
Evaluation of right ventricular systolic function in patients with rheumatic mitral stenosis by tissue tricuspid annular displacement technique
Introduction
Rheumatic heart disease (RHD) is a heart disease caused by an abnormal autoimmune response triggered by group A streptococcal infection. Although the rapid economic development has controlled the prevalence of RHD to a certain extent, it still accounts for a certain proportion in the global cardiovascular patient population. In developing countries, RHD is one of the main causes of cardiovascular morbidity and mortality—about 300,000 people die from it globally every year (1,2). Rheumatic mitral stenosis (RMS) is one of the common manifestations of RHD (3). The mitral stenosis in RMS patients will further affect the overall function of the heart, especially having a significant impact on right heart function. Due to mitral stenosis, the pressure in the left atrium rises, leading to an increase in pulmonary venous pressure, which in turn affects the afterload of the right ventricle. Long-term load changes can cause adaptive changes in the right ventricle and may eventually lead to right heart dysfunction (1,4,5).
The prognosis of RMS is closely related to the severity of the disease and the timeliness and effectiveness of treatment. Untreated RMS patients may encounter serious complications such as heart failure, arrhythmia, and embolic events. This will not only significantly reduce the quality of life of patients but also pose a serious threat to life (6-8). Although the protection of left heart function still occupies a core position in the current treatment of RMS, the damage to right heart function cannot be ignored. The right ventricle plays an important role in regulating pulmonary circulation. In some pathological states, right heart dysfunction may have a significant impact on the overall prognosis (9). Therefore, early and accurate assessment of right heart function is of great significance for comprehensively understanding the condition of RMS, formulating treatment strategies, and improving prognosis (10).
Echocardiography, as an economical, non-invasive, and easily accessible auxiliary examination method, is the preferred imaging examination method in the diagnosis and treatment system of cardiovascular diseases (11-13). In particular, the measurement of right ventricular global longitudinal strain (RVGLS) based on speckle tracking echocardiography (STE) has been widely used to evaluate the myocardial systolic function of the right ventricle (12,14,15). However, due to technical limitations and individual differences, it is not always possible to obtain clear right ventricular images. In view of this, this study introduces the tissue mitral annular displacement (TMAD) parameter, focusing on the myocardial speckle displacement in the tricuspid annulus area, to explore its application value in evaluating the right heart function of RMS patients.
The purpose of this study was to analyze the relationship between the TMAD technology and RVGLS in evaluating the right ventricular systolic function of patients with RHD, and to deeply explore the potential of TMAD as a simple index for evaluating right heart function, so as to more comprehensively understand the impact of RHD on heart function, and at the same time provide scientific basis for clinical implementation of treatment strategies and improvement of patient prognosis. We present this article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2296/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (No. 2022-KY-E-132); all participants signed the informed consent form. From June 2022 to June 2023, 83 patients diagnosed with RMS by echocardiography at The First Affiliated Hospital of Guangxi Medical University were prospectively and consecutively selected as the case group. The exclusion criteria for participants in this group were as follows: presence of non-rheumatic lesions; patients with acute or chronic heart failure; those with acute or chronic infections; abnormal liver and kidney functions; the presence of blood system diseases; and patients with malignant tumors. Meanwhile, 56 physical examinees matched with the case group in age and gender were selected from the healthy physical examination population of our hospital and included in the normal control group. After excluding 14 people due to non-compliance with exclusion criteria images and 11 people for reasons such as having systemic diseases, a total of 64 RMS patients and 50 normal individuals were finally enrolled. The height and weight data of the two groups of research participants were collected, and then the body mass index (BMI) was calculated. The continuous-wave Doppler technique of echocardiography was used to acquire the mitral valve blood-flow spectrum and measure the pressure half-time. The mitral valve orifice area was then estimated by combining this with two-dimensional (2D) echocardiographic tracing. Subsequently, the severity of RMS was graded in strict accordance with the relevant criteria in the 2022 guidelines of the American Society of Echocardiography (ASE) (13).
Echocardiographic measurements
For echocardiographic measurements, a Philips EPIQ 7C color Doppler ultrasound diagnostic instrument (Philips, Amsterdam, Netherlands) was used, equipped with an S5-1 probe (with a frequency of 1–5 MHz). The participants assumed the left lateral decubitus position and the electrocardiogram was connected simultaneously. Using this probe, a complete 2D echocardiogram assessment was conducted. Firstly, the 2D imaging mode was selected, the cardiac cycle was adjusted to 3–5 beats (5 beats for patients with persistent atrial fibrillation). The participants were asked to hold their breath at the end of expiration, obtain the section in the four-chamber heart view and optimize the view to fully display the right ventricular contour, and then perform data acquisition. In the end, all 114 participants successfully obtained complete and clear 2D multi-cardiac cycle images.
Image analysis
The saved 2D multi-cardiac cycle images were re-imported into the Philips Qlab workstation for analysis. The region of interest (ROI) was manually outlined and placed on the septal side, free wall side, and apex of the tricuspid annulus. The software automatically recognizes and delineates the endocardial and epicardial boundaries of the right ventricle, and software will automatically obtain the RVGLS. In addition, TMAD was obtained by clicking the TMAD mode, and then manually outlining by placing sampling points on the free wall side at the tricuspid annular level, the ventricular septal side, and the apex of the right ventricle, respectively. The software automatically calculates the parameter values of each point and calculates the TMAD at the right ventricular free wall (TMAD1), TMAD at the ventricular septum (TMAD2), TMAD at the midpoint of the tricuspid valve (TMADm) displacement, and percentage of right ventricular longitudinal shortening (TMADm%).
At the same time, in the four-chamber heart view, the endocardial boundary of the right ventricle was outlined respectively at the end-systolic and end-diastolic stages. The area of the right ventricle was measured. The right ventricular fractional area change (RVFAC) was calculated by the formula: RVFAC = (end-diastolic area − end-systolic area)/end-diastolic area × 100%.
Reproducibility of right heart parameter measurements
Ultrasound images were taken of 30 randomly selected patients. Two experienced attending physicians performed two repeated measurements on these images at different times. The collected data were subjected to intraclass correlation coefficient (ICC) test to analyze the reliability within and between observers.
Statistical analysis
The data were analyzed using SPSS 25 (IBM Corp., Armonk, NY, USA). Initially, the normality of the data was assessed using the Shapiro-Wilk test. For continuous variables that conformed to a normal distribution, mean and standard deviation were used for description. The chi-square test was used for comparing the gender composition rates, and the independent sample t-test was used for comparing between two groups. The receiver operating characteristic (ROC) curve was drawn, and the area under the ROC curve (AUC) of RVGLS and TMAD was calculated. The optimal cut-off value was selected to analyze the diagnostic efficacy of the observation indicators at this cut-off value. An AUC value >0.9 was considered to indicate high diagnostic efficacy of the parameter; 0.7< AUC value <0.9 was considered indicative of medium diagnostic efficacy; and AUC value <0.7 was considered to indicate low diagnostic efficacy. The correlation between two parameters in the same group was analyzed by Pearson correlation analysis. |r|>0.8 was considered indicative of extremely high correlation between two variables; 0.6<|r|≤0.8 was considered indicative of high correlation between two variables; 0.4<|r|≤0.6 was considered indicative of moderate correlation between two variables; 0.2<|r|≤0.4 was considered indicative of low correlation between two variables, and |r|≤0.2 was considered indicative of no correlation between two variables. A significance level of α=0.05 and bilateral P<0.05 were considered statistically significant differences.
Results
Clinical characteristics
In this study, a total of 64 patients diagnosed with RMS and 50 normal controls were enrolled. The participant inclusion process is shown in Figure 1. The basic clinical characteristics of all participants were statistically analyzed. As shown in Table 1, it is evident that there were no significant differences in gender composition ratio, age, height, weight, and BMI among the participants, and the differences were not statistically significant. However, there was a statistically significant difference in the composition ratio of mitral stenosis among the participants. Among the RMS patients, 100% had moderate or severe mitral stenosis, and patients with severe stenosis comprised approximately 58% of the total RMS patient population in our cohort.
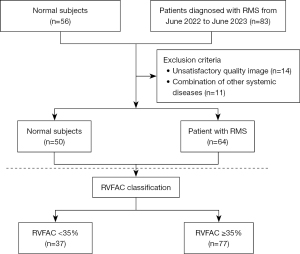
Table 1
| Parameters | Controls (n=50) | RHD (n=64) | P value |
|---|---|---|---|
| Age (years) | 51.06±13.4 | 54.23±7.92 | 0.90 |
| Female | 38 [76] | 48 [75] | 0.12 |
| Height (cm) | 159.72±8.90 | 158.42±8.28 | 0.42 |
| Weight (kg) | 57.46±7.88 | 55.06±9.69 | 0.16 |
| BMI (kg/m2) | 22.50±2.25 | 21.86±3.09 | 0.22 |
| RMS | 0 [0] | 64 [100] | <0.001* |
| Moderate | 0 [0] | 27 [42] | <0.001* |
| Severe | 0 [0] | 37 [58] | <0.001* |
Data are presented as mean ± SD or frequency [%]. Moderate: mitral valve orifice area is between 1.5 and 2.5 cm2 (inclusive); severe: mitral valve orifice area <1.5 cm2. *, P<0.05 compared with control group. BMI, body mass index; RHD, rheumatic heart disease; RMS, rheumatic mitral stenosis; SD, standard deviation.
Echocardiographic parameters
The parameters such as RVFAC, RVGLS, and TMAD obtained after echocardiography examination of patients with RMS were compared with those of the normal control group. The results showed that the values of the above indicators in RMS patients were all lower than those in the normal control group (P values were all less than 0.001) (see Figures 2,3 for details).
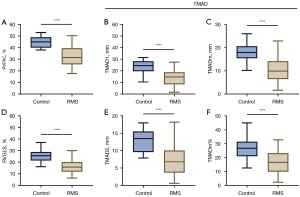
Correlation analysis between RAFAC and RVGLS and TMAD
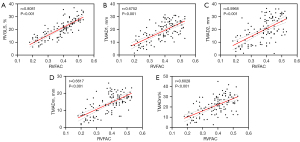
Correlation analysis was performed on the RVGLS and TMAD parameters of all participants with the RVFAC parameter. The results showed that RVFAC was highly correlated with RVGLS, TMAD1, TMADm, and TMADm% (r=0.8081, 0.6752, 0.6617, 0.6028, all P<0.001). It was moderately correlated with TMAD2 (r=0.5968, P<0.001) (Figure 4).
Evaluation of diagnostic efficacy of RVGLS and TMAD
According to the guideline recommendations jointly issued by the ASE and the European Association of Echocardiography (EAE) (16), all participants were divided into the normal right ventricular systolic function group (RVFAC ≥35%) and the right ventricular systolic dysfunction group (RVFAC <35%) according to their RVFAC. The ROC curve was drawn, and the AUC of RVGLS and TMAD parameters was calculated to evaluate their diagnostic efficacy.
The results showed that both RVGLS and TMAD had high diagnostic efficacy in the application of identifying right ventricular systolic dysfunction in participants (see Figure 5 and Table 2 for details).
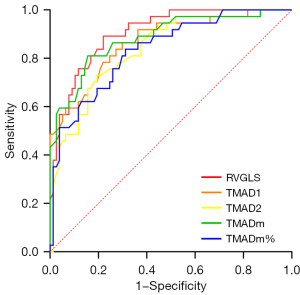
Table 2
| Parameters | AUC value (95% CI) | P value | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| RVGLS | 0.9066 (0.8532–0.9601) | <0.001 | 18.90% | 77.9 | 89.2 |
| TMAD1 | 0.8687 (0.7976–0.9399) | <0.001 | 17.55 mm | 70.1 | 86.5 |
| TMAD2 | 0.8415 (0.7680–0.9151) | <0.001 | 8.15 mm | 80.5 | 73.0 |
| TMADm | 0.8849 (0.8169–0.9528) | <0.001 | 12.95 mm | 84.4 | 81.1 |
| TMADm% | 0.8349 (0.7574–0.9123) | <0.001 | 20.90% | 68.8 | 83.8 |
AUC, area under the ROC curve; CI, confidence interval; ROC, receiver operating characteristic; RVGLS, right ventricular global longitudinal strain; TMAD, tissue mitral annular displacement; TMAD1, TMAD at the right ventricular free wall; TMAD2, TMAD at the ventricular septum; TMADm, TMAD at the midpoint of the tricuspid valve; TMADm%, percentage of right ventricular longitudinal shortening.
Reproducibility of right heart parameter measurements
The reproducibility of echocardiographic measurements was evaluated by ICC analysis. As shown in Table 3, both interobserver and intraobserver variability for all parameters demonstrated excellent reliability.
Table 3
| Parameters | Interobserver variability | Intraobserver variability | |||
|---|---|---|---|---|---|
| ICC (95% CI) | P value | ICC (95% CI) | P value | ||
| RVGLS | 0.873 (0.703–0.972) | <0.001 | 0.906 (0.788–0.963) | <0.001 | |
| TMAD1 | 0.978 (0.870–0.982) | <0.001 | 0.982 (0.902–0.986) | <0.001 | |
| TMAD2 | 0.970 (0.931–0.966) | <0.001 | 0.978 (0.900–0.980) | <0.001 | |
| TMADm | 0.893 (0.774–0.957) | <0.001 | 0.950 (0.877–0.988) | <0.001 | |
| TMADm% | 0.922 (0.828–0.961) | <0.001 | 0.947 (0.883–0.991) | <0.001 | |
CI, confidence interval; ICC, intraclass correlation coefficient; RVGLS, right ventricular global longitudinal strain; TMAD, tissue mitral annular displacement; TMAD1, TMAD at the right ventricular free wall; TMAD2, TMAD at the ventricular septum; TMADm, TMAD at the midpoint of the tricuspid valve; TMADm%, percentage of right ventricular longitudinal shortening.
Technical operation time
The operation time for measuring the RVGLS and TMAD parameters of 30 participants by ultrasound for the first time was timed. The time consumed for measuring these two parameters was compared to see if there was a difference, with the results shown in Table 4.
Table 4
| Operation time (min) | RVGLS | TMAD | P value |
|---|---|---|---|
| Mean ± SD | 6.25±1.76 | 1.45±0.45 | <0.001* |
| Minimum value | 4 | 1 | <0.001* |
| Maximum value | 9 | 3 | <0.001* |
*, P<0.05 compared with RVGLS group. RVGLS, right ventricular globe longitudinal strain; SD, standard deviation; TMAD, tissue mitral annular displacement.
Discussion
Our research mainly yielded the following three findings: first, the right ventricular myocardial contractility of patients with RMS is significantly weakened compared to the normal group. Second, RVGLS and TMAD technology have a good correlation. When performing TMAD technology detection, the time consumed and the requirement for the clarity of regional images are lower than those for detecting RVGLS. This indicates that in practical clinical applications, TMAD technology has higher efficiency and convenience. Third, RVGLS has a quite high diagnostic ability with an AUC value of 0.9066. At the same time, the four parameters under TMAD technology also perform well, showing that TMAD technology also has good diagnostic power.
The pathological changes of RMS mainly involve the mitral valve, which easily causes valve thickening and adhesion, and then leads to valvular stenosis, ultimately resulting in left heart hemodynamic disorders and having a great impact on systemic blood circulation (16,17). Therefore, in the diagnosis and treatment process of RMS, previous studies have mostly focused on the evaluation of left heart function in patients, and in clinical treatment, more attention is paid to the protection of left heart function (for example, patients often use surgical procedures such as mitral valve replacement to improve left heart blood flow). With the in-depth study of RMS, researchers have gradually realized that protecting right heart function also has important value for good prognosis of RMS patients (10).
The right atrium receives venous blood from the whole body through the superior and inferior vena cava, and then transfers the blood into the pulmonary circulation for blood exchange to continue to supply energy to the body. With the progressive development of RMS, the increase in left atrial pressure load will not only lead to congestion in the pulmonary circulation but also gradually involve the right heart system. The increase in right heart afterload forces the right ventricular wall to have a compensatory increase in tension, which in turn leads to right ventricular hypertrophy and dilation. Due to the continuous expansion of the right ventricle, the tricuspid annulus also enlarges, further leading to tricuspid insufficiency. In echocardiography, it is usually manifested as tricuspid insufficiency and the increase in pulmonary artery pressure estimated by the tricuspid regurgitation spectrum. Under the condition of long-term pulmonary hypertension, the enlargement of the tricuspid annulus and tricuspid insufficiency will greatly increase the risk of right heart failure and even death from total heart failure (18,19). Therefore, during the treatment of RMS, accurate and early identification of patients with subclinical right ventricular myocardial dysfunction is of great value for guiding the focus of clinical decision-making in disease treatment and improving the prognosis of patients.
The effectiveness of the RVGLS value measured based on speckle tracking technology in evaluating right ventricular myocardial function has been widely recognized in the field (20). However, due to limitations such as individual differences in patient’s body position, body shape, bone condition, and heart morphology, as well as differences in the proficiency of ultrasound physicians in mastering the technology, consistent accurate imaging of the right ventricle is challenging. When obtaining the overall strain of the right ventricle, it is usually difficult to obtain a very clear and complete right ventricular myocardial image, rendering RVGLS unable to fully exert its maximum value in evaluating right ventricular myocardial function (21). The TMAD technology is also based on speckle tracking technology. Different from RVGLS, TMAD focuses on the myocardial speckle displacement in the tricuspid annulus area. By detecting the TMAD1, TMAD2, and TMADm of the patient and the TMADm%, the myocardial motion is evaluated. Obtaining images of this area is simpler and easier than obtaining complete right ventricular images.
In this study, statistical analysis was performed on the ultrasound image data of 114 participants (64 patients with RMS and 50 normal controls). When comprehensively evaluating the right heart function of the participants, we adopted the RVFAC parameter. As a 2D echocardiographic parameter, RVFAC has limitations including assumptions about the geometric shape of the right ventricle, dependence on the identification of the endocardial border, and the inability to assess regional functional heterogeneity. In this study, we selected RVFAC as a reference because it remains a basic assessment method recommended by current clinical guidelines (22,23). We found that there was a significant difference in right heart function between patients with RMS and the normal control group. The values of right heart function evaluation parameters RVFAC, RVGLS, and TMAD in patients were all lower than those in normal people, indicating that the right ventricular myocardial contractility of patients with RMS was significantly weakened compared to the normal group. Through correlation analysis of the derived parameters of RVGLS and TMAD technology, this study found that RVGLS and TMAD technology had a good correlation. This shows that in daily work, TMAD technology can be used as a simple index to evaluate the right ventricular myocardial function of patients with RMS, providing the same clinical reference significance as RVGLS and early indication of the possibility of right heart systolic dysfunction in clinical patients with RMS. In addition, after classifying all participant data according to RVFAC and performing ROC curve analysis, it was found that RVGLS had a quite high diagnostic ability (AUC =0.9066). At the same time, the four parameters under TMAD technology also had good performance, showing that TMAD technology also had good diagnostic power. This conclusion is similar to the research results obtained by Sun et al. using TMAD technology to identify the right ventricular contractility of uremic patients (24). However, in the results of this study, the diagnostic efficacy shown by TMAD was slightly inferior to that of RVGLS. This may be related to the difference in the principle of data acquisition technology. TMAD data is obtained by measuring the displacement of the tricuspid tissue annulus, which is different from RVGLS that directly focuses on myocardial movement. Strictly speaking, TMAD should be an indirect measurement method of right ventricular myocardial contractility. In the original data acquisition stage of this study, great attention was paid to the image acquisition of the overall shape of the right ventricle, so this may be an important factor affecting the evaluation of the diagnostic efficacy of the two parameters.
To further verify the practicability and efficiency of these parameters in actual clinical work, we conducted a comparative analysis of the measurement and operation time of the two parameters RVGLS and TMAD. The results show that although there were differences in the operation habits and experience levels of different physicians, the measurement time of RVGLS and TMAD was within the acceptable range of daily inspection time and has good consistency. Moreover, when performing TMAD technology detection, the time consumed and the requirement for the clarity of regional images are lower than those for detecting RVGLS. This indicates that even under high clinical work pressure, the TMAD technology can still be efficiently integrated into the daily diagnosis process and provide physicians with quick and relatively accurate assessment results.
Although three-dimensional (3D) echocardiography and cardiac magnetic resonance (CMR) imaging can assess right ventricular volume and ejection fraction more accurately, especially CMR, which is currently the gold standard and can provide more comprehensive information on right ventricular function, its application in routine clinical practice has not been widely adopted due to limitations in equipment availability, examination time-consuming nature, and cost factors. 3D echocardiography also has significant advantages in evaluating right ventricular function, but it has high requirements for image quality and may be difficult to obtain ideal images in some patients. Therefore, this study selected two techniques based on 2D echocardiography, namely TMAD and RVGLS, which have the advantages of simple operation, low cost, and high accessibility. Focusing on the development of convenient techniques suitable for primary medical scenarios, in future research, we plan to conduct more in-depth verification in combination with CMR or 3D echocardiography.
Study limitations
The limitations of this study are as follows: first, the sample size is limited and it is a single-center study, which may affect the universality and reliability of the conclusion. Second, this study only targets patients with RMS and normal controls, and the applicability to patients with other heart diseases needs further study. Third, affected by patient individual differences (such as body position, body shape, bone condition, heart morphology, etc.) and the proficiency of ultrasound physicians, the measurement results may have certain errors. Fourth, other factors that may affect the right ventricular myocardial function of RMS patients are not fully considered.
Conclusions
We have verified the accuracy of TMAD technology and RVGLS in evaluating the right ventricular systolic function of patients with RMS, and the measurement results of the two were highly correlated with RVFAC. This indicates that both TMAD technology and RVGLS are effective tools for evaluating the right ventricular function of RMS patients. In view of the advantages of TMAD technology in terms of operational simplicity and measurement efficiency, it may be a better choice for early clinical identification of subclinical right ventricular myocardial dysfunction in patients with RMS.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-2296/rc
Funding: The study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2296/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethics Committee of The First Affiliated Hospital of Guangxi Medical University (No. 2022-KY-E-132). Written informed consent was provided by all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet 2012;379:953-64. [Crossref] [PubMed]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021. Erratum in: J Am Coll Cardiol 2021;77:1958-9. [Crossref] [PubMed]
- Kumar RK, Antunes MJ, Beaton A, Mirabel M, Nkomo VT, Okello E, Regmi PR, Reményi B, Sliwa-Hähnle K, Zühlke LJ, Sable CAmerican Heart Association Council on Lifelong Congenital Heart Disease and Heart Health in the Young. Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Contemporary Diagnosis and Management of Rheumatic Heart Disease: Implications for Closing the Gap: A Scientific Statement From the American Heart Association. Circulation 2020;142:e337-57. Erratum in: Circulation 2021;143:e1025-6. [Crossref] [PubMed]
- Ruan R, Liu X, Zhang Y, Tang M, He B, Zhang QW, Shu T. Global, Regional, and National Advances Toward the Management of Rheumatic Heart Disease Based on the Global Burden of Disease Study 2019. J Am Heart Assoc 2023;12:e028921. [Crossref] [PubMed]
- Wunderlich NC, Beigel R, Siegel RJ. Management of mitral stenosis using 2D and 3D echo-Doppler imaging. JACC Cardiovasc Imaging 2013;6:1191-205. [Crossref] [PubMed]
- Silbiger JJ. Advances in Rheumatic Mitral Stenosis: Echocardiographic, Pathophysiologic, and Hemodynamic Considerations. J Am Soc Echocardiogr 2021;34:709-722.e1. [Crossref] [PubMed]
- Choi YJ, Choi JY, Lee J, Choi BG, Park S, Kang DO, Park EJ, Kim JB, Roh SY, Choi CU, Kim JW, Kim EJ, Rha SW, Park CG, Yong HS, Baek MJ, Na JO. Prognostic Value of Pulmonary Artery Systolic Pressure in Severe Rheumatic Mitral Stenosis. Circ Cardiovasc Imaging 2024;17:e016302. [Crossref] [PubMed]
- Hardegree EL, Sachdev A, Fenstad ER, Villarraga HR, Frantz RP, McGoon MD, Oh JK, Ammash NM, Connolly HM, Eidem BW, Pellikka PA, Kane GC. Impaired left ventricular mechanics in pulmonary arterial hypertension: identification of a cohort at high risk. Circ Heart Fail 2013;6:748-55. [Crossref] [PubMed]
- Han L, Zhang B, Zhu J, Mei J, Zou L, Xu Z. A study on mechanical properties of pulmonary artery in pulmonary hypertension secondary to rheumatic mitral stenosis. Chinese Journal of Thoracic and Cardiovascular Surgery 2001;17:4-6.
- Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward CAmerican Heart Association Council on Clinical Cardiology. Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018;137:e578-622. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713; quiz 786-8. [Crossref] [PubMed]
- Egbe AC, Miranda WR, Connolly HM. Role of Echocardiography for Assessment of Cardiac Remodeling in Congenitally Corrected Transposition of Great Arteries. Circ Cardiovasc Imaging 2022;15:e013477. [Crossref] [PubMed]
- Pandian NG, Kim JK, Arias-Godinez JA, Marx GR, Michelena HI, Chander Mohan J, Ogunyankin KO, Ronderos RE, Sade LE, Sadeghpour A, Sengupta SP, Siegel RJ, Shu X, Soesanto AM, Sugeng L, Venkateshvaran A, Campos Vieira ML, Little SH. Recommendations for the Use of Echocardiography in the Evaluation of Rheumatic Heart Disease: A Report from the American Society of Echocardiography. J Am Soc Echocardiogr 2023;36:3-28. [Crossref] [PubMed]
- Nyberg J, Jakobsen EO, Østvik A, Holte E, Stølen S, Lovstakken L, Grenne B, Dalen H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc Imaging 2023;16:1516-31. [Crossref] [PubMed]
- Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013;6:711-21. [Crossref] [PubMed]
- Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011;12:167-205. [Crossref] [PubMed]
- Magne J, Pibarot P, Sengupta PP, Donal E, Rosenhek R, Lancellotti P. Pulmonary hypertension in valvular disease: a comprehensive review on pathophysiology to therapy from the HAVEC Group. JACC Cardiovasc Imaging 2015;8:83-99. [Crossref] [PubMed]
- Sakkuru PKR, Velam V, Durgaprasad R, Chanda N, Maddala RNM, Yandrapu MN. Assessment of immediate effects of percutaneous balloon mitral valvuloplasty on right ventricular and pulmonary functions in severe rheumatic mitral stenosis patients using speckle tracking echocardiography and spirometry. J Cardiovasc Thorac Res 2020;12:280-5. [Crossref] [PubMed]
- Egbe AC, Miranda WR, Jain CC, Andi K, Abozied O, Younis A, Kandlakunta S, Salama AA, Stephens EH, Connolly HM. Prognostic Performance of Right Ventricular Global Longitudinal Strain Measurements in Patients With Ebstein Anomaly. J Am Coll Cardiol 2023;82:503-13. [Crossref] [PubMed]
- Keelan J, Pasumarti N, Crook S, Decost G, Wang Y, Crystal MA, Shah A, Bacha E, Mercer-Rosa L, DiLorenzo M. Right Ventricular Strain in Patients With Ductal-Dependent Tetralogy of Fallot. J Am Soc Echocardiogr 2023;36:654-65. [Crossref] [PubMed]
- Muraru D, Haugaa K, Donal E, Stankovic I, Voigt JU, Petersen SE, Popescu BA, Marwick T. Right ventricular longitudinal strain in the clinical routine: a state-of-the-art review. Eur Heart J Cardiovasc Imaging 2022;23:898-912. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Sun J, Wang Y, Zhang J, Rui H, Sun Y, Wang Z. Evaluation of Right Ventricular Systolic Function with Automated Motion Tracking of Tricuspid Annular Displacement in Patients with Uremia. Chinese Journal of Ultrasound in Medicine 2016;32:28-30.


