
Association of computed tomography-imaged common iliac vein compression with inferior vena cava thrombosis: a propensity score-matched analysis
Introduction
Inferior vena cava (IVC) thrombosis is an underdiagnosed type of venous thromboembolism (VTE), which can result in death if not promptly treated (1,2). IVC thrombosis is initially clinically silent and may become evident only after sudden and fatal pulmonary embolism (PE) (2,3). The primary cause of provoked IVC thrombosis is long-term implantation of an IVC filter, with a prevalence ranging from 1.8% to 32.6% (3-5). Additionally, lower extremity deep vein thrombosis (LEDVT) is a contributing factor for IVC thrombosis, as 2.6–4.0% of patients with LEDVT experience concomitant IVC thrombosis (1,2,6,7). However, the true incidence of IVC thrombosis is difficult to determine and may be underestimated (1,2).
Previous studies have shown that common iliac vein (CIV) compression significantly contributes to the development of thrombosis involving the CIV and secondary LEDVT (8-10). However, it appears to act as a protective factor against the migration of LEDVT to PE (11). These findings indicate that a compressed CIV might act as a physical barrier that impedes blood flow but prevents thrombus migration, which could increase LEDVT risk and decrease PE incidence (9-11). Hence, investigating the association between CIV compression and IVC thrombosis could have important clinical implications; for instance, the use of IVC filters in low-risk patients may be reduced and decisions regarding thrombosis management more accurately informed. A recent study provided evidence for a reduction in IVC thrombosis due to CIV compression (7). However, this study was limited by the nonquantitative assessment of CIV compression, with the only outcome being whether severe CIV compression was a protective factor for IVC thrombosis. On the basis of previous findings indicating that PE is less common in those with moderate and severe CIV compression (12), it was hypothesized that IVC thrombosis may also be influenced by the degree of CIV compression; however, this hypothesis not been thoroughly tested.
This study aimed to assess whether the degree of CIV compression could predict IVC thrombosis risk in patients with ipsilateral LEDVT and to characterize its association with IVC thrombosis risk on a continuous scale. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1831/rc).
Methods
Data source, study population, and design
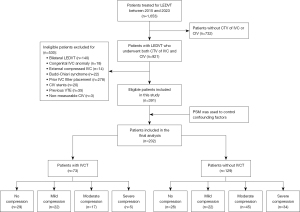
This retrospective case-control study was performed using imaging and medical records obtained from Nanjing First Hospital. Two reviewers, who were blinded to the clinical outcomes, screened the medical database and picture archiving and communication systems to identify eligible candidates from patients who were treated for LEDVT between January 5, 2015, and July 11, 2023. The suspected diagnosis of LEDVT was initially verified by medical history, physical examination, and hematological testing (D-dimer), with confirmation performed via compression ultrasound. If the diagnosis was inconclusive, supplementary venography via the dorsal vein of the foot was performed. IVC thrombosis was identified via computed tomography venography (CTV) and/or cavography performed prior to further endovascular treatment. The inclusion criterion for this study was LEDVT patients who underwent CTV of both the IVC and CIV. The exclusion criteria included bilateral LEDVT, Budd-Chiari syndrome, congenital IVC anomaly, IVC filter placement prior to CTV examination, IVC externally compressed by neighboring pathologic processes, a history of VTE, previous contralateral or ipsilateral CIV stenting, and nonmeasurable CIV (a flowchart of patient inclusion is shown in Figure 1). Overall, 1,653 individuals with LEDVT were identified. Among them, 921 had CTVs of the IVC and CIV, and 391 (mean age 60.42±16.67 years; 53.96% male) were initially included in this study. Among these patients, 73 (18.67%) patients diagnosed with IVC thrombosis were included in the IVC thrombosis group, whereas 318 (81.33%) without IVC thrombosis were included in the non-IVC thrombosis group. The protocol was approved by the institutional review board of Nanjing First Hospital (No. KY20200117-01), and the requirement for written informed consent was waived due to the retrospective nature of the analysis. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Patient baseline demographics, clinical characteristics, and radiographic variables
The clinical characteristics of the eligible patients were collected and analyzed. These included demographics (age and sex), onset time (i.e., the time of symptoms onset to hospital admission) of deep vein thrombosis (DVT), D-dimer value, concurrent PE, and comorbidities such as hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease (CAD), and cerebral vascular disease (CVD). Risk factors for LEDVT were also assessed, including trauma, major surgery history, immobilization (i.e., the duration lasted more than 12 hours), autoimmune diseases (e.g., antiphospholipid antibody syndrome, systemic lupus erythematosus, and rheumatoid arthritis), disc herniation, cancer, inflammation (e.g., pneumonia, urinary infection, and skin or soft tissue infections), and varicose veins. Additionally, imaging information on CTV was analyzed to evaluate thrombus segments (iliofemoral DVT and femoropopliteal DVT), thrombus limbs (left- and right-sided LEDVT), and CIV characteristics (minimum diameter, compression percentage, and level).
Imaging protocol, measurement modalities, and definitions
The initial diagnosis of IVC thrombosis was based on radiographic reports and was later verified by the interventional radiologists, who confirmed the presence of filling defects in deep veins and the IVC. CTV was conducted within 48 hours of LEDVT diagnosis via a single CT machine (128-slice dual-source CT; SOMATOM Definition Flash, Siemens Healthineers, Erlangen, Germany). These images were acquired during a single inspiratory breath hold and reconstructed with slices ranging from 1.0 to 5.0 mm in thickness to facilitate the diagnosis and measurement of CIV compression. An 18-gauge intravenous line was used to administer the contrast agent (iopromide; Bayer, Leverkusen, Germany) through the cubital vein.
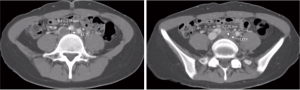
CIV compression was evaluated by quantitative and qualitative measures based on CTV images (13). To limit interrater measurement variability, multiple training sessions and frequent image review meetings were held to ensure the consistency of the measurement technique, and a third rater determined the final scores if necessary. Continuous CTV images were assessed to obtain the point of maximum CIV compression. The quantitative evaluation included the CIV minimum diameter and compression percentage. The compression percentage of the CIV diameter was calculated as follows: 1 − D1/D2 × 100%, where D1 is the minimum CIV diameter at the point of maximum compression, and D2 is the minimum diameter at the CIV caudal to the compression (12) (as shown in Figure 2). The degree of CIV compression was qualitatively assessed based on the calculated compression percentage. The compression degree was classified into four categories: no compression (0–25%), mild (26–50%), moderate (51–75%), and severe (>75%).
Statistical analysis
All statistical analyses were performed using the SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and R statistical language software version 4.2.3 (The R Foundation for Statistical Computing, Vienna, Austria). The distribution of continuous variables was assessed using the Kolmogorov-Smirnov test. Data with a normal distribution are presented as the mean and standard deviation (SD), while data without a normal distribution are presented as the median and interquartile range (IQR). The Student t-test and Mann-Whitney test were used to assess the correlation between two groups for normally distributed data and nonnormally distributed data, respectively. The interrater reliability for collateral grading was measured by the Lin concordance correlation coefficient (CCC). Violin plots were generated with GraphPad Prism version 9.0 (Dotmatics, Boston, MA, USA). To minimize the potential bias between the two study groups, a 1:2 matching propensity score matching (PSM) analysis (caliper width 0.2) without replacement was performed to mitigate the effect of selection bias and adjust for potential confounding factors, including the covariates of age, male sex, hypertension, and iliofemoral DVT. The baseline characteristics of the pre- and postmatched groups were listed. To determine the association between CIV compression and IVC thrombosis, univariable and multivariable logistic regression analyses were performed with odds ratios (ORs), adjusted ORs, and 95% confidence intervals (CIs). Model 1 revealed an unadjusted association between the CIV minimum diameter or CIV compression percentage and the diagnosis of IVC thrombosis. Model 2 incorporated adjustments for age, male sex, hypertension, and iliofemoral DVT. Model 3 was applied after PSM to minimize confounding factors and ensure more reliable estimates. Model 4 was used to assess the association between the degree of CIV compression and IVC thrombosis, evaluating how different levels of compression impact risk. We also applied restricted cubic spline (RCS) to flexibly model the dose-response association between CIV compression and IVC thrombosis risk. Knots were chosen at the 5th, 35th, 65th, and 95th percentiles as per Harrell’s recommendations. The likelihood ratio test was used for potential nonlinearity. Statistical significance was determined as a P value <0.05 (two-tailed).
Results
Baseline demographics and characteristics of included patients with LEDVT
Data on the baseline demographics, clinical variables, and radiographic characteristics of patients with and without IVC thrombosis are presented in Table 1. The median LEDVT onset time was 7 days (IQR 3.00–14.00 days), and the mean D-dimer value was 7.96±10.15 µg/mL. Concurrent PE was observed in 52.69% (206/391) of patients. The leading comorbidities and risk factors were hypertension, which affected 38.62% (151/391) of patients, and immobilization, which affected 14.83% (58/391) of patients. The incidence of left-sided LEDVT was almost twice as high as that of right-sided LEDVT (66.75% vs. 33.25%), and iliofemoral DVT was found in 68.54% (268/391) of patients. Overall, the incidence of IVC thrombosis with left-sided LEDVT was significantly greater than that with right-sided LEDVT (73.97% vs. 26.03%). The reproducibility of CIV minimum diameter and CIV caudal diameter was consistent between the two raters, with CCCs of 0.80 and 0.87, respectively. The median minimum diameter and compression percentage of the CIV were 4.90 mm (IQR 2.95–8.70 mm) and 52.69% (IQR 24.12–73.02%), respectively.
Table 1
| Characteristic | All eligible for propensity score matching | Propensity score matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n=391) | IVCT (n=73) | Non-IVCT (n=318) | P value | All (n=202) | IVCT (n=73) | Non-IVCT (n=129) | P value | |||
| Demographics | ||||||||||
| Age, years | 60.42±16.67 | 53.59±16.39 | 61.99±16.36 | <0.01 | 54.61±16.02 | 53.59±16.39 | 55.19±15.84 | 0.50 | ||
| Male | 211 (53.96) | 47 (64.38) | 164 (51.57) | <0.05 | 126 (62.38) | 47 (64.38) | 79 (61.24) | 0.66 | ||
| Onset time, days | 7 (3.00, 14.00) | 7 (3.00, 14.00) | 7 (3.00, 15.00) | 0.90 | 7 (3.00, 15.00) | 7 (3.00, 15.00) | 7 (3.75, 15.00) | 0.77 | ||
| D-dimer value, μg/mL | 7.96±10.15 | 9.99±11.01 | 7.50±9.90 | 0.06 | 8.70±10.31 | 9.99±11.01 | 7.97±9.86 | 0.50 | ||
| Concurrent PE | 206 (52.69) | 35 (47.95) | 171 (53.77) | 0.37 | 115 (56.93) | 35 (47.95) | 80 (62.02) | 0.05 | ||
| Thrombus segments‡ | ||||||||||
| Iliofemoral DVT | 268 (68.54) | 66 (90.41) | 202 (63.52) | <0.01 | 180 (89.11) | 66 (90.41) | 114 (88.37) | 0.66 | ||
| Femoropopliteal DVT | 125 (31.97) | 23 (31.51) | 102 (32.08) | 0.93 | 54 (26.73) | 23 (31.51) | 31 (24.03) | 0.25 | ||
| Thrombus limbs | ||||||||||
| Left side | 261 (66.75) | 54 (73.97) | 207 (65.09) | 0.15 | 137 (67.82) | 54 (73.97) | 83 (64.34) | 0.16 | ||
| Right side | 130 (33.25) | 19 (26.03) | 111 (34.91) | 65 (32.18) | 19 (26.03) | 46 (35.66) | ||||
| Comorbidities | ||||||||||
| Hypertension | 151 (38.62) | 20 (27.40) | 131 (41.19) | 0.03 | 52 (25.74) | 20 (27.40) | 32 (24.81) | 0.69 | ||
| Diabetes mellitus | 70 (17.9) | 9 (12.32) | 61 (19.18) | 0.17 | 26 (12.87) | 9 (12.33) | 17 (13.18) | 0.86 | ||
| Hyperlipidemia | 7 (1.79) | 1 (1.37) | 6 (1.89) | >0.99 | 1 (0.5) | 1 (1.37) | 0 (0.00) | 0.36 | ||
| CAD | 44 (11.25) | 7 (9.59) | 37 (11.64) | 0.17 | 18 (8.91) | 7 (9.59) | 11 (8.53) | 0.80 | ||
| CVD | 50 (12.79) | 7 (9.59) | 43 (13.52) | 0.36 | 16 (7.92) | 7 (9.59) | 9 (6.98) | 0.51 | ||
| Risk factors for LEDVT | ||||||||||
| Trauma | 10 (2.56) | 1 (1.37) | 9 (2.83) | 0.09 | 2 (0.99) | 1 (1.37) | 1 (0.78) | >0.99 | ||
| Major surgery history | 12 (3.07) | 1 (1.37) | 11 (3.46) | 0.58 | 9 (4.46) | 1 (1.37) | 8 (6.20) | 0.21 | ||
| Immobilization | 58 (14.83) | 14 (19.18) | 44 (13.84) | 0.25 | 31 (15.35) | 14 (19.18) | 17 (13.18) | 0.26 | ||
| Autoimmune diseases† | 27 (6.91) | 8 (10.96) | 19 (5.97) | 0.29 | 16 (7.92) | 8 (10.96) | 8 (6.20) | 0.23 | ||
| Disc herniation | 19 (4.86) | 1 (1.37) | 18 (5.66) | 0.22 | 10 (4.95) | 1 (1.37) | 9 (6.98) | 0.15 | ||
| Cancer | 45 (11.51) | 9 (12.32) | 36 (11.32) | 0.06 | 21 (10.40) | 9 (12.33) | 12 (9.30) | 0.50 | ||
| Inflammation | 17 (4.35) | 3 (4.11) | 14 (4.40) | >0.99 | 8 (3.96) | 3 (4.11) | 5 (3.88) | >0.99 | ||
| Varicose veins | 20 (5.12) | 3 (4.11) | 17 (5.35) | 0.90 | 9 (4.46) | 3 (4.11) | 6 (4.65) | >0.99 | ||
| CIV compression degree | ||||||||||
| CIV minimum diameter, mm | 4.90 (2.95, 8.70) |
6.72 (4.57, 8.78) |
4.02 (2.49, 8.60) |
<0.01 | 5.04 (3.09, 8.66) |
6.72 (4.57, 8.78) |
3.99 (2.48, 8.03) |
<0.01 | ||
| CIV compression percentage, % | 52.69 (24.12, 73.02) |
37.71 (20.91, 55.86) |
54.79 (27.12, 77.29) |
<0.01 | 49.81 (23.51, 70.61) |
37.71 (20.91, 55.86) |
55.33 (28.65, 78.05) |
<0.01 | ||
Data are presented as mean ± SD, n (%) or median (interquartile range). †, including antiphospholipid antibody syndrome, systemic lupus erythematosus, and rheumatoid arthritis. ‡, including patients who have both iliofemoral DVT and femoropopliteal DVT, isolated distal DVT was not listed. CAD, cardiologic artery disease; CVD, cerebral venous disease; DVT, deep vein thrombosis; IVCT, inferior vena cava thrombosis; CIV, common iliac vein; LEDVT, lower extremity deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation.
Univariable analysis revealed that patients with IVC thrombosis were younger (53.59±16.39 vs. 61.99±16.36 years; P<0.01), had a lower incidence of hypertension (27.40% vs. 41.19%; P=0.03), and had a larger CIV minimum diameter (6.72 vs. 4.02 mm; P<0.01) but were more likely to be male (64.38% vs. 51.57%; P<0.05), have iliofemoral DVT (90.41% vs. 63.52%, P<0.01), and have a smaller CIV compression percentage (37.71% vs. 54.79%; P<0.01). Multivariate logistic regression analysis revealed that iliofemoral DVT and an increased CIV minimum diameter were significantly positively associated with IVC thrombosis, whereas older age and CIV compression percentage were negatively associated with IVC thrombosis. Unadjusted Model 1 revealed a close association of CIV minimum diameter (OR =1.10; 95% CI: 1.03–1.18; P<0.01) and CIV compression percentage (OR =0.99; 95% CI: 0.98–0.99; P<0.01) with IVC thrombosis. After adjustments were made for age, male sex, hypertension, and iliofemoral DVT, Model 2 indicated that the associations of CIV minimum diameter (adjusted OR =1.10; 95% CI: 1.02–1.18; P<0.01) and CIV compression percentage (adjusted OR =0.99; 95% CI: 0.98–0.99; P<0.01) with IVC thrombosis remained consistent.
PSM balancing of potential confounders
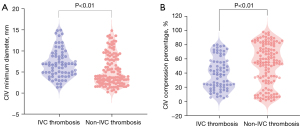
The analysis included 202 patients, with 73 patients in the IVC thrombosis group and 129 in the non-IVC thrombosis group. After PSM, the median CIV minimum diameter was 5.04 mm (IQR 3.09–8.66 mm), whereas the compression percentage was 49.81% (IQR 23.51–70.61%). Violin plots of the minimum diameter and compression percentage of the CIV for patients with and without IVC thrombosis are provided in Figure 3. Patients with IVC thrombosis exhibited a significantly greater median CIV minimum diameter (6.72 vs. 3.99 mm; P<0.001) and a lower compression percentage (37.71% vs. 55.33%; P<0.01) compared to those without IVC thrombosis. According to Model 3, the association remained significant, indicating that a lower CIV minimum diameter and higher compression percentage significantly reduced the risk of IVC thrombosis. Among the patients, no CIV compression, mild CIV compression, moderate CIV compression, or severe CIV compression was found in 28.22% (57/202), 21.78% (44/202), 30.69% (62/202), and 19.31% (39/202) of patients, respectively. Patients with IVC thrombosis had a significantly lower incidence of moderate and severe CIV compression than did those without IVC thrombosis. Model 4 analysis confirmed that moderate compression (OR =0.36; 95% CI: 0.17–0.78; P=0.01) and severe compression (OR =0.14; 95% CI: 0.05–0.42; P<0.01) significantly reduced the risk of IVC thrombosis.
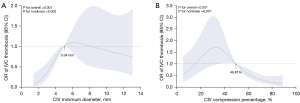
To further investigate the relationship of CIV minimum diameter and CIV compression percentage on a continuous scale with the risk of IVC thrombosis, we fitted the RCS with four knots. As shown in Figure 4A,4B, our analysis revealed a nonlinear dose-response association (U-shaped fitting curve) of the minimum CIV diameter and CIV compression percentage with IVC thrombosis risk (nonlinear P<0.05). Moreover, the fitting curve indicated the presence of a threshold effect for both variables (both P values <0.01). An CIV minimum diameter level below 5.04 mm, a smaller CIV minimum diameter was negatively associated with the incidence of IVC thrombosis, demonstrating continuously decreasing odds. Similarly, at compression percentages above 49.81%, a higher compression percentage was negatively associated with the incidence of IVC thrombosis, with continuously decreasing odds.
Discussion
In this retrospective case-control study, CIV compression was linked to the occurrence of IVC thrombosis in patients with ipsilateral LEDVT. A higher degree of CIV compression was found to be a protective factor against the risk of IVC thrombosis when the CIV minimum diameter was <5.04 mm or the compression percentage was >49.81%. Neither the degree of no compression nor the degree of mild compression reduced the risk of IVC thrombosis. Conversely, moderate and severe compression was associated with a reduced risk of IVC thrombosis.
IVC thrombosis has been reported to occur in association with various systemic and local disorders (13). Linnemann et al. reported that 3.0% of patients from the MAISTHRO (MAin-ISar-THROmbosis) database experienced IVC thrombosis (13). Although thrombosis limited to the IVC is rare, in majority of cases have an extension or origin from the iliac veins or lower extremity veins. Therefore, we conducted a study of 391 patients diagnosed with ipsilateral LEDVT, with 68.54% arising from iliofemoral veins, and iliofemoral DVT was identified as a risk factor for IVC thrombosis. The clinical presentation of IVC thrombosis may vary according to the level of thrombosis (from the iliac confluence to the right atrium) and the degree of occlusion of the cava (5,14). Since IVC thrombosis is considered a subset of DVT within the spectrum of VTE disease, it is likely to present symptoms similar to those of LEDVT. Compared with LEDVT, a diagnosis that relies solely on clinical presentations is fraught with challenges because the most common symptoms—limb edema, discomfort, and venous varicosity—are common and nonspecific. Although collateral vessels in the abdominal wall, flank, or bilateral inguinal region may lead to suspicion of IVC thrombosis (2,5), these findings are often ambiguous and complicate timely diagnosis. Therefore, IVC thrombosis may be concealed in LEDVT, potentially leading to catastrophic consequences. Evaluating the predictive factors for IVC thrombosis in patients with LEDVT has considerable implications, for instance, its use in the development of individualized screening plans.
Despite the scarcity of recommendations on the best diagnostic modality for IVC thrombosis, Alkhouli et al. suggested that screening is reasonable in patients with suspected IVC thrombosis with LEDVT and high-risk features (1). Sonography, while initial, is usually operator dependent, and the visualization of IVC thrombosis can be hindered by overlying bowel gas, fat in obese patients, or body position (2). CTV, magnetic resonance imaging (MRI), and transcatheter venography have been shown to overcome these inherent limitations with their respective strengths (2,15). MRI and magnetic resonance angiography can determine the presence, size, and location of IVC thrombosis. However, this is a concern for patients with IVC filters because a high magnetic field can potentially affect the filter. CTV, with its advancements in postprocedural reconstruction and 3D rendering, allows for isotropic data collection and superior spatial resolution, offering detailed representations of both normal and abnormal IVC structures. Nevertheless, in the absence of high-quality evidence demonstrating health economic benefits, routine CTV for IVC thrombosis is not recommended by current guidelines. Our study primarily relied on CTV to diagnose IVC thrombosis and evaluate the degree of CIV involvement, which could help identify those patients who would benefit most from CTV. Moreover, CTV can be used to evaluate images for related findings that may indicate underlying defects.
Congenital anomalies of the IVC, such as hypoplasia or aplasia (accounting for 60%), and venous aneurysms may increase the risk of thrombosis (13). Other common findings that contribute to the development of IVC thrombosis include Budd-Chiari syndrome (50%), external IVC compression due to neighboring pathologic processes, and IVC occlusion stemming from filter-related thrombosis (13,16). Our study was initially designed to investigate the impact of CIV compression on IVC thrombosis; hence patients with IVC anomalies, Budd-Chiari syndrome, filter use prior to CTV examination, or external compression were excluded. According to previous studies (12,13), a diversity factors may contribute to thrombogenesis (17). These risk factors are likely to have complex interactions in contributing to incidence, and IVC thrombosis is ultimately dependent upon patient characteristics and disease history (2). Our previous study (16) found that sex, age, proximal LEDVT, bilateral LEDVT, and D-dimer >4.72 µg/mL were predisposing risk factors for IVC thrombosis. However, CIV compression was not considered. In present study, in our work, we expanded on previous CIV compression studies in this field and found that compressed CIV was closely associated with IVC thrombosis risk, which is in line with the findings of Liu et al. (6). The significant negative relationship between CIV compression and the incidence of IVC thrombosis persisted even after the above-mentioned potential confounders were controlled for, suggesting that CIV compression may be an independent risk factor for IVC thrombosis. In addition, numerous studies have shown that malignant disease and autoimmune disease are predictive of immediate IVC thrombosis recurrence [hazard ratio (HR) =5.8, 95% CI: 1.7–19.8; P=0.01] (18,19). However, the differences were not significant between the two groups.
CIV compression has been widely acknowledged to be a key factor in LEDVT and PE incidence. Previous studies (5-11) have shown that the risk of LEDVT in the left limb is approximately twofold greater than that in the right limb, whereas the risk of PE is twofold lower than that in the right limb. Our study is in line with these findings, indicating a significantly higher prevalence of LEDVT in the left limb as compared with the right limb (66.75% vs. 33.25%). This finding suggests that CIV compression might act as a physical barrier, rendering CIV compression more susceptible to LEDVT but limiting the extension of thrombi into the pulmonary artery. However, the association between CIV compression and secondary IVC thrombosis has only been examined in a handful of studies (6). Liu et al. (6) reported that severe CIV compression was protective factor for the risk of IVC thrombosis, which is consistent with our results. In our study, we observed a significantly higher incidence of IVC thrombosis in the left limb than in the right limb (73.97% vs. 26.03%), which appears to conflict with previous findings. This discrepancy might be attributed to potential selection bias, as 732 patients with LEDVT were excluded due to an absence of CTV.
Regarding the relationship between CIV compression and IVC thrombosis, our study further investigated the impact of the degree of CIV compression on a continuous scale. We found that an increasing degree of CIV compression was not consistently correlated with a reduced risk of IVC thrombosis. However, when the CIV minimum diameter was <5.04 mm or the compression percentage was >49.81%, an increasing degree of CIV compression as a protective factor against IVC thrombosis was associated with a decreased risk of IVC thrombosis. This finding has not been previously reported and aligned with the results of the RCS analysis. No and mild CIV compression was not associated with significantly reduced IVC thrombosis risk, whereas moderate and severe CIV compression was found to potentially reduce the incidence of IVC thrombosis. We speculate that no or a mildly compressed CIV, with only a slight reduction in lumen size, may not be effective in preventing thrombi from passing through.
The study involved several limitations that should be noted. First, we employed a single-center, retrospective design involving a relatively low number of patients with LEDVT who received CTV; hence, selection bias might have been introduced. Despite this, this study had a larger sample size compared to previous studies (6). In addition, the clinical applicability of our findings, especially in identifying patients who may benefit from CTV, depends on the availability of prior CT imaging to evaluate the extent of CIV compression. In the absence of such imaging, accurately assessing the severity of compression becomes more challenging, which may restrict the practical implementation of our conclusions in clinical decision-making. Second, our assumption that IVC thrombosis originated exclusively from confirmed ipsilateral LEDVT might have overlooked the possibility of thrombi developing from other conditions. We did attempt to eliminate the possible effects by excluding patients with IVC anomalies, IVC filter-related thrombosis, Budd-Chiari syndrome, or external compression. Third, five patients were administered anticoagulation measures immediately upon diagnosis and prior to CTV examination, which might have prevented clot propagation and subsequent IVC thrombosis, potentially affecting the D-dimer values. These factors might also have slightly impacted the reliability of the results. Nevertheless, this study remains the first to evaluate the degree of CIV compression on a continuous scale. Further large-scale prospective studies are necessary to longitudinally evaluate the occurrence of IVC thrombosis and validate these findings.
Conclusions
This study identified iliofemoral DVT and increasing CIV minimum diameter as risk factors for the occurrence of IVC thrombosis, whereas increasing age and CIV compression percentage were protective factors. Compared with no or mild compression, moderate and severe CIV compression was associated with significantly reduced IVC thrombosis. An increasing degree of CIV compression was consistently associated with a decreased risk of IVC thrombosis when the minimum diameter was <5.04 mm or when the compression was >49.81%, indicating that CIV compression is a protective factor against IVC thrombosis. These findings may be helpful to better understand the role of CIV compression in IVC thrombosis.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1831/rc
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1831/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the institutional review board of Nanjing First Hospital (No. KY20200117-01). The study was performed in accordance with the Declaration of Helsinki and its subsequent amendments. Requirement for written informed consent was waived due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alkhouli M, Morad M, Narins CR, Raza F, Bashir R. Inferior Vena Cava Thrombosis. JACC Cardiovasc Interv 2016;9:629-43. [Crossref] [PubMed]
- Shi W, Dowell JD. Etiology and treatment of acute inferior vena cava thrombosis. Thromb Res 2017;149:9-16. [Crossref] [PubMed]
- Xiao N, Karp J, Lewandowski R, Cuttica M, Schimmel D, Martin K, Desai KR. Inferior Vena Cava Thrombosis Risk in 1582 Patients with Inferior Vena Cava Filters. Radiology 2022;303:300-2. [Crossref] [PubMed]
- Gong M, Liu Z, Kong J, Zhao B, Jiang R, He X, Wang T, Gu J. Incidence and risk factors for inferior vena cava filter thrombosis detected at time of filter retrieval in patients with lower extremity deep vein thrombosis: a multicenter retrospective cohort study. Quant Imaging Med Surg 2023;13:8313-25. [Crossref] [PubMed]
- Avgerinos ED, El-Shazly O, Jeyabalan G, Al-Khoury G, Hager E, Singh MJ, Makaroun MS, Chaer RA. Impact of inferior vena cava thrombus extension on thrombolysis for acute iliofemoral thrombosis. J Vasc Surg Venous Lymphat Disord 2016;4:385-91. [Crossref] [PubMed]
- Liu XR, Zhou W, Chen F. Severe compression of left iliac vein is a protective factor for the risk of inferior vena cava thrombosis. J Vasc Surg Venous Lymphat Disord 2022;10:1107-12. [Crossref] [PubMed]
- Roncato C, Lefant PY. Images in clinical medicine. Thrombosis of the inferior vena cava and dilated veins of the trunk. N Engl J Med 2011;364:2535. [Crossref] [PubMed]
- Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg 1965;52:816-21. [Crossref] [PubMed]
- Chen F, Den J, Yuan QW, Zhou WM, Xiong JX, Zhou W. Compression of left common iliac vein is independently associated with left-sided deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2013;1:364-9. [Crossref] [PubMed]
- Chen D, Chen F, Li MF, Huang JG, Tang XH, Zhou WM. Left iliac vein compression is not associated with infrainguinal deep venous thrombosis but is associated with iliac vein involvement. J Vasc Surg Venous Lymphat Disord 2018;6:689-95. [Crossref] [PubMed]
- Chan KT, Popat RA, Sze DY, Kuo WT, Kothary N, Louie JD, Hovsepian DM, Hwang GL, Hofmann LV. Common iliac vein stenosis and risk of symptomatic pulmonary embolism: an inverse correlation. J Vasc Interv Radiol 2011;22:133-41. [Crossref] [PubMed]
- Narayan A, Eng J, Carmi L, McGrane S, Ahmed M, Sharrett AR, Streiff M, Coresh J, Powe N, Hong K. Iliac vein ompression as risk factor for left- versus right-sided deep venous thrombosis:case-control study. Radiology 2012;265:949-57. [Crossref] [PubMed]
- Linnemann B, Schmidt H, Schindewolf M, Erbe M, Zgouras D, Grossmann R, Schambeck C, Lindhoff-Last E. Etiology and VTE risk factor distribution in patients with inferior vena cava thrombosis. Thromb Res 2008;123:72-8. [Crossref] [PubMed]
- Jaff MR. Wait--The Inferior Vena Cava Is Thrombosed? Now What? JACC Cardiovasc Interv 2016;9:644-5. [Crossref] [PubMed]
- Rossi FH, Kambara AM, Rodrigues TO, Rossi CBO, Izukawa NM, Pinto IMF, Thorpe PE. Comparison of computed tomography venography and intravascular ultrasound in screening and classification of iliac vein obstruction in patients with chronic venous disease. J Vasc Surg Venous Lymphat Disord 2020;8:413-22. [Crossref] [PubMed]
- Gong M, Kong J, Shi Y, Zhao B, Liu Z, He X, Gu J. Risk factors and a predictive model for nonfilter-associated inferior vena cava thrombosis in patients with lower extremity deep vein thrombosis. Front Cardiovasc Med 2022;9:1083152. [Crossref] [PubMed]
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I4-8. [Crossref] [PubMed]
- McAree BJ, O'Donnell ME, Fitzmaurice GJ, Reid JA, Spence RA, Lee B. Inferior vena cava thrombosis: a review of current practice. Vasc Med 2013;18:32-43. [Crossref] [PubMed]
- Kraft C, Schuettfort G, Weil Y, Tirneci V, Kasper A, Haberichter B, Schwonberg J, Schindewolf M, Lindhoff-Last E, Linnemann B. Thrombosis of the inferior vena cava and malignant disease. Thromb Res 2014;134:668-73. [Crossref] [PubMed]


