imaging-guided fine needle aspiration or biopsy is minimally invasive, cost-effective, convenient, and safer, leading to its growing use in clinical practice.
Previous research has mainly compared the diagnostic efficacy or safety of two biopsy techniques for pancreatic lesions (7-9), with limited studies directly comparing three methods. Additionally, there is a significant gap in cost-effectiveness analysis for pancreatic biopsy techniques. Therefore, the objective of this study was to compare the diagnostic efficacy, postoperative complication rates, and cost-effectiveness of three methods: percutaneous ultrasound-guided core needle biopsy (US-CNB), percutaneous computed tomography-guided core needle biopsy (CT-CNB), and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2670/rc).
Methods
Patient selection
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This retrospective study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2023-329). The collection of informed consent from patients was deemed unnecessary and waived due to the retrospective nature of the study. This study was conducted at the Department of Medical Ultrasound, West China Hospital, Sichuan University. From January 2018 to January 2023, consecutive patients who underwent US-CNB, CT-CNB, or EUS-FNA for suspected malignant pancreatic lesions based on imaging procedure results from CT, magnetic resonance imaging (MRI), and/or EUS were included in this study. Patients with inadequate follow-up data were excluded. We calculated the power of the Wald tests for assessing whether the parameters are equal to zero (null hypothesis) at a 5% significance level (two-sided). The final sample size in this study ensures an adequate level of precision.
Data on lesion size, location, cytologic or histological report, histological diagnosis after surgical resection or clinical/radiological follow-up findings, and short-term complications after pancreatic biopsy (≤7 days after the procedure, or until discharge if this occurred before day 7). Lesion size was primarily determined using contrast-enhanced CT/MRI, as it was the standard imaging modality in all cases. The measurements were taken at the largest cross-sectional diameter of the lesion. Post-biopsy complications were categorized into minor and major according to the Society of Interventional Radiology’s guidelines (10).
Sampling techniques
During the biopsy procedure, US systems with abdominal probes were utilized, including Hitachi HI VISION Preirus (Hitachi, Tokyo, Japan), the Philips IU-22 (Philips Healthcare, Chicago, IL, USA), and the Mindray Resona7 (Mindray, Shenzhen, China). Lesion size, location, echogenicity, shape, and depth were re-evaluated to ensure accurate biopsy planning. Color Doppler imaging was used to assess the vascular supply of the lesion and proximity to major vessels to minimize the risk of vascular injury. Depending on the lesion’s location, patients were positioned supine or in the right lateral decubitus position, and the optimal puncture site was marked. Standard protocols for skin disinfection were followed, and local anesthesia with 1% lidocaine was administered. Under real-time ultrasound guidance, a 17G coaxial puncture needle (Bard Peripheral Vascular Inc., Tempe, AZ, USA) was advanced to the lesion’s periphery. The coaxial cannula was stabilized, the stylet was removed, and an 18G tissue biopsy needle (Bard Peripheral Vascular Inc.) was used to obtain 2–3 tissue samples, depending on the tissue quality and bleeding. Specimens were fixed in 10% formalin and sent for pathological analysis.
Before the biopsy, a CT scan (SOMATOM Definition AS+ 64; Siemens Healthineers, Erlangen, Germany) was performed to visualize the pancreatic lesion and assess blood flow, ensuring major vessels and necrotic areas were avoided. The biopsy site was marked on the skin, followed by standard disinfection and draping. After administering 1% lidocaine for local anesthesia, a small incision was made. A 17G coaxial puncture needle (Bard Peripheral Vascular Inc.) was positioned at the lesion’s edge, and a CT scan confirmed its placement. After verifying the needle placement, the stylet was removed, and an 18G biopsy needle (Bard Peripheral Vascular Inc.) was advanced through the coaxial cannula to obtain a tissue. The specimen was then fixed in 10% formalin and sent for pathological analysis.
During the biopsy, the patient was typically positioned in the left lateral decubitus position under general anesthesia. An EUS (UM-G20-29R; Olympus, Tokyo, Japan) was inserted into the stomach or duodenal to assess the pancreatic lesion, including its location, size, pancreatic duct dilation, and surrounding structures. Based on this assessment, a 22G puncture needle (ECHO-3-22; COOK, Bloomington, IN, USA) was directed towards the lesion. A 5 mL syringe was used to create continuous suction, employing a negative pressure technique to collect the specimen. The specimen was sent to the pathology department for further processing.
Evaluation of FNA/CNB and follow-up findings
The successful acquisition of cytological or histological specimens was considered a technical success. Samples were considered ‘diagnostic’ when a formal pathological report was issued; they were considered ‘non-diagnostic’ if the collected sample was deemed inadequate for proper cytological analysis. Cytological/histological results of FNA/CNB were reviewed by experienced physicians blinded to the clinical and biologic data of patients. According to the Papanicolaou Society of Cytopathology (PSC) (11), FNA cytology diagnoses are categorized into six classes: nondiagnostic (I); benign (II); atypical (III); neoplastic, benign (IV A); neoplastic, other (IV B); suspicious (V); and malignant (VI). Cytologically negative cases were defined as categories II and IV A, representing specimens with no evidence of malignancy. Cytologically positive cases included categories IVB and VI, reflecting samples with malignant or high-risk neoplastic features. Categories III and V, associated with higher malignancy risk (9), were also considered positive, following D’Onofrio et al.’s (12) approach. CNB pathological diagnoses were classified into nondiagnostic, benign, atypical, and malignant. Benign was considered negative, whereas atypical and malignant were classified as positive, based on previously published medical literature standards (13).
The final diagnosis was confirmed by histopathology of resected specimens (surgical pathology) or by clinical and radiological follow-up of at least six months. Criteria for a positive result included the following: (I) malignancy confirmed by postoperative pathology; (II) significant lesion reduction after anti-tumor therapy; or (III) over 50% lesion enlargement or new metastases during follow-up. Negative results were determined by the following: (I) benign pathology after surgery; (II) lesion reduction or disappearance without anti-tumor therapy; (III) spontaneous resolution of pancreatic abnormalities during follow-up; or (IV) no lesion growth over at least six months without anti-tumor treatment. Intraductal papillary mucinous neoplasms (IPMN) encompasses both benign and malignant neoplasms. The final diagnosis of IPMN was based on postoperative pathological findings in patients who had undergone surgery. For non-surgical cases, assessment followed the 2017 International IPMN Guidelines (Fukuoka Consensus) (14), with particular attention to High-Risk Stigmata. Additionally, during follow-up, the development of Worrisome Features was closely monitored.
Cost-effectiveness analysis
This study focused on direct medical costs, including biopsy procedures, pathology diagnostics, and post-biopsy complication management, excluding indirect costs such as productivity loss and travel (15). Pathology costs encompass routine microscopy diagnosis and immunohistochemistry (IHC) tests for certain patients. IHC staining helps identify specific protein expressions, aiding diagnosis in selected cases. Diagnostic accuracy is used as the effectiveness metric to calculate the cost/effectiveness ratio (C/E) and incremental cost-effectiveness ratio (ICER). According to World Health Organization (WHO) guidelines, willingness-to-pay (WTP) threshold for cost-effectiveness analysis in China is set at 196,500 yuan (three times the per capita GDP in 2018), and a method is cost-effective if its ICER is below this threshold (16,17). A one-way sensitivity analysis, adjusting biopsy costs by ±10%, ensures robust conclusions.
Statistical analysis
Statistical analysis was conducted using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Quantitative data were presented as mean ± standard deviation, with differences in age and lesion size compared using analysis of variance (ANOVA). Categorical variables were expressed as frequencies and percentages. The chi-square test or Fisher’s exact test was used to compare the technical success rates, sample adequacy, diagnostic accuracy, sensitivity, specificity, and complication rates among the three groups. Subgroup analyses based on lesion size and location were performed using Fisher’s exact test to compare diagnostic accuracy, sample adequacy, and complication rates. Statistical significance was determined using Bonferroni correction, with a P<0.0167 considered statistically significant.
Results
Participant characteristics
Between January 2018 and January 2023, 424 patients underwent biopsy for suspected malignant pancreatic lesions using US-CNB, CT-CNB, or EUS-FNA. After excluding 25 patients due to insufficient follow-up data, 399 patients (mean age, 58.27±13.32 years) were analyzed (Figure 1). Of these, 258 underwent EUS-FNA, 86 underwent US-CNB, and 55 underwent CT-CNB. Lesion sizes in the EUS-FNA group (3.32±1.32 cm) were significantly smaller compared to those in the US-CNB (4.80±2.25 cm) and CT-CNB (4.37±1.74 cm) groups (P<0.001). Additionally, lesions in the EUS-FNA group (78.70%) were more frequently located in the pancreatic head or neck compared to the US-CNB (44.19%) or CT-CNB group (47.27%) (P<0.001) (Table 1).
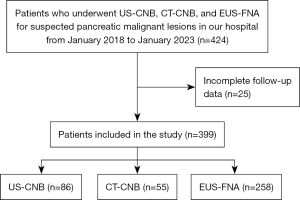
Table 1
| Variable | US-CNB | CT-CNB | EUS-FNA | P value* |
|---|---|---|---|---|
| Age (years) | 59.70±12.35 | 60.71±9.21 | 58.52±10.89 | 0.132 |
| Gender | ||||
| Male | 44 (51.16) | 32 (58.20) | 155 (60.08) | 0.349 |
| Female | 42 (48.84) | 23 (41.80) | 103 (39.92) | |
| Lesion location | ||||
| Head/neck | 38 (44.19) | 26 (47.27) | 203 (78.70) | <0.001… |
| Body/tail | 47 (55.81) | 29 (52.73) | 55 (21.30) | |
| Lesion size (cm) | 4.80±2.25 | 4.37±1.74 | 3.32±1.32 | <0.001… |
| <2 | 5 (5.80) | 4 (7.27) | 45 (17.40) | 0.014… |
| ≥2 | 81 (94.20) | 51 (92.73) | 213 (82.60) |
Data are presented as mean ± standard deviation or number (percentage, %); *, P value <0.0167, the difference is statistically significant; …, there is a statistical difference in US-CNB vs. EUS-FNA group, CT-CNB vs. EUS-FNA group. CT-CNB, computed tomography-guided core needle biopsy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; US-CNB, ultrasound-guided core needle biopsy.
Sample analysis
All 399 cases had a 100% sample acquisition rate. The satisfaction rates for sample adequacy were 97.70% for US-CNB, 90.90% for CT-CNB, and 74.03% for EUS-FNA. Notably, the satisfaction rate for EUS-FNA was significantly lower compared to the other two methods (P<0.001).
In the US-CNB group, 83 cases were diagnosed as malignant, all of which were confirmed as malignant through surgery or follow-up. One initially negative case was later confirmed as benign during follow-up. In the CT-CNB group, 43 cases were identified as malignant and confirmed through surgery or follow-up. Four atypical cases were classified as malignant per the study’s criteria and later confirmed during follow-up, whereas three initially benign cases were confirmed as benign during follow-up (Table S1). In the EUS-FNA group, cytology identified 112 malignant, 22 neoplastic, 22 suspicious, and nine atypical specimens, all classified as positive for malignancy. Surgery or follow-up confirmed all but one case as malignant. Of 26 negative FNA cases, 10 were later diagnosed as malignant, with the rest confirmed as benign (Table S2). The final diagnoses of all patients are listed in Table 2.
Table 2
| Final diagnosis | US-CNB | CT-CNB | EUS-FNA |
|---|---|---|---|
| Malignant, n (%) | 84 (97.67) | 49 (89.10) | 196 (75.97) |
| Adenocarcinoma | 71 | 44 | 159 |
| NETs | 3 | 2 | 21 |
| G1 | 1 | 0 | 9 |
| G2 | 1 | 2 | 9 |
| G3 | 1 | 0 | 3 |
| NECs | 3 | 1 | 0 |
| Adenosquamous carcinoma | 1 | 0 | 2 |
| Lymphoma | 2 | 0 | 3 |
| Metastasis | 3 | 1 | 7 |
| Malignant IPMN* | 0 | 0 | 1 |
| Other malignant& | 1 | 1 | 3 |
| Benign, n (%) | 2 (2.33) | 6 (10.90) | 62 (24.03) |
| Benign IPMN* | 0 | 2 | 3 |
| SPN | 0 | 1 | 1 |
| Serous cystadenoma | 0 | 0 | 1 |
| Chronic pancreatitis | 0 | 0 | 5 |
| Autoimmune pancreatitis | 1 | 2 | 9 |
| Tuberculosis | 0 | 0 | 1 |
| Other benign | 1 | 1 | 42 |
*, IPMN encompasses both benign and malignant neoplasms. &, this category refers to cases where pathological examination failed to provide a definitive malignant diagnosis, but follow-up imaging results or clinical progression indicated features suggestive of malignancy. CT-CNB, computed tomography-guided core needle biopsy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; IPMN, intraductal papillary mucinous neoplasms; NETs, neuroendocrine tumor; NECs, neuroendocrine carcinomas; SPN, solid pseudopapillary neoplasm; US-CNB, ultrasound-guided core needle biopsy.
Diagnostic value analysis
The analysis demonstrated that the diagnostic accuracy of the US-CNB (97.70%) and CT-CNB (90.90%) groups was significantly higher than that of the EUS-FNA group (69.80%), with a statistically significant difference (P<0.001). However, there were no statistically significant differences in sensitivity or specificity among the US-CNB, CT-CNB, and EUS-FNA groups (Table 3).
Table 3
| Variable | US-CNB | CT-CNB | EUS-FNA | P value* |
|---|---|---|---|---|
| Accuracy, % | 97.70 | 90.90 | 69.80 | <0.001… |
| Sensitivity, % | 100 | 100 | 94.30 | 0.02 |
| Specificity, % | 100 | 100 | 94.10 | 0.88 |
| Satisfactory rate, % | 97.70 | 90.90 | 74.03 | <0.001… |
| Technical success, % | 100 | 100 | 100 | >0.999 |
*, P value <0.0167 is considered statistically significant; …, there is a statistical difference in US-CNB vs. EUS-FNA group, CT-CNB vs. EUS-FNA group. CT-CNB, computed tomography-guided core needle biopsy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; US-CNB, ultrasound-guided core needle biopsy.
Procedure-related complication rates
The overall complication rates following biopsy procedures were 15.12% for US-CNB, 16.36% for CT-CNB, and 10.47% for EUS-FNA, with no statistically significant differences among these groups (P=0.319), as detailed in Table 4.
Table 4
| Variable | US-CNB | CT-CNB | EUS-FNA | P value* |
|---|---|---|---|---|
| Complication rate, n (%) | 13 (15.12) | 9 (16.36) | 27 (10.47) | 0.319 |
| Pain | 12 | 8 | 26 | |
| Bleeding | 1 | 1 | 1 |
*, P value <0.0167 is considered statistically significant. CT-CNB, computed tomography-guided core needle biopsy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; US-CNB, ultrasound-guided core needle biopsy.
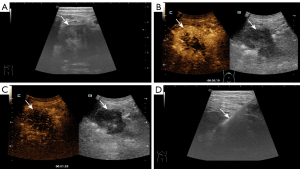
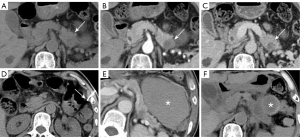
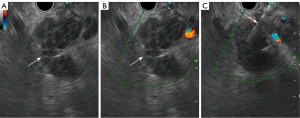
Pain was the most common complication: 13 cases in the US-CNB group, eight in CT-CNB, and 26 in EUS-FNA. Except for one major bleeding case in the EUS-FNA group, all were minor and managed with observation or symptomatic treatment. Each group had one case of bleeding. In the US-CNB group, one patient experienced hemorrhagic fluid from an abdominal drain (placed pre-procedure due to significant ascites) the day after the biopsy. This patient’s hemoglobin dropped from 124 g/L to 115 g/L, but the bleeding was controlled with tranexamic acid and aprotinin finally (Figure 2). In CT-CNB, one patient’s hemoglobin dropped from 120 to 97 g/L post-procedure. A follow up abdominal CT revealed a new 10.6 cm × 7.8 cm hematoma in the left upper abdomen, not present before. This patient was treated with tranexamic acid and aprotinin. Four weeks later, the second CT-scan showed that the hematoma had reduced to 2.4 cm × 1.8 cm (Figure 3). In EUS-FNA, one patient developed intermittent hematemesis and melena the day after the procedure, with hemoglobin dropping from 118 to 92 g/L. The patient was treated with pharmacological hemostatics and a red blood cell transfusion, leading to resolution of the bleeding (Figure 4).
Subgroup analysis
In both lesions <2 and ≥2 cm, diagnostic accuracy and sampling satisfaction rates were significantly higher in the US-CNB and CT-CNB groups compared to EUS-FNA. Subgroup analyses by lesion location similarly showed that the US-CNB and CT-CNB groups had superior diagnostic accuracy and sampling satisfaction rates compared to EUS-FNA, with statistically significant differences. Further details are presented in Table S3.
Cost-effectiveness analysis
Table 5 shows the costs, C/Es, and ICERs for each biopsy technique. The per capita costs were 2,510.71 yuan for US-CNB, 4,772.38 yuan for CT-CNB, and 6,519.30 yuan for EUS-FNA. Compared to EUS-FNA, the ICER for US-CNB was −14,367.7 yuan, and for CT-CNB was −8,279.22 yuan per correct diagnosis, both below the WTP threshold. Since needle biopsy costs have the greatest impact on the overall total, we adjusted this variable by 10% in our sensitivity analysis. As the cost of biopsy fluctuates within a certain range, the ICERs remains at a level below the established threshold standard (Tables S4,S5).
Table 5
| Group | Cost/person (yuan) | Accuracy | C/E | ICER |
|---|---|---|---|---|
| US-CNB | 2,510.71 | 97.70% | 2,569.81 | −14,367.7 |
| CT-CNB | 4,772.38 | 90.90% | 5,250.15 | −8,279.22 |
| EUS-FNA | 6,519.30 | 69.80% | 9,339.97 | – |
C/E, cost/effectiveness ratio; CT-CNB, computed tomography-guided core needle biopsy; EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; ICER, incremental cost-effectiveness ratio; US-CNB, ultrasound-guided core needle biopsy.
Discussion
This study evaluated the diagnostic efficacy, safety, and cost-effectiveness of US-CNB, CT-CNB, and EUS-FNA for pancreatic suspicious malignant lesions. The results demonstrated that US-CNB and CT-CNB had superior diagnostic accuracy (97.70% vs. 90.90% vs. 69.80%, P<0.001) and sampling satisfaction (97.70% vs. 90.90% vs. 74.03%, P<0.001) compared to EUS-FNA, with no significant difference in postoperative complications among groups. Moreover, cost-effectiveness analysis showed that US-CNB and CT-CNB were more cost-effective and economically advantageous than EUS-FNA.
Currently, several methods exist for obtaining pancreatic tissue samples. EUS-FNA is commonly the first choice for diagnosing pancreatic lesions, combining endoscopy and ultrasound to provide clear images by bypassing interference from gastrointestinal gas or fat (18). FNA samples are limited by providing predominantly cytological material with minimal tissue architecture, which is crucial for diagnosing conditions such as lymphoma, neuroendocrine tumors, and autoimmune pancreatitis (19). This limitation may hinder identifying key features such as lymphoplasmacytic infiltrates, angioinvasion, or stromal patterns necessary for accurate diagnosis, staging, and treatment planning. Combining FNA with core needle biopsy or IHC is essential to ensure comprehensive pathological evaluation and guide optimal therapeutic strategies. Rapid onsite evaluation (ROSE) helps ensure sample adequacy by allowing real-time assessment by a cytopathologist. Previous research has indicated that the presence of ROSE during EUS-FNA of pancreatic lesions is a factor that affects diagnostic outcomes (20). Ecka et al. reported that ROSE significantly reduces unsatisfactory samples (5.6% vs. 29.3%, P=0.001) (21). Without ROSE, this study found sample adequacy and diagnostic accuracy rates of 71.1% and 67.6%, respectively. In cases where pathology results conflict with clinical suspicion of malignancy, a second biopsy or further investigation is necessary, despite the increased risk of complications and psychological or financial burden on patients (22). CNB samples are of higher quality than FNA, providing more tissue for comprehensive histological and IHC analysis (13). Studies confirm that CNB yields sufficient tissue for accurate diagnosis, with superior accuracy compared to FNA for pancreatic diseases (23-25). This study also found significantly higher diagnostic accuracy in the US-CNB and CT-CNB groups compared to EUS-FNA. Similar results were reported by Sur et al. (25), with CT-guided pancreatic biopsy showing over 90% accuracy, consistent with our findings.
To assess if pancreatic lesion size and location affect the diagnostic efficacy and safety, we categorized based on lesion location and size, and compared diagnostic accuracy, sample adequacy, and complication rates within subgroups. The results showed US/CT-CNB had higher accuracy and sample adequacy than EUS-FNA, regardless of lesion size or location. In clinical practice, EUS-guided biopsy is preferred for small lesions due to the challenges percutaneous biopsy faces, particularly with lesion localization and guidance. The higher resolution of EUS enables more precise localization, providing clear advantages for targeting small lesions. In contrast to our findings, the study by van Riet et al. (26) indicated that there was no significant difference in diagnostic accuracy between US-CNB and EUS-FNA, regardless of lesion size or location.
Ultrasound-guided percutaneous biopsy is portable, cost-effective, and avoids ionizing radiation (27,28). In contrast, EUS-FNA time-consuming, more expensive, and requires moderate sedation (29). CT-guided biopsy, though widely used, is reserved for cases where US- or EUS-guided biopsies fail or when lesions are inaccessible by these two methods (30). Although CT provides clear cross-sectional views and accurate distance measurements (24), its use is limited by radiation exposure to both the patient and the operator. In contrast, US-guided biopsy avoids ionizing radiation, making it safer and more acceptable. Furthermore, US-guided biopsy, with real-time monitoring and enhanced safety via color Doppler, avoids this risk (13). Although CEUS-CNB was not used in this study, it is widely used for abdominal organs, especially in liver biopsy (31,32). Further research is needed on its use for pancreatic lesions.
Although imaging-guided pancreatic biopsy is generally safe, complications still occur. Studies have shown that complication rates for percutaneous biopsy range from 0% to 21.4% (33,34), whereas those for EUS-guided biopsy range from 3.6% to 22% (35). This study found that the postoperative complication rates for US-CNB, CT-CNB, and EUS-FNA are 15.12%, 16.36%, and 10.47% respectively, with no significant differences (P=0.319). Previous reports on complications related to pancreatic biopsy have shown a wide range of incidence rates, which may stem from differing definitions. In Ross et al.’s study (36), performing EUS-FNA alongside retrograde cholangiopancreatography contributed to higher complication rates. Although our study was retrospective, all biopsy patients in our study were hospitalized, closely monitored, and underwent tests and imaging if complications were suspected. The entire process was carefully documented in the patients’ hospital records to ensure accuracy. Previous studies have reported severe complications from US- or CT-guided CNB (12,30,34). However, in this study, only mild complications were observed in the US/CT-CNB group. The most common issue was postoperative pain, lasting less than 24 hours and relieved by analgesics. Two patients experienced bleeding, resolved with hemostatic medication. The absence of severe complications may be due to the coaxial technique, which minimizes tissue damage and bleeding. Additionally, injecting embolic agents through the coaxial cannula helps prevent potential postoperative bleeding (37,38). A prospective study involving 355 patients reported that the incidence of severe complications related to pancreatic lesions after EUS-FNA was 2.54% (39). In this study, the EUS-FNA group had 26 patients with mild complications and one with severe complication, lower than previously reported rates.
US-CNB, CT-CNB, and EUS-FNA each have advantages and disadvantages. When selecting a biopsy method, diagnostic accuracy, safety, and cost-effectiveness are key considerations. This study assessed biopsy cost-effectiveness and found US/CT-CNB to be more economical. However, EUS-FNA is often preferred due to ease of use, physician familiarity, and patient preference. EUS-FNA’s high-resolution imaging and less invasive nature make it more acceptable, despite the cost advantage of US/CT-guided biopsies.
This retrospective study compared US-CNB, CT-CNB, and EUS-FNA for diagnosing suspected pancreatic malignant lesions, considering diagnostic efficacy, safety, and cost-effectiveness. However, it had several limitations. First, the retrospective design limited standardized long-term follow-up, with incomplete radiographic and clinical data. Additionally, the retrospective design of this study inherently restricted our ability to standardize the selection of biopsy methods, which would have contributed a certain degree of selection bias. Furthermore, the follow-up period was short, potentially hindering a full assessment of long-term effects. In addition, the cost-effectiveness analysis may have lacked certain factors, with results varying by region and healthcare system.
Conclusions
Imaging-guided biopsy is a reliable and effective method for obtaining pancreatic tissue samples. For suspected malignant pancreatic lesions, US-CNB and CT-CNB provide superior sample adequacy and diagnostic accuracy compared to EUS-FNA. Additionally, US-CNB and CT-CNB are more cost-effective than EUS-FNA in diagnosing these lesions.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2670/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2670/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2023-329) and the requirement for individual consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Partyka O, Pajewska M, Kwaśniewska D, Czerw A, Deptała A, Budzik M, et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers (Basel) 2023;15:3634. [Crossref] [PubMed]
- Li HY, Cui ZM, Chen J, Guo XZ, Li YY. Pancreatic cancer: diagnosis and treatments. Tumour Biol 2015;36:1375-84. [Crossref] [PubMed]
- Moutinho-Ribeiro P, Iglesias-Garcia J, Gaspar R, Macedo G. Early pancreatic cancer - The role of endoscopic ultrasound with or without tissue acquisition in diagnosis and staging. Dig Liver Dis 2019;51:4-9. [Crossref] [PubMed]
- Neff CC, Simeone JF, Wittenberg J, Mueller PR, Ferrucci JT Jr. Inflammatory pancreatic masses. Problems in differentiating focal pancreatitis from carcinoma. Radiology 1984;150:35-8. [Crossref] [PubMed]
- D’Onofrio M, De Robertis R, Barbi E, Martone E, Manfrin E, Gobbo S, Puntel G, Bonetti F, Pozzi Mucelli R. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol 2016;26:1801-7. [Crossref] [PubMed]
- Chai WL, Kuang XF, Yu L, Cheng C, Jin XY, Zhao QY, Jiang TA. Percutaneous ultrasound and endoscopic ultrasound-guided biopsy of solid pancreatic lesions: An analysis of 1074 lesions. Hepatobiliary Pancreat Dis Int 2023;22:302-9. [Crossref] [PubMed]
- Caymaz I, Afandiyeva N. Diagnostic Evaluation of Solid Pancreatic Lesions: Endoscopic Ultrasound-Guided Fine Needle Aspiration Versus Percutaneous Ultrasound-Guided Core Needle Biopsy. Cardiovasc Intervent Radiol 2023;46:1596-602. [Crossref] [PubMed]
- Syed A, Babich O, Rao B, Singh S, Carleton N, Gulati A, Kulkarni A, Garg M, Farah K, Kochhar G, Morrissey S, Mitre M, Kulkarni A, Dhawan M, Silverman JF, Pharaon M, Thakkar S. Endoscopic ultrasound-guided fine-needle aspiration vs core needle biopsy for solid pancreatic lesions: Comparison of diagnostic accuracy and procedural efficiency. Diagn Cytopathol 2019;47:1138-44. [Crossref] [PubMed]
- Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199-202. [Crossref] [PubMed]
- Pitman MB, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: A review. Cancer Cytopathol 2014;122:399-411. [Crossref] [PubMed]
- D’Onofrio M, Beleù A, De Robertis R. Ultrasound-guided percutaneous procedures in pancreatic diseases: new techniques and applications. Eur Radiol Exp 2019;3:2. [Crossref] [PubMed]
- Tyng CJ, Almeida MF, Barbosa PN, Bitencourt AG, Berg JA, Maciel MS, Coimbra FJ, Schiavon LH, Begnami MD, Guimarães MD, Zurstrassen CE, Chojniak R. Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World J Gastroenterol 2015;21:3579-86. [Crossref] [PubMed]
- Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738-53. [Crossref] [PubMed]
- Shiina T, Goto-Hirano K, Takura T, Daida H. Cost-effectiveness of follow-up invasive coronary angiography after percutaneous coronary stenting: a real-world observational cohort study in Japan. BMJ Open 2022;12:e061617. [Crossref] [PubMed]
- Wen F, Zheng H, Zhang P, Zhou J, Chen H, Zhou K, Li Q, Bi F. Patient-based cost-effectiveness analysis of FOLFIRI versus FOLFOX7 for advanced gastric adenocarcinoma in China: A 4-year prospective randomised phase II study. Eur J Cancer Care (Engl) 2020;29:e13196. [Crossref] [PubMed]
- Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol 2020;16:1189-98. [Crossref] [PubMed]
- Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer 2003;99:285-92. [Crossref] [PubMed]
- Matsubayashi H, Sasaki K, Ono S, Abe M, Ishiwatari H, Fukutomi A, Uesaka K, Ono H. Pathological and Molecular Aspects to Improve Endoscopic Ultrasonography-Guided Fine-Needle Aspiration From Solid Pancreatic Lesions. Pancreas 2018;47:163-72. [Crossref] [PubMed]
- van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc 2019;89:329-39. [Crossref] [PubMed]
- Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of a tertiary hospital. Diagn Cytopathol 2013;41:1075-80. [Crossref] [PubMed]
- Cho IR, Jeong SH, Kang H, Kim EJ, Kim YS, Cho JH. Comparison of contrast-enhanced versus conventional EUS-guided FNA/fine-needle biopsy in diagnosis of solid pancreatic lesions: a randomized controlled trial. Gastrointest Endosc 2021;94:303-10. [Crossref] [PubMed]
- Stella SF, Van Borsel M, Markose G, Nair SB. Image-Guided Percutaneous Biopsy for Pancreatic Lesions: 10-Year Experience in a Tertiary Cancer Center. Can Assoc Radiol J 2019;70:199-203. [Crossref] [PubMed]
- Liu J, Huang W, Wang S, Wu Z, Wang Z, Ding X, Wang Z. Comparison of core needle biopsy and fine-needle aspiration methods in CT-guided percutaneous sampling of pancreatic tumors. J Cancer Res Ther 2023;19:904-9. [Crossref] [PubMed]
- Sur YK, Kim YC, Kim JK, Lee JH, Yoo BM, Kim YB. Comparison of Ultrasound-Guided Core Needle Biopsy and Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Pancreatic Lesions. J Ultrasound Med 2015;34:2163-9. [Crossref] [PubMed]
- van Riet PA, Erler NS, Bruno MJ, Cahen DL. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: a systemic review and meta-analysis. Endoscopy 2021;53:411-23. [Crossref] [PubMed]
- Francica G, Meloni MF, Riccardi L, de Sio I, Terracciano F, Caturelli E, Iadevaia MD, Amoruso A, Roselli P, Chiang J, Scaglione M, Pompili M. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol 2018;105:96-101. [Crossref] [PubMed]
- Jeong S, Park SB, Kim SH, Hwang JH, Shin J. Clinical significance of contrast-enhanced ultrasound in chronic kidney disease: a pilot study. J Ultrasound 2019;22:453-60. [Crossref] [PubMed]
- Huang Y, Shi J, Chen YY, Li K. Ultrasound-Guided Percutaneous Core Needle Biopsy for the Diagnosis of Pancreatic Disease. Ultrasound Med Biol 2018;44:1145-54. [Crossref] [PubMed]
- Gruber-Rouh T, Langenbach MC, Eichler K, Vogl TJ, Yel I, Beeres M. CT-guided percutaneous biopsy of suspect pancreatic lesions: radiological and clinical outcome. Clin Radiol 2019;74:899.e7-899.e12. [Crossref] [PubMed]
- Miller DL, Abo A, Abramowicz JS, Bigelow TA, Dalecki D, Dickman E, Donlon J, Harris G, Nomura J. Diagnostic Ultrasound Safety Review for Point-of-Care Ultrasound Practitioners. J Ultrasound Med 2020;39:1069-84. [Crossref] [PubMed]
- Sutherland EL, Choromanska A, Al-Katib S, Coffey M. Outcomes of ultrasound-guided renal mass biopsies. J Ultrasound 2018;21:99-104. [Crossref] [PubMed]
- Kahriman G, Ozcan N, Dogan S, Ozmen S, Deniz K. Percutaneous ultrasound-guided core needle biopsy of solid pancreatic masses: Results in 250 patients. J Clin Ultrasound 2016;44:470-3. [Crossref] [PubMed]
- Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR. CT- and US-guided biopsy of the pancreas. Radiology 1993;187:99-104. [Crossref] [PubMed]
- Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol 2003;98:2663-8. [Crossref] [PubMed]
- Ross WA, Wasan SM, Evans DB, Wolff RA, Trapani LV, Staerkel GA, Prindiville T, Lee JH. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc 2008;68:461-6. [Crossref] [PubMed]
- Lin CY, Ou MC, Liu YS, Chuang MT, Shan YS, Tsai HM, Wang CK, Tsai YS. A CT-guided fat transversing coaxial biopsy technique for pancreatic lesion biopsy that avoids major organs and vessels. Saudi J Gastroenterol 2017;23:341-7. [Crossref] [PubMed]
- Yu H, Zhang C, Liu S, Jiang G, Li S, Zhang L, Kang E, Zhang B, Xu W. Application value of coaxial biopsy system in needle cutting biopsy for focal ground glass-like density nodule. J Cancer Res Ther 2018;14:1509-14. [Crossref] [PubMed]
- Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006;63:622-9. [Crossref] [PubMed]


