
Ultrasound detection of biliary atresia splenic malformation syndrome from prenatal to postnatal: a case description
Introduction
Biliary atresia splenic malformation syndrome (BASM), proposed by Davenport et al. in 1993, refers to biliary atresia accompanied by splenic malformation and various anatomical anomalies, including situs inversus, vascular anomalies, intestinal malrotation, pancreatic anomalies, and other cardiac or pulmonary malformations (1-3). Biliary atresia has a regional incidence ranging from 0.3 to 3.7 per 10,000 live births (2,4), with BASM occurring in approximately 5–15% of biliary atresia cases (3,5). Aside from cirrhosis and hepatic failure, severe cardiac malformations and secondary hepatopulmonary syndrome largely determine the prognosis (6,7). Most BASM cases are not correctly diagnosed until surgery; prenatal or neonatal ultrasound diagnosis of this syndrome has been rarely reported. In the case reported herein, we observed several characteristic images of BASM upon neonatal transabdominal ultrasound, and have described detailed cautions for prenatal diagnosis of BASM.
Case presentation
A 13-day-old female neonate, delivered at 37 weeks of gestation, was referred to Shengjing Hospital of China Medical University due to neonatal jaundice. During prenatal ultrasound at the district hospital, a gallbladder measuring 1.1 cm × 0.2 cm was visualized, along with persistent right umbilical vein (Figure 1A). Meanwhile, the inferior vena cava (IVC) crossed over the abdominal aorta from left to right at the kidney level (Figure 1B). On day 17 after birth, transabdominal ultrasound revealed a dysplastic gallbladder measuring 1.1 cm × 0.2 cm, which did not contract after feeding (Figure 2A). The liver exhibited a “mirror-image” of normal anatomy (Figure 2B), the pancreas was significantly shorter than usual (Figure 2C), and polysplenia, characterized by four lobulated spleens, was noted in the left hypochondrium (Figure 2D). Based on these findings, BASM was suspected. Whole-exome sequencing identified deleterious variants in the ABCB4 and MMP21 genes, which have been individually implicated in several hepatobiliary diseases (8) and heterotaxy along with laterality defects (9,10).

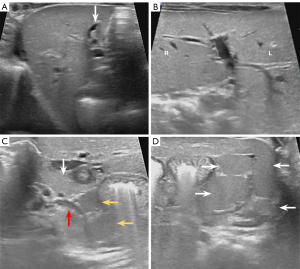
The infant underwent surgery on the 39th post-natal day. During the laparoscopic bile duct exploration, the liver was noted to be irregularly textured and dark green. An empty gallbladder was situated to the left of the ligamentum teres hepatis with a thick and tough wall. Intraoperative cholangiography revealed that the small gallbladder could be visualized by the contrast agent, but the intrahepatic bile duct, the biliary tract system, and the duodenum could not be visualized, and there was no contrast agent in the abdominal cavity. Therefore, type III biliary atresia was diagnosed. Laparotomy showed that the portal vein was compressing the duodenum approximately 0.5 cm distal to the pylorus (Figure 3A). Multiple nodular spleens were visible in the left upper quadrant of the abdomen (Figure 3B). Additionally, the Treitz ligament was not visualized, and the Ladd band crossed the duodenum and initial segment of the jejunum. Subsequently, hepaticojejunostomy, duodenoplasty, and a Ladd operation were performed. Despite surgical intervention, the total bilirubin level persisted in increasing from 124.7 to 157.9 µmol/L. Concurrently, conjugated bilirubin increased from 101.9 to 132.1 µmol/L, and unconjugated bilirubin increased from 22.8 to 25.8 µmol/L. The infant was discharged 37 days after surgery to prevent viral infection, but succumbed to liver failure and pulmonary infection at 6 months of age.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was provided by the patient’s parents for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
BASM is a rare but catastrophic congenital anomaly (1,3,5). To the best of our knowledge, there have been no reports of prenatal ultrasound diagnosis of BASM to date. In this case, specific ultrasound signs appeared before delivery, potentially providing clues for future prenatal and postnatal diagnosis of BASM.
Currently, the dysplastic gallbladder can be used to diagnose neonatal biliary atresia. The main gallbladder anomalies include the absence of a gallbladder, a small gallbladder, irregular gallbladder wall, and abnormal gallbladder contraction (2,4,11-14). In our previous study, we proposed criteria for high-risk gallbladders for prenatal prediction of biliary atresia (15) for the first time, which include three conditions: (I) nonvisualization of the gallbladder, (II) gallbladder length ≤1.4 cm, or (III) a width-to-length ratio of the gallbladder <0.2 when the gallbladder length is >1.4 cm throughout pregnancy. In this case, both prenatal and postnatal ultrasound findings of the gallbladder indicated the possibility of biliary atresia.
In addition, heterotaxy of the liver was diagnosed postnatally, suggesting that the prenatal diagnosis of persistent right umbilical vein was inaccurate. During the prenatal ultrasound examination, sonographers neglected to evaluate the overall appearance and proportion of the left and right lobes of the liver, thus overlooking heterotaxy of the liver. In a 28-year retrospective study conducted by Davenport et al. (3), a liver with a “mirror-image” appearance and a left-sided gallbladder has been described in BASM. Therefore, when high-risk gallbladders and heterotaxy of the liver are concurrently detected, BASM should be considered.
Vascular anomalies are frequent in BASM, particularly the interruption of IVC and preduodenal portal vein (2,3,5). To the best of our knowledge, this is the first reported case of a left IVC associated with BASM. Therefore, in suspected BASM cases, scanning and evaluation of the vena cava are crucial.
Another significant abnormality in BASM patients is splenic malformation, which includes polysplenia, asplenia, a double spleen, and right-sided spleen (2,3,5,6,16). When there is a high suspicion of BASM, the spleen should be carefully examined. High-frequency probes are used during newborn ultrasound examinations, which can clearly display the location, quantity, and morphology of the spleen. The fetal spleen, with an echo slightly lower than that of the liver, is commonly located on the left or behind the gastric bubble in the upper-left region of the abdomen. The splenic artery is also crucial for scanning the spleen (17), and prenatal diagnosis of polysplenia (18) and asplenia (19) have been reported before. Therefore, prenatal detection of splenic malformation is a feasible approach for diagnosing BASM.
The fetus was diagnosed with a short pancreas and intestinal malrotation postnatally. Pancreatic abnormalities include a short pancreas and an annular pancreas (2,3), due to an aberrant fusion of the dorsal and ventral buds (20). The incidence of a short pancreas in BASM is nearly 4% (2,3), with most cases identified during surgery or autopsy (2,21). Scanning the fetal pancreas is a challenge for sonographers (20). The “whirlpool sign” is a typical manifestation of intestinal malrotation combined with midgut volvulus. Several other features, including ascites, echogenic bowels, and intestinal dilatation, can also assist in diagnosis (22). However, in the absence of intestinal abnormalities, sonographers rarely proactively scan the mesenteric blood vessels. Consequently, for patients suspected of BASM, scanning the structure of the pancreas and intestines, as well as using mesenteric Doppler, is necessary.
It is necessary to perform detailed fetal echocardiography once BASM is suspected. According to previous studies, BASM fetuses with cardiac anomalies have a higher mortality rate than those without cardiac anomalies (3,6). Additionally, the incidence of cardiac anomalies in BASM is up to 45% (2). During prenatal ultrasound examinations, several diseases such as ventricular septal defect, tetralogy of Fallot, and hypoplastic left heart can be discovered (3).
A comprehensive imaging evaluation of the malformations will undoubtedly benefit surgical decision-making and planning; however, it requires doctors to have a thorough understanding of the syndrome. Table 1 presents the ultrasound features of several anomalies in BASM (2,4,11-15,18,20,22-27). If an accurate diagnosis can be obtained before delivery, it will facilitate earlier decisions regarding treatment options. Parents will be fully informed of the severity of the fetal disease. It also enables early intervention and treatment, which can minimize the impact on the fetus. The patients can then receive treatment as soon as they are born, rather than after jaundice persisting. In addition, an early surgery can reduce the progress of liver failure (28).
Table 1
| Malformations | Prenatal ultrasound features | Postnatal ultrasound features |
|---|---|---|
| Biliary atresia | Absence of gallbladder, small gallbladder, irregular gallbladder wall, abnormal gallbladder contraction (2,4,11-15) | |
| Other potential features (23,24) | Other potential features (11-14) | |
| (I) Dilated right hepatic artery | (I) The triangular cord sign | |
| (II) Biliary cysts | (II) Hepatic subcapsular flow | |
| (III) Ascites | (III) Hilar lymphadenopathy | |
| (IV) Echogenic bowel | (IV) Increased liver stiffness in elastography techniques | |
| Splenic malformation | ||
| Polysplenia | Several splenic arteries (18) | Nodular or globular nodules, with an echo similar to the spleen |
| Asplenia | No identifiable spleen | |
| Vascular anomalies | ||
| The interruption of IVC | Absence of proximal segment of IVC | |
| Dilated azygous vein or hemiazygos vein | ||
| Pancreatic abnormalities | ||
| Short pancreas | Difficult to discover (20) | Shorter than usual |
| Annular pancreas | Distention of the stomach and duodenum | |
| The duodenum surrounded by pancreatic tissue (25,26) | ||
| Intestinal malrotation | Inverse SMA/SMV orientation (27) | |
| Intestinal dilatation | ||
| Whirlpool sign when combined with midgut volvulus (22) | ||
IVC, inferior vena cava; SMA/SMV, superior mesenteric artery/superior mesenteric vein.
Conclusions
Neonatal ultrasound can accurately diagnose BASM and provide more comprehensive information for surgical planning. When prenatal ultrasound detects an abnormal gallbladder along with portal vein abnormalities, BASM should be considered as a potential diagnosis. More detailed scans should be conducted when BASM is suspected.
Acknowledgments
None.
Footnote
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-2085/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was provided by the patient’s parents for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davenport M, Savage M, Mowat AP, Howard ER. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery 1993;113:662-8. [PubMed]
- Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet 2009;374:1704-13. [Crossref] [PubMed]
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzić N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006;149:393-400. [Crossref] [PubMed]
- Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun 2016;73:1-9. [Crossref] [PubMed]
- Guttman OR, Roberts EA, Schreiber RA, Barker CC, Ng VLCanadian Pediatric Hepatology Research Group. Biliary atresia with associated structural malformations in Canadian infants. Liver Int 2011;31:1485-93. [Crossref] [PubMed]
- Nio M, Wada M, Sasaki H, Tanaka H, Watanabe T. Long-term outcomes of biliary atresia with splenic malformation. J Pediatr Surg 2015;50:2124-7. [Crossref] [PubMed]
- Kimura T, Hasegawa T, Sasaki T, Okada A, Mushiake S. Rapid progression of intrapulmonary arteriovenous shunting in polysplenia syndrome associated with biliary atresia. Pediatr Pulmonol 2003;35:494-8. [Crossref] [PubMed]
- Reichert MC, Lammert F. ABCB4 Gene Aberrations in Human Liver Disease: An Evolving Spectrum. Semin Liver Dis 2018;38:299-307. [Crossref] [PubMed]
- Guimier A, Gabriel GC, Bajolle F, Tsang M, Liu H, Noll A, et al. MMP21 is mutated in human heterotaxy and is required for normal left-right asymmetry in vertebrates. Nat Genet 2015;47:1260-3. [Crossref] [PubMed]
- Perles Z, Moon S, Ta-Shma A, Yaacov B, Francescatto L, Edvardson S, Rein AJ, Elpeleg O, Katsanis N. A human laterality disorder caused by a homozygous deleterious mutation in MMP21. J Med Genet 2015;52:840-7. [Crossref] [PubMed]
- Zhou L, Shan Q, Tian W, Wang Z, Liang J, Xie X. Ultrasound for the Diagnosis of Biliary Atresia: A Meta-Analysis. AJR Am J Roentgenol 2016;206:W73-82. [Crossref] [PubMed]
- Tam PKH, Wells RG, Tang CSM, Lui VCH, Hukkinen M, Luque CD, De Coppi P, Mack CL, Pakarinen M, Davenport M. Biliary atresia. Nat Rev Dis Primers 2024;10:47. [Crossref] [PubMed]
- Dike PN, Mahmood N, Harpavat S. Recent advances in the use of ultrasound and related techniques in diagnosing and predicting outcomes in biliary atresia. Curr Opin Pediatr 2021;33:515-20. [Crossref] [PubMed]
- Gong Z, Lin L, Lu G, Wan C. Development and validation of a model for early diagnosis of biliary atresia. BMC Pediatr 2023;23:549. [Crossref] [PubMed]
- Zeng K, Yang Z, Chen L, Sun W, Wang Y, Chen C, Cai A. Prediction of Fetal Biliary Atresia Based on Second and Third-Trimester Ultrasound Characteristics. Ultraschall Med 2023;44:307-17. [Crossref] [PubMed]
- Schwarz KB, Haber BH, Rosenthal P, Mack CL, Moore J, Bove K, Bezerra JA, Karpen SJ, Kerkar N, Shneider BL, Turmelle YP, Whitington PF, Molleston JP, Murray KF, Ng VL, Romero R, Wang KS, Sokol RJ, Magee JC. Extrahepatic anomalies in infants with biliary atresia: results of a large prospective North American multicenter study. Hepatology 2013;58:1724-31. [Crossref] [PubMed]
- Abuhamad AZ, Robinson JN, Bogdan D, Tannous RJ. Color Doppler of the splenic artery in the prenatal diagnosis of heterotaxic syndromes. Am J Perinatol 1999;16:469-73. [Crossref] [PubMed]
- Meyers ML, Crombleholme T. Prenatal MRI Diagnosis of Hirschsprung's Disease at 29 Weeks' Gestational Age in a Fetus with Heterotaxy and Polysplenia Syndrome. Fetal Diagn Ther 2016;40:235-40. [Crossref] [PubMed]
- Konstantinidou A, Sifakis S, Koukoura O, Mantas N, Agrogiannis G, Patsouris E. Pancreatic aplasia in a fetus with asplenia-cardiovascular defect-heterotaxy (Ivemark syndrome). Birth Defects Res A Clin Mol Teratol 2008;82:601-4. [Crossref] [PubMed]
- Kivilevitch Z, Achiron R, Perlman S, Gilboa Y. The Normal Fetal Pancreas. J Ultrasound Med 2017;36:1997-2005. [Crossref] [PubMed]
- Deveci MS, Deveci G. Biliary atresia splenic malformation syndrome--is it a result of embryonically midline rotational defects? A case report. J Pediatr Surg 2000;35:1377-80. [Crossref] [PubMed]
- Yang L, Chen H, Lv G, Li F, Liao J, Ke L. Evaluation of ultrasonography in fetal intestinal malrotation with midgut volvulus. Ginekol Pol 2022;93:296-301. [Crossref] [PubMed]
- Shen O, Rabinowitz R, Yagel S, Gal M. Absent gallbladder on fetal ultrasound: prenatal findings and postnatal outcome. Ultrasound Obstet Gynecol 2011;37:673-7. [Crossref] [PubMed]
- He F, Feng S, Xiu Y, Zhang Y, Wang Y, Zhang Z, Chen L. Dysmorphic Gallbladder Found on Prenatal Ultrasound as a Hint for Biliary Atresia. J Ultrasound Med 2023;42:1345-51. [Crossref] [PubMed]
- Zhang B, Zhang W, Hu Y, Pang H, Yang H, Luo H. Evaluation of prenatal and postnatal ultrasonography for the diagnosis of fetal double bubble sign. Quant Imaging Med Surg 2024;14:6386-96. [Crossref] [PubMed]
- Piglia EBMD, Penna CRR, Tobias J, Oliveira D, Marchiori E. The main radiologic findings in annular pancreas. Radiol Bras 2019;52:275-6. [Crossref] [PubMed]
- Binu V, Nicholson C, Cundy T, Gent R, Piotto L, Taranath A, Goh DW. Ultrasound imaging as the first line of investigation to diagnose intestinal malrotation in children: Safety and efficacy. J Pediatr Surg 2021;56:2224-8. [Crossref] [PubMed]
- Xu X, Dou R, Zhao S, Zhao J, Gou Q, Wang L, Zhan J. Outcomes of biliary atresia splenic malformation (BASM) syndrome following Kasai operation: a systematic review and meta-analysis. World J Pediatr Surg 2022;5:e000346. [Crossref] [PubMed]


