
Performance of S-MAR in metal artifact reduction for intracranial aneurysm patients after endovascular embolization: a cross-sectional spectral computed tomography study
Introduction
Currently, endovascular embolization is the primary approach for the treatment of most intracranial aneurysms at many medical institutions (1-3). Digital subtraction angiography (DSA) is the gold standard for assessing aneurysmal occlusion after coiling (4). It provides high spatial resolution, three-dimensional (3D) imaging, and is resistant to device-related artifacts from coils, stents, and flow disruptors (4). However, due to the invasive nature, lengthy procedure time, and potential complications of DSA, non-invasive imaging techniques are generally preferred during the follow-up period.
Computed tomography angiography (CTA) is a widely available, cost effective, and non-invasive imaging modality with short examination times, making it a common tool for diagnosis (5). However, the imaging of coiled aneurysm is severely hampered by beam-hardening artifacts from the platinum coil mass, and as a result, some researchers view CTA as unsuitable for follow-up (5,6). Recent advancements have led to the introduction of new methods to address this limitation. Previous dual-energy computed tomography (CT) and spectral CT studies have shown that beam-hardening, blooming, and metallic artifacts are reduced in monochromatic images as energy levels increase (7,8). Virtual monochromatic imaging (VMI) at high kilo electron volt (keV) levels (>100 keV) has been shown to reduce metal artifacts in most metal implants, especially orthopedic implants (9,10). While VMI at higher keV levels effectively reduces metal artifacts, this improvement often comes at the expense of a lower contrast-to-noise ratio (CNR), posing challenges for optimal vessel visualization (11-14). Skull and atherosclerosis are common factors that interfere with imaging quality in cerebral CTA. Thus, it is difficult to achieve both metal artifact reduction (MAR) and optimal vessel depiction in coiled cerebral CTA.
Previous studies have shown that a subtraction method, in which non-enhanced data is subtracted from enhanced CTA data, improves the in-stent lumen visualization of coronary arteries, and has a performance consistent with that of DSA (15-17). Subtraction images can effectively erase background information, reducing the effects of metal implants, bones, and atherosclerosis, and thus enhancing vessel visualization.
The use of VMI or the MAR technique in conventional dual CT and spectral CT has had some effect on artifacts decreasing; however, such techniques usually have a high keV (>100 keV) condition, and thus are difficult to apply to CTA images. Additionally, DSA is not a suitable method for clinical follow-up. Thus, these limitations need to be addressed.
Because spectral CT has both high spatial and temporal resolution, these images, which include conventional computed tomography images (CI), VMI images, and virtual non-contrast (VNC) images, are reconstructed in the same phase. To address the limitations of conventional CTA in coiled aneurysm imaging, S-MAR combines VMI and subtraction techniques with MAR software to derive a subtraction image by subtracting VMI with MAR (VMIMAR) images from VNC images with MAR (VNCMAR).
A previous study initially applied S-MAR and showed that it had good performance in the 40-keV condition, and thus represents a breakthrough in addressing the issue of reducing artifacts while maintaining good vessel contrast simultaneously (18). S-MAR can elevate image quality and contribute to accurate luminal assessment, which may enhance aneurysm monitoring and treatment planning. However, in the above-mentioned study, no comparison was made between the diagnostic accuracy of S-MAR and conventional DSA. Thus, research needs to be conducted to determine whether S-MAR can accurately represent luminal measurements. In addition, the above-mentioned study focused on the performance of S-MAR at 40 keV to maintain a high CNR, but did not examine its performance at other keV levels. Identifying the optimal keV range for S-MAR could refine imaging protocols, ensuring accurate vessel assessment without compromising diagnostic accuracy.
The present study aimed to evaluate the diagnostic accuracy of S-MAR compared to DSA, and investigate the effects of different keV levels on its performance, addressing a critical gap in post-coiling aneurysm imaging. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2640/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. [2021]0633). Due to the retrospective nature of the study, the requirement of written informed consent was waived.
Patient demographic characteristics
The data of the patients included in this study were retrieved from the medical database of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. From November 2021 to February 2023, 94 patients who underwent cerebral CTA performed on spectral CT were included in the study. Of the patients, 21 were men, and 73 were women. The patients had a mean age of 56.53±10.46 years (range, 23–82 years). Additionally, eight of the patients had two coils and two patients had three coils; thus, a total of 106 coils were found. Patients with meta clips or other metal implants were excluded from the study. The average coil diameter was 7.50 mm (range, 2.3–18.8 mm). In total, 67 patients underwent endovascular embolotherapy using metal coils with stent-assisted therapy. Notably, 50 patients have underwent DSA and CTA examinations and the time interval between the two examinations is no more than six months. The coils were located as follows: internal carotid artery: 52; middle cerebral artery: 28, anterior cerebral artery: 7, anterior communicating artery: 9, vertebrobasilar artery: 7, and posterior communicating artery: 3. Patients were divided into two groups based on the presence of a stent: group A (stent-assisted, n=67) and group B (coils only, n=27). The patients were also classified into the following three groups based on coil diameter: group 1 (coil diameter ≤5 mm; n=11), group 2 (coil diameter >5 and ≤8 mm; n=64), and group 3 (coil diameter >8 mm; n=31).
Data acquisition
A dual-layer spectral CT (IQon spectral CT, Philips Healthcare, Cleveland, OH, USA) was used for all the examinations. For each patient, an 18-gauge catheter was inserted into the antecubital vein to administer intravenous contrast (Iodixanol 320), after which 30–40 mL of saline was injected. The injection protocol was determined according to the patient’s body mass index (0.6 mL/kg). Specifically, 50–60 mL of contrast was injected at a rate of 4–5 mL/s, with an injection time of 10 s. The scanning protocol was as follows: direction: caudal–cranial; monitor plane: 4th cervical vertebra; threshold value: 100 Hounsfield units (HU); voltage: 120 vp; collimation: 64×0.625 mm; pitch: 0.985; rotation time: 0.50 s; field of view: 190–210 mm; image matrix: 512×512; slice thickness: 1.0 mm; and slice increment: 0.75 mm.
First, all the images were reconstructed from spectral-based images (SBIs) using a brain standard B filter. Subsequently, the reconstructed images were generated using a post-processing workstation for the purpose of analysis. The VMI images, which had a range that extended from 40 to 110 keV and increased in steps of 10 keV, along with the VNC images, were derived from the SBI dataset. VMI can be synthesized through a process that involves decomposing two fundamental materials, reconstructing the density maps of bone and water within the projection domain, and then linearly combining the mass density maps at every energy level. Alternatively, they can be generated directly by linearly combining the low and high kilovolt peak CT images in the image domain. Conversely, the VNC images were created by subtracting the iodine map from the dual-energy enhanced CT image. All the images were then processed using the commercial MAR software for orthopedic implants (O-MAR, Philips Healthcare, Cleveland, OH, USA) to produce the VMIMAR and VNCMAR images, respectively.
Subtraction procedure
The S-MAR images were derived by subtracting the VMIMAR and VNCMAR images, based on the subtraction of the CT value. In this study, VMIMAR images ranging from 40–110 keV were chosen as enhanced images for subsequent analysis because low vessel contrast and a low CNR were observed in the VMI (>110 keV) images.
The following procedure was employed: (I) two sets of images were arranged according to the mixed Z position (based on the spatial location of each layer); (II) the weight of the VMIMAR image and VNCMAR image was “1” and “–1”, respectively; (III) a bias HU of “1000” was used; (IV) for the purpose of further analysis, thin multiplanar reformation and reconstruction images were created using techniques such as volume rendering (VR) and maximum intensity projection (MIP).
Quantitative image analysis
All the measurements were performed by two radiologists using a post-processing workstation (IQon, IntelliSpace Portal, version 9.0). Coil diameter was measured in the CI at the appropriate window level (WL) and window width (WW) to ensure that there were no halos around the coils. Subsequently, the metrical data were measured three times, and the mean value was calculated. The vessel maximum diameter (VDmax), minimum diameter (VDmin), and mean diameter (VDmean) of the CI and S-MAR images were automatically calculated using the post-processing workstation, selecting the same part of the target vessel. Manual adjustments were made if significant measurement errors were observed. Two independent and experienced radiologists, one with 13 years of experience in head and neck radiology and the other with six years of experience in the same field, determined the VDmax, VDmin, and VDmean measurements from DSA. The radiologists were not provided with detailed information about the images. The mean values were then calculated based on these measurements.
The image assessment was carried out using the region of interest (ROI) on the plane that exhibited the highest degree of metal-induced susceptibility artifacts. To more accurately locate the vessels, instead of using the CI, the S-MAR image was employed for the placement of the ROI. ROI1 was positioned at the center of the adjacent cerebral vessel to the greatest extent possible, taking care to avoid any plaques or coils. While ROI2, which had a fixed size of 50 square millimeters, was positioned in the same slice in the peripheral brain. It was positioned as close to the coil as possible to ensure that it was not influenced by the artifacts. The “copy-and-paste” function was used to replicate ROI1 and ROI2 across the remaining images to ensure the ROIs were uniformly placed at identical positions on all sets of images corresponding to each coil. Each ROI was measured three separate times by the two radiologists, and the mean value was then calculated. The CNR and background noise [Noise(Background)] were employed to assess the image quality of both the CI and S-MAR images. Noise(Background) was the standard deviation (SD) of the attenuation values present in the cerebral parenchyma. The CT(Vessel) and CT(Background) were defined as the CT values of the target vessel and peripheral parenchyma, respectively. It is important to note that when choosing the ROI, it is crucial to refrain from selecting areas where the noise level of the peripheral cerebral parenchyma approaches zero. This ensures more accurate and reliable measurements for the objective evaluation of the image quality.
The CNR and Noise(Background) of the CI and S-MAR images were calculated to evaluate the overall degree of contrast in the cerebral artery images. The CNR was calculated as follows:
For comparison, the CI were aligned with the S-MAR images by selecting the same vessel segment. The parent artery or sever stenosed artery was designated as the target vessel. The VDmax, VDmin, and VDmean were automatically calculated by the post-processing workstation.
Qualitative image analysis
A five-point CA score scale was used to evaluate the degree of metal artifacts as follows: 5 points: the artifacts completely obstruct visualization, making adjacent vessel assessment impossible; 4 points: the artifacts almost entirely obstruct visualization, significantly hampering the assessment of practically all neighboring vessels; 3 points: moderate artifacts partially affect the assessment of adjacent vessels; 2 points: minimal artifacts rarely affect the assessment of adjacent vessels; and 1 point: no artifacts, with clear visualization of adjacent vessels. Two radiologists with expertise in head and neck radiology independently scored the CI and S-MAR images. They were blinded to each other’ scores and had no knowledge of the specific details of the images. If discrepancies in scoring arose, a consensus was reached through discussion between the observers. The CA score was calculated for both the CI and S-MAR images.
Statistical analysis
The statistical analysis was carried out using SPSS Statistics 22.0 (IBM Corporation, Armonk, NY, USA). The Mann-Whitney test was used to analyze the differences in the quantitative and qualitative image analysis parameters between the CI and S-MAR images. The inter-method agreement for the VDmax, VDmin, and VDmean between the CI, S-MAR, and DSA images was assessed using the intraclass correlation coefficient (ICC). Scores on the ICC scale were categorized as follows: ≤0.40, poor correlation; 0.41–0.54, fair correlation; 0.55–0.69, moderate correlation; 0.70–0.84, good correlation; and 0.85–1.00, excellent correlation. A P value <0.001 was considered statistically significant. All the quantitative parameters are expressed as interquartile ranges (25th–75th percentiles). The inter-observer agreement was assessed using kappa statistics. Spearman’s rank correlation coefficient (rs) was employed to evaluate the correlations. Specifically, it was used to determine the relationships between the coil diameter and the CA score, as well as between the presence of a stent and the CA score. Coefficient values on the kappa scale were categorized as follows: ≤0.40, poor agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, excellent agreement.
The sample size was based on the sample sizes of previous similar studies for which sample sizes >30 were common. Thus, the sample size of 94 patients with 106 coils in this study was assumed to be an adequate sample size.
Results
Artifact reduction
The inter-observer agreement for the CA score was high (k=0.86). There was no significant difference in the CA scores between group A (stent-assisted) and group B (coils only) (P>0.001). A strong correlation was found between coil diameter and the CA score in the CI (rs =0.649, P<0.001). S-MAR (40–90 keV) reduced the values of CT(Vessel), CT(Background), and Noise(Background), while increasing the CNR. S-MAR (40–70 keV) maintained good vessel contrast and had a higher CNR than both the CI and S-MAR images (80–110 keV). However, S-MAR (100–110 keV) is not recommended due to its low vessel contrast (Table 1). Compared to the CI, S-MAR was able to significantly reduce artifacts and noise, and increase the CNR. With less interference, the anatomical details became much clearer, thereby heightening the radiologists’ confidence in making accurate diagnoses. In addition, the performance of S-MAR (40–70 keV) was better than that of S-MAR (80–90 keV) (P<0.001) (Figure 1). These results indicate that 40–70 keV is a suitable range.
Table 1
| Objective parameters | CT(Vessel) | CT(Background) | Noise(Background) | CNR |
|---|---|---|---|---|
| CI | 282.50 [236.43, 337.50] | 33.00 [18.36, 40.97] | 26.93 [18.36, 40.97] | 9.63 [5.36, 15.02] |
| S-MAR (40–50 keV) | 253.95 [201.00, 290.25] | 14.15 [3.75, 30.18] | 8.68 [5.44, 12.08] | 25.12 [17.46, 46.47] |
| S-MAR (60–70 keV) | 203.87 [167.53, 240.34] | 11.56 [3.05, 23.57] | 7.54 [4.38, 6.34] | 15.74 [9.5, 20.16] |
| S-MAR (80–90 keV) | 160.34 [135.64, 186.52] | 9.67 [2.56, 21.67] | 7.12 [4.11, 5.89] | 10.07 [6.98, 12.87] |
| S-MAR (100–110 keV) | 125.31 [106.19, 149.94] | 7.22 [2.01, 17.64] | 6.57 [3.87, 4.67] | 7.55 [4.75, 9.1] |
| P value | – | – | – | P1,2 <0.001 |
Data are expressed as median [interquartile range]. P1 represents the comparison between CI and S-MAR (40–50 keV); P2 represents the comparison between CI and S-MAR (60–70 keV). S-MAR (40–70 keV) elevated image quality compared with CI. CI, conventional computed tomography images; CNR, contrast-to-noise ratio; CT, computed tomography; CT(Background), CT value of the background; CT(Vessel), CT value of the vessel; keV, kilo electron volt; Noise(Background), noise of background; S-MAR, a combination of metal artifact reduction, virtual monochromatic imaging, and the subtraction algorithm.

Coil diameter effect
Under the CA score scale, a score of 2 points indicates scarce artifacts with minimal effect on the assessment of adjacent vessels. Statistical differences were observed between the three groups (P<0.001), showing that the performance of S-MAR was influenced by coil diameter. S-MAR (40–70 keV) performed well in groups 1 and 2, with all cases achieving a CA score ≤2, as illustrated in the representative case (Figure 2). In group 3, S-MAR (60–70 keV) performed better than S-MAR (40–50 keV) (Table 2), suggesting that higher keV levels help to further reduce artifacts. Five cases (16.7% of group 3), in which the coil diameters >12 mm, had CA scores of 3, indicating that the effect of a large coil diameter cannot be underestimated.
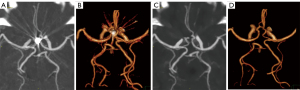
Table 2
| Subjective parameters (CA score) | Number | CI | S-MAR | |||||
|---|---|---|---|---|---|---|---|---|
| 40 keV | 50 keV | 60 keV | 70 keV | 80 keV | 90 keV | |||
| Group 1 (diameter ≤5 mm) | 11 | 4 [3, 4] | 1 [1, 1] | 1 [1, 1] | 1 [1, 1] | 1 [1, 1] | 2 [2, 2] | 2 [2, 2] |
| Percentage of CA score ≤2 (%) | – | – | 100 | 100 | 100 | 100 | 100 | 100 |
| Group 2 (5< diameter ≤8 mm) | 64 | 5 [5, 5] | 1 [1, 1] | 1 [1, 1] | 1 [1, 1] | 1 [1, 1] | 2 [2, 2] | 2 [2, 2] |
| Percentage of CA score ≤2 (%) | – | – | 100 | 100 | 100 | 100 | 81.3 | 76.6 |
| Group 3 (diameter >8 mm) | 31 | 5 [5, 5] | 2 [1, 3] | 2 [1, 2] | 2 [1, 2] | 2 [1, 2] | 3 [2, 3] | 3 [2, 3] |
| Percentage of CA score ≤2 (%) | – | – | 74.2 | 83.6 | 87.1 | 90.3 | 35.5 | 25.8 |
Data are expressed as median [interquartile range]. The performance of S-MAR (40–70 keV) was better than S-MAR (80–90 keV). S-MAR (40–70 keV) reduced metal artifacts thoroughly in groups 1 and 2, and S-MAR (60–70 keV) in group 3 showed better than S-MAR (40–50 keV). CA, coil artifact; CI, conventional computed tomography images; keV, kilo electron volt; S-MAR, a combination of metal artifact reduction, virtual monochromatic imaging, and the subtraction algorithm.
S-MAR (40–50 keV) showed almost equivalent vessel contrast to CI in vascular reconstruction. Some loss of vessel contrast was noted in S-MAR (60–70 keV), but this did not significantly affect vascular visualization (Figure 3). S-MAR (40–70 keV) successfully eliminated CA score while maintaining high vessel contrast, and had vessel visualization consistent to that of DSA. Conversely, S-MAR (80–90 keV) showed remaining artifacts and poor vessel depiction (Figure 3), which confirmed that this range is not optimal.

Quantitative image analysis
S-MAR images had better diagnostic accuracy in the VDmax, VDmin, and VDmean measurements than CI. The ICCs were 0.534 (0.476, 0.689), 0.658 (0.561, 0.717), and 0.617 (0.573, 0.687) for CI and DSA, respectively, and 0.845 (0.783, 0.884), 0.947(0.876, 0.954), and 0.956 (0.875, 0.962) for S-MAR and DSA, respectively (P<0.001) (Table 3). The CI often displayed a rough and uneven lumen caused by metal blooming artifacts, which led to the overestimation of luminal vessel diameter and the false estimation of residual intra-aneurysmal contrast enhancement (Figure 4A). Conversely, S-MAR images not only removed the metal coil but also eliminated artifacts, providing a smooth lumen and accurate vessel diameter measurement (Figure 4B).
Table 3
| Quantitative analysis | CI | S-MAR | DSA | Intraclass correlation coefficient (95% confidence interval) | |
|---|---|---|---|---|---|
| CI vs. DSA | S-MAR vs. DSA | ||||
| VDmax | 4.39 (2.01, 5.64) | 3.37 (2.88, 4.32) | 3.43 (2.64, 4.38) | 0.534 (0.476, 0.689) | 0.845 (0.783, 0.884) |
| VDmin | 2.58 (1.87, 3.28) | 3.10 (2.52, 3.87) | 3.02 (2.43, 4.10) | 0.658 (0.561, 0.717) | 0.947 (0.876, 0.954) |
| VDmean | 3.33 (2.73, 4.98) | 3.10 (2.74, 4.16) | 3.19 (2.61, 4.25) | 0.617 (0.573, 0.687) | 0.956 (0.875, 0.962) |
Data are expressed as median [interquartile range]. A P value <0.001 was considered statistically significant. CI, conventional computed tomography images; DSA, digital subtraction angiography; VDmax, vessel maximum diameter; VDmean, vessel mean diameter; VDmin, vessel minimum diameter; S-MAR, a combination of metal artifact reduction, virtual monochromatic imaging, and the subtraction algorithm.

However, S-MAR was not perfect in all cases. For large coils (i.e., those with a metal coil diameter >12 mm), the coil was removed, and the true vessel was visualized in the S-MAR images (Figure 5A,5B). Artifact-induced unconnected lumen and severe luminal stenosis were corrected (Figure 5C,5D). However, some artifacts persisted, which led to the overestimation of mild luminal stenosis visually. Conversely, no such obvious stenosis was observed in DSA (Figure 5E). It appears that beam-hardening, photon starvation, scattering, and edge effects caused by large coils, which lead to significant signal discontinuity, or CT detector loss, which leads to numerous dark or bright artifacts in line with the edge of the metal, cannot be easily eliminated or compensated by the current MAR algorithm.

Discussion
Study’s contributions
This was the first trial study to investigate the diagnostic accuracy of S-MAR in comparison to DSA. The results showed that S-MAR is an effective method for removing metal CA score and enhancing vessel visualization both qualitatively and quantitatively. The underestimation and overestimation of the vessel lumen observed in CI caused by metal blooming artifacts were corrected, and the false estimation of residual intra-aneurysmal contrast enhancement was avoided. Beam hardening, photon starvation, scattering, and edge effects caused by coils were reduced or erased, improving image quality and enhancing diagnostic confidence. This study may help radiologist sand therapists to make accurate vessel assessments, and enhance aneurysm monitoring and treatment planning in clinical practice.
Metal artifacts in CT imaging are primarily caused by beam hardening, photon starvation, scattering, and edge effects. Previous studies have shown that the size of metal implants is a significant factor in reducing metal artifacts (19,20). The present study also found a strong correlation between artifact degree and coils size. In a previous study, 5 mm was found to be a cut-off point in the performance of S-MAR (40 keV) (18). In the present study, 8 mm was found to be another cut-off point in the performance of S-MAR (40–70 keV) based on the improvement in the CA score. In the majority of clinical cases where the coil diameter was ≤8 mm, S-MAR not only efficiently diminished the artifacts caused by metal coils compared to CI, but also remarkably enhanced both the subjective and objective quality of the images. However, its effect was relatively limited for large coils (a metal coil diameter >12 mm) (Figure 5D), and the remaining minor artifacts may still lead to an overestimation of luminal stenosis (Figure 4).
Related work
In traditional dual-layer CT or spectral CT research, VMI of 130–200 keV have been shown to reduce metal artifacts, improving the visibility of adjacent muscle, bone, and organs (10,21,22). High keV images effectively reduce mild metal artifacts and introduce less severe secondary artifacts than MAR algorithms, but are unable to satisfactorily reduce severe metal artifacts caused by implants with high density (14). However, high keV levels are not suitable for cerebral CTA due to poor vessel contrast. Many MAR algorithms have been shown to be useful in reducing the effects of metal artifacts from coil masses (11-14,23). Additionally, subtraction algorithm has also been shown to enhance in-stent lumen visualization, while reducing the influence of background information (17,24).
In the present study, all images from each case were derived from the same SBI, ensuring no misregistration during the subtraction process. S-MAR uses a low keV condition and optimizes the advantage of MAR software and subtraction algorithm, which can reduce metal artifacts more effectively without generating secondary artifacts, and preserve a high CNR with excellent vessel contrast. S-MAR (40 keV) was shown to provide good vessel visualization in a previous study (18). In the present study, S-MAR (40–70 keV) demonstrated excellent performance in groups 1 and 2 (a metal coil diameter ≤8 mm). For larger coils (a metal coil diameter >8 mm), S-MAR (60–70 keV) is recommended. However, for giant coils (a metal coil diameter >12 mm), the effectiveness of S-MAR was relatively limited.
Stent-assisted coiling and flow-diverter stents are common treatments for intracranial aneurysms (3,25,26). Stents are metal implants; however, they were not found to be strongly correlated with artifacts in this study. Thus, stent-assisted coiling treatment does not increase metal artifacts, which can provide a positive reference for the selection of aneurysm endovascular embolization therapy. Metal coils and stents were erased in S-MAR, while the lumen of blood vessels remained. Vessel morphology was affected by the shape of the stents, as seen in the representative case (Figure 3). In clinical work, magnetic resonance angiography is regarded as another conventional method during follow-up (24). However, a loss of blood flow signal was observed in some cases due to the metal coils and stents, especially for metal implants of a large size or large range. Thus, S-MAR is a better choice for stent-assisted or large metal coil cases.
Limitations
This study had a number of limitations. First, there was no post-processing station to automatically determine the VDmax, VDmin, and VDmean data for DSA, which might have resulted in manual measurement errors by different observers. However, these data were collected by two radiologists, who were blinded to each other’ scores and performed the measurements several times to calculate the average to avoid potential bias. Second, while S-MAR showed great performance for groups 1 and 2, its effect was less satisfactory for large coils (with a diameter >12 mm), suggesting that further research is needed to explore alternative methods for this issue. Third, many factors, such as the size, shape, and material of the metal implants, determine the severity and visibility of metal artifacts. This study focused on the influence of the metal size characteristic, using the average diameter as the index of measurement, which might not fully account for the effect of irregular shapes. It would be helpful to measure coils shape using a 3D volume model instead of a plane figure. More precise algorithmic improvements could also distinguish between various alloy compositions to remove metal artifacts more thoroughly, which would optimize S-MAR for larger coils. Fourth, the patients included in this retrospective study were derived from a systematic sample in a set period, and there might be potential biases related to the maldistribution of sex and the different locations of the metal coils. These issues could be addressed by expanding the sample size and the use of random sampling.
Conclusions
S-MAR has significant advantages in metal artifacts reducing and vessel visualization improvement, and accurate luminal assessment. Its improved artifact reduction performance can increase diagnostic confidence, and may enhance aneurysm monitoring and treatment planning.
Acknowledgments
The authors would like to express their sincere appreciation to all the members and their families for their unwavering support and encouragement throughout this journey.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2640/rc
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2024-2640/coif). All authors report that this study was funded by the National Natural Science Foundation Project (No. 82371945). S.G. is an engineer at Philips Healthcare. He provided image interpretation and language editing support in this study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. [2021]0633). Due to the retrospective nature of the study, the requirement of written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 2014;13:393-404. [Crossref] [PubMed]
- Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015;385:691-7. [Crossref] [PubMed]
- Chai CL, Pyeong Jeon J, Tsai YH, Whittaker P, Macdonald RL, Lindgren AE, Ayling OGS, Acioly MA, Cohen-Gadol A, Huang YH. Endovascular Intervention Versus Surgery in Ruptured Intracranial Aneurysms in Equipoise: A Systematic Review. Stroke 2020;51:1703-11. [Crossref] [PubMed]
- Pierot L, Costalat V, Moret J, Szikora I, Klisch J, Herbreteau D, Holtmannspötter M, Weber W, Januel AC, Liebig T, Sychra V, Strasilla C, Cognard C, Bonafé A, Molyneux A, Byrne JV, Spelle L. Safety and efficacy of aneurysm treatment with WEB: results of the WEBCAST study. J Neurosurg 2016;124:1250-6. [Crossref] [PubMed]
- Soize S, Gawlitza M, Raoult H, Pierot L. Imaging Follow-Up of Intracranial Aneurysms Treated by Endovascular Means: Why, When, and How? Stroke 2016;47:1407-12. [Crossref] [PubMed]
- Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, de Groot JC, Groen RJ, Mooij JJ, Oudkerk M. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis--systematic review and meta-analysis. Radiology 2011;258:134-45. [Crossref] [PubMed]
- Machida H, Tanaka I, Fukui R, Shen Y, Ishikawa T, Tate E, Ueno E. Dual-Energy Spectral CT: Various Clinical Vascular Applications. Radiographics 2016;36:1215-32. [Crossref] [PubMed]
- Dolati P, Eichberg D, Wong JH, Goyal M. The Utility of Dual-Energy Computed Tomographic Angiography for the Evaluation of Brain Aneurysms After Surgical Clipping: A Prospective Study. World Neurosurg 2015;84:1362-71. [Crossref] [PubMed]
- Zhang X, Pan T, Lu SS, Hong XN. Application of Monochromatic Imaging and Metal Artifact Reduction Software in Computed Tomography Angiography after Treatment of Cerebral Aneurysms. J Comput Assist Tomogr 2019;43:948-52. [Crossref] [PubMed]
- Mellander H, Fransson V, Ydström K, Lätt J, Ullberg T, Wassélius J, Ramgren B. Metal artifact reduction by virtual monoenergetic reconstructions from spectral brain CT. Eur J Radiol Open 2023;10:100479. [Crossref] [PubMed]
- Shim E, Kang Y, Ahn JM, Lee E, Lee JW, Oh JH, Kang HS. Metal Artifact Reduction for Orthopedic Implants (O-MAR): Usefulness in CT Evaluation of Reverse Total Shoulder Arthroplasty. AJR Am J Roentgenol 2017;209:860-6. [Crossref] [PubMed]
- Zhou Y, Lei L, Wang Z, Cao W, Qin M, Dong S, Dang J, Zhou Z. Utility of spectral CT with orthopedic metal artifact reduction algorithms for (125)I seeds implantation in mediastinal and hepatic tumors. Quant Imaging Med Surg 2024;14:698-710. [Crossref] [PubMed]
- Bannas P, Li Y, Motosugi U, Li K, Lubner M, Chen GH, Pickhardt PJ. Prior Image Constrained Compressed Sensing Metal Artifact Reduction (PICCS-MAR): 2D and 3D Image Quality Improvement with Hip Prostheses at CT Colonography. Eur Radiol 2016;26:2039-46. [Crossref] [PubMed]
- Selles M, van Osch JAC, Maas M, Boomsma MF, Wellenberg RHH. Advances in metal artifact reduction in CT images: A review of traditional and novel metal artifact reduction techniques. Eur J Radiol 2024;170:111276. [Crossref] [PubMed]
- Li Q, Lv F, Li Y, Li K, Luo T, Xie P. Subtraction CT angiography for evaluation of intracranial aneurysms: comparison with conventional CT angiography. Eur Radiol 2009;19:2261-7. [Crossref] [PubMed]
- Ma G, Yu Y, Duan H, Dou Y, Jia Y, Zhang X, Yang C, Chen X, Han D, Guo C, He T. Subtraction CT angiography in head and neck with low radiation and contrast dose dual-energy spectral CT using rapid kV-switching technique. Br J Radiol 2018;91:20170631. [Crossref] [PubMed]
- Qin L, Gu S, Chen C, Zhang H, Zhu Z, Chen X, Han Q, Yan F, Yang W. Initial exploration of coronary stent image subtraction using dual-layer spectral CT. Eur Radiol 2019;29:4239-48. [Crossref] [PubMed]
- Zheng H, Yang M, Jia Y, Zhang L, Sun X, Zhang Y, Nie Z, Wu H, Zhang X, Lei Z, Jing W. A Novel Subtraction Method to Reduce Metal Artifacts of Cerebral Aneurysm Embolism Coils. Clin Neuroradiol 2022;32:687-94. [Crossref] [PubMed]
- Bolstad K, Flatabø S, Aadnevik D, Dalehaug I, Vetti N. Metal artifact reduction in CT, a phantom study: subjective and objective evaluation of four commercial metal artifact reduction algorithms when used on three different orthopedic metal implants. Acta Radiol 2018;59:1110-8. [Crossref] [PubMed]
- Wellenberg RHH, Hakvoort ET, Slump CH, Boomsma MF, Maas M, Streekstra GJ. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur J Radiol 2018;107:60-9. [Crossref] [PubMed]
- Neuhaus V, Grosse Hokamp N, Zopfs D, Laukamp K, Lennartz S, Abdullayev N, Maintz D, Borggrefe J. Reducing artifacts from total hip replacements in dual layer detector CT: Combination of virtual monoenergetic images and orthopedic metal artifact reduction. Eur J Radiol 2019;111:14-20. [Crossref] [PubMed]
- Chae HD, Hong SH, Shin M, Choi JY, Yoo HJ. Combined use of virtual monochromatic images and projection-based metal artifact reduction methods in evaluation of total knee arthroplasty. Eur Radiol 2020;30:5298-307. [Crossref] [PubMed]
- Katsura M, Sato J, Akahane M, Kunimatsu A, Abe O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018;38:450-61. [Crossref] [PubMed]
- Wang X, Benson JC, Jagadeesan B, McKinney A. Giant Cerebral Aneurysms: Comparing CTA, MRA, and Digital Subtraction Angiography Assessments. J Neuroimaging 2020;30:335-41. [Crossref] [PubMed]
- D Vera D. Cardenas SA, Ortiz AF, Rodriguez AM, Ferreira CA, Serrano S, Reyes A, Galvis M, Vargas O, Mantilla DE. Safety and Efficacy of Endovascular Coils and Non-Flow-Diverting Stents for Management of Unruptured Intracranial Aneurysms: A Location-Specific Outcomes Analysis. World Neurosurg 2024;182:e734-41. [Crossref]
- Cagnazzo F, di Carlo DT, Cappucci M, Lefevre PH, Costalat V, Perrini P. Acutely Ruptured Intracranial Aneurysms Treated with Flow-Diverter Stents: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol 2018;39:1669-75. [Crossref] [PubMed]


