
Utilizing temporal information to assess metabolic heterogeneity: a study of 18F-FDG dynamic positron emission tomography as a treatment response biomarker in small cell lung cancer
Introduction
Small cell lung cancer (SCLC) represents the most prevalent neuroendocrine tumor of the lung, accounting for approximately 15% of all lung cancer cases (1). The majority of SCLC patients are diagnosed at an extensive stage (2). Despite significant advancements in the diagnosis and treatment of lung cancer in recent years, up to 40% of patients with extensive-stage SCLC (ES-SCLC) do not achieve an objective response (OR) following first-line treatment (3). Consequently, it is imperative to predict treatment response in patients prior to treatment to facilitate the development of subsequent individualized treatment plans.
Fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is a crucial tool for the comprehensive assessment of cancer patients. However, the prognostic value of conventional metabolic parameters, such as maximum standard uptake value (SUVmax), in predicting treatment outcomes in SCLC remains controversial across different studies (4-7). Heterogeneity between tumors significantly impacts treatment outcomes (8). Conventional metabolic parameters may inadequately assess the metabolic heterogeneity of tumors, leading to suboptimal prediction of treatment response in SCLC. Therefore, novel methods to evaluate metabolic heterogeneity between tumors are essential for predicting treatment response in patients with ES-SCLC.
Compared to conventional PET scans, dynamic PET imaging (DPI) with 18F-FDG can track the uptake process of the radiotracer, providing detailed information on substrate delivery and metabolism, and more directly reflect the biochemical uptake process of 18F-FDG (9). In DPI, the temporal behavior of each pixel can be used to describe time-activity curve (TAC). The TAC, which calculates the mean activity of pixels in the target region over time, reflects the distribution and metabolism of the radiotracer in the target tissue or organ, offering valuable reference information for disease diagnosis and treatment evaluation (10). Previous studies have demonstrated that DPI parameters based on 18F-FDG can aid in predicting the prognosis of breast cancer patients (11). However, research in SCLC remains limited.
We hypothesize that the TAC features from DPI with 18F-FDG can better reflect tumor metabolic heterogeneity and correlate more closely with treatment response in SCLC compared to conventional PET parameters. Therefore, this study aimed to explore the relationship between TAC features and treatment response following first-line treatment in patients with ES-SCLC, with the goal of assisting in the formulation of subsequent treatment plans and achieving precise, personalized treatment. A flow chart showing the study procedure is presented in Figure 1. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1687/rc).
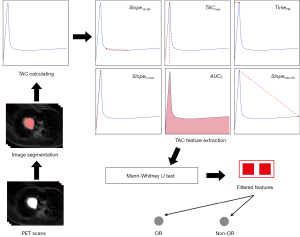
Methods
Patients
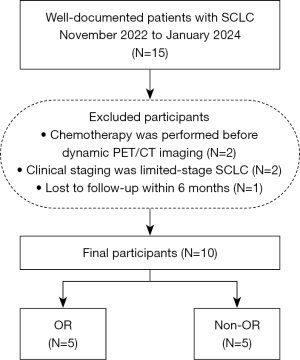
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Cancer Hospital & Shenzhen Hospital of Chinese Academy of Medical Sciences (approval No. KYLH2022-1). All patients signed a written informed consent form before the DPI. In total, 15 patients with pathologically confirmed SCLC in the Cancer Hospital & Shenzhen Hospital from November 2022 to January 2024 were prospectively enrolled. To reduce potential confounding factors arising from variations in prior treatment regimens or disease stages, the patient inclusion criteria were as follows: (I) histologically confirmed SCLC; (II) complete DPI data and clinical data; and (III) having accepted the first-line treatment from the National Comprehensive Cancer Network guidelines. The exclusion criteria were as follows: (I) chemotherapy was performed before DPI (N=2); (II) clinical staging was limited-stage SCLC (N=2); and (III) lost to follow-up within a 6-month period (N=1). Finally, 10 patients were included in the study. Given the incorporation of immunotherapy in the first-line treatment regimen for ES-SCLC, this study defines complete response (CR) and partial response (PR) according to the Immune Response Evaluation Criteria in Solid Tumors (iRECIST) criteria as OR (12). Figure 2 presents a schematic representation delineating the process of patient inclusion, exclusion, and subsequent grouping.
Dynamic PET/CT imaging
All participants observed a minimum fasting period of 6 hours prior to undergoing PET/CT imaging (Discovery MI PET/CT, GE Healthcare, Milwaukee, WI, USA). Initial imaging comprised a whole-body CT scan, encompassing the area from the head to the mid-femur, with participants in a supine position. CT parameters included a tube voltage of 120 kV, a tube current setting of 180–350 mA, a pitch of 1.375:1, and a noise index of 13. Following the administration of 18F-FDG (mean 259 MBq, range 227–298 MBq), PET scans of the chest region were promptly initiated. The duration of the total dynamic scans was 65 minutes, segmented into 28 frames: 6 frames of 10 seconds each, 4 frames of 30 seconds each, 4 frames of 60 seconds each, 4 frames of 120 seconds each, and 10 frames of 300 seconds each. Subsequently, an additional whole-body static PET scan was conducted upon completion of the dynamic acquisition. Attenuation correction utilized CT data, whereas reconstruction employed the Block sequential regularized expectation maximization reconstruction algorithm with 25 iterations and 2 subsets.
Image segmentation
The PET images were imported into ITK-SNAP software (Version 4.0.2; https://www.itksnap.org/) for segmentation. Initially, a senior radiologist with 16 years of PET imaging experience (Y.L.) determined the tumor boundaries on the PET images, using the PET/CT fusion images as a reference. Following this, a junior radiologist with 4 years of PET imaging experience (Y.W.) meticulously delineated the tumor boundaries layer by layer to construct the region of interest (ROI).
To ensure the accuracy and consistency of the segmentation, the senior radiologist subsequently reviewed all the ROIs created by the junior radiologist. This final review also ensured that the lesion coverage was comprehensive across all frames of the dynamic PET images and that the effects of respiration or minor motion were within acceptable limits.
As the segmentation process was conducted manually, without the use of automation, the combination of multi-level expertise and the systematic review process helped to minimize errors and inter-reader variability.
TAC calculation
The quantification of radiotracer uptake within DPI can be affected by a plethora of variability sources, including but not limited to, scanner characteristics such as resolution, image reconstruction methodologies, and physiological factors such as patient movement. The current study employs a framework predicated on the extraction of the mean radioactivity intensity within the lesion ROI. The initial phase of processing involves reconstructing the dynamics frames and segmenting the lesion ROIs as described previously. Following this, we applied Eq. [1], where the radioactivity content within each ROI is quantified for every 3-dimensional image slice corresponding to each time frame. In this context, represents the radioactivity intensity associated with voxel within the ROI at the specific time point . Upon the calculation of radioactivity distributions throughout all temporal frames, the temporal dynamics of radiotracer accumulation within the lesion ROI can be readily obtained from the DPI.
TAC feature extraction and Ki value
Derived from the TAC computed for the ROI, a number of 6 features were extracted, delineated as follows: the gradient of the TAC curve spanning the interval from 10 to 30 minutes (Eq. [2]),
the area under the TAC curve (Eq. [3]) that elucidates the cumulative quantity of radioactive pharmaceuticals within a temporal frame,
the initial slope of the curve from its beginning to its peak (Eq. [4]),
the slop from the curve’s peak to the 60-minute (Eq. [5]),
the temporal juncture at which the curve reaches its peak, for short, and lastly, the peak value attained by the curve, for short.
The determination of the Ki value in dynamic PET employing a 2-compartment model necessitates the analysis of rate constants (13).
Statistical analyses
Statistical analyses were performed in R (Version 4.3.3, https://www.r-project.org/). Given the small sample size, the Mann-Whitney U test was employed to assess the significance of differences in continuous variables related to metabolic parameters and clinical features. Fisher’s exact test was utilized to evaluate the significance of differences between discrete variables. Statistical significance was defined as P<0.05. Statistically significant features were selected to distinguish between the OR group and the non-OR group. Subsequently, the threshold range was determined for the two groups. For each threshold, the sensitivity and specificity were calculated, which were then utilized to compute the area under the receiver operating characteristic curve (AUC).
Results
Clinical data
Table 1 presents a comparative analysis of clinical characteristics between the OR group and the non-OR group, each comprising 5 patients. Age and gender distribution were similar across groups, with the OR group averaging 58.00±6.51 years and the non-OR group 60.60±14.11 years (P=0.691), and a predominant male representation in both groups. Smoking history and absence of brain metastasis were consistent across groups, demonstrating no significant differences (P>0.999). Treatment regimens varied, with a balanced distribution between etoposide, cisplatin, and durvalumab, versus etoposide, carboplatin, and serplulimab within each group (P=0.483). Efficacy outcomes highlighted that all patients in the OR group achieved a PR, contrasting with the non-OR group where 80% had stable disease (SD) and 20% showed progressive disease (PD). Lesion size differences between groups were not statistically significant (P=0.295).
Table 1
| Characteristics | OR (N=5) | Non-OR (N=5) | P value |
|---|---|---|---|
| Age (years) | 58.00±6.51 | 60.60±14.11 | 0.691 |
| Sex | >0.999 | ||
| Male | 4 (80.00) | 5 (100.00) | |
| Female | 1 (20.00) | 0 (0.00) | |
| Smoking (yes) | 2 (40.00) | 2 (40.00) | >0.999 |
| Brain metastasis (yes) | 0 (0.00) | 0 (0.00) | >0.999 |
| Treatment | 0.483 | ||
| Etoposide + cisplatin + durvalumab | 2 (40.00) | 3 (60.00) | |
| Etoposide + carboplatin + serplulimab | 3 (60.00) | 2 (40.00) | |
| Efficacy | – | ||
| Partial response | 5 (100.00) | 0 (0.00) | |
| Stable disease | 0 (0.00) | 4 (80.00) | |
| Progressive disease | 0 (0.00) | 1 (20.00) | |
| Lesion size (cm) | 6.00±0.759 | 4.72±1.538 | 0.295 |
Data are presented as mean ± standard deviation or n (%). Lesion size = maximum diameter of the tumor lesion. OR, objective response.
Metabolic features
Table 2 presents a comparative analysis of the metabolic features between patients who exhibited an OR and those who did not. Among the TAC features, demonstrated a statistically significant difference between the OR and non-OR groups (P=0.011), indicating a potential predictive value for treatment response. The other remaining features did not show significant differences between the groups (P>0.05). Conventional metabolic features analyzed include SUVmax, SUVmin, SUVmean, metabolic tumor volume (MTV), and total lesion glycolysis (TLG). None of these features demonstrated statistically significant differences between the OR and non-OR groups (P>0.05). Nonetheless, there was a notable trend observed in MTV (P=0.095). The absolute quantitative metabolic parameter, Ki, was also considered, but it did not show any statistical difference. The numerical values of 6 TAC features were normalized and depicted using line graphs across the 10 included patients (Figure 3).
Table 2
| Metabolic features | OR (N=5) | Non-OR (N=5) | P value |
|---|---|---|---|
| Slope10–30 | 0.18±0.18 | −0.02±0.04 | 0.011* |
| TACmax | 33.1±27.38 | 12.3±23.85 | 0.335 |
| TimeTM | 19.14±5.15 | 20.72±13.76 | 0.417 |
| Slope0–max | 728.73±326.06 | 400.56±194.84 | 0.105 |
| AUCT | 31.34±40.65 | 67.20±79.55 | 0.265 |
| Slopemax–60 | 0.29±0.58 | −0.03±0.55 | 0.417 |
| SUVmax | 10.28±2.63 | 11.94±2.26 | 0.548 |
| SUVmin | 4.11±1.05 | 4.78±0.91 | 0.548 |
| SUVmean | 6.26±1.96 | 7.00±1.29 | 0.548 |
| MTV | 67.99±12.71 | 38.48±24.45 | 0.095 |
| TLG | 543.93±272.59 | 338.72±237.69 | 0.151 |
| Ki | 0.03±0.01 | 0.02±0.02 | 0.548 |
Data are presented as mean ± standard deviation. An asterisk (*) indicates a P value less than 0.05, denoting statistical significance. AUC, area under the receiver operating characteristics curve; MTV, metabolic tumor volume; OR, objective response; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; SUVmin, minimum standardized uptake value; TAC, time-activity curve; TLG, total lesion glycolysis.
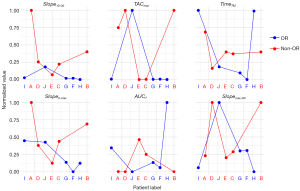
In Figure 4, the feature within the TAC profiles of 10 patients is visualized, with patients A–E classified into the non-OR group and patients F–J categorized into the OR group. A discernible disparity in this specific characteristic between the two cohorts is readily apparent upon visual inspection.

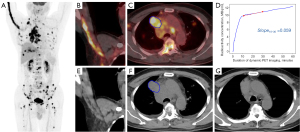
Based on the feature , the calculated AUC for distinguishing between the OR group and the non-OR group is 0.960. There were two optimal cutoff points identified: (I) threshold =0.070, with a sensitivity of 1.000 and a specificity of 0.800; (II) threshold =–0.018, with a sensitivity of 0.800 and a specificity of 1.000. Figure 5 presents the case of a patient whose TAC exhibits a pattern that deviates from the typical OR patterns (Figure 5D). This observation can potentially be attributed to the extensive metastatic dissemination throughout the patient’s body, coupled with the encroachment upon multiple veins within the right upper extremity (Figure 5B), culminating in the absence of discernible peak uptake within the TAC during the initial 10-minute interval of the DPI process. After excluding the patient, the optimal cutoff value was determined to be –0.018.
Discussion
Herein we derived a predictive biomarker of treatment response from DPI with 18F-FDG. Our analysis revealed that the TAC feature surpasses conventional metabolic parameters in reflecting the metabolic heterogeneity between tumors, thereby offering a more accurate prognostication of treatment outcomes in patients with ES-SCLC.
Predicting the efficacy of treatment for ES-SCLC remains a significant challenge in clinical practice. In recent years, treatment regimens for ES-SCLC have gradually shifted from platinum-based chemotherapy to combined chemotherapy and immunotherapy (14-16). Historically, metabolic parameters used to predict the efficacy of SCLC treatments were often tailored to single chemotherapy regimens and exhibited variability across different datasets. Lee et al. (17) discovered that patients with ES-SCLC who had higher mean SUVmax values had significantly shorter overall survival (OS) compared to those with lower mean SUVmax values [9.5 months, 95% confidence interval (CI): 4.9–13.9] vs. 17.7 months (95% CI: 12.0–20.1); P=0.007). However, a study by Kim et al. (18) indicated no significant differences in OS and progression-free survival (PFS) between high and low SUVmax groups in a baseline PET/CT analysis of 82 SCLC patients. Similarly, Oh et al. (19) posited that SUVmax is not an independent predictor of SCLC progression, but rather, total MTV is. Patients with high total MTV had poorer prognoses compared to those with low total MTV [hazard ratio for death: 2.11 (95% CI: 1.31–3.39); P=0.002]. A meta-analysis encompassing 38 studies also demonstrated that MTV has superior prognostic value over other PET parameters in SCLC (20). The majority of patients included in these studies received chemotherapy alone.
Hashimoto et al. (21) included 46 patients with ES-SCLC who underwent combined chemotherapy and immunotherapy. Their multivariate analysis revealed that MTV was an independent predictor of PFS, rather than SUVmax. However, according to their study, neither MTV nor other conventional metabolic indicators could predict disease control. The findings of our study corroborate these results. Although MTV, aside from , performed best in evaluating different efficacy groups, it did not reach statistical significance. Among all the parameters reflecting tumor metabolic heterogeneity, was the only feature that showed statistical significance between the OR and non-OR groups.
Molecular biomarkers such as circulating tumor DNA and tumor mutational burden have demonstrated significant promise in advancing individualized patient stratification (22). However, their application often necessitates specialized assays, significant financial investment, and extended processing time. In contrast, TAC slope can be extracted from routine dynamic PET imaging, potentially providing immediate clinical insights without additional resource demands. The parameter () accounts for the information of the mid-phase retention. The radiotracer activity changes within the first 10 minutes post-injection are more susceptible to the influence of blood perfusion. Conversely, the changes in radiotracer activity tend to stabilize after 30 minutes post-injection. The can be derived from scans conducted between 10 and 30 minutes post-injection, facilitating a protocol that substantially decreases the required scan duration. This reduction in scan time can enhance scanning efficiency and lower the overall costs associated with large-scale studies. Additionally, a shorter scan duration offers the added benefit of minimizing the likelihood of motion artifacts, thereby potentially improving image quality. Therefore, we designed the feature of the TAC () to better capture the metabolic uptake characteristics of the target lesion. This feature serves as a more robust indicator for assessing heterogeneity between tumors and aids in predicting the OR in ES-SCLC (AUC: 0.960).
Previous research on dynamic TAC has predominantly concentrated on gliomas, with feature extraction primarily confined to parameters such as peak time, which does not comprehensively reflect the metabolic heterogeneity (23-25). Chitalia et al. (11) utilized an unsupervised clustering algorithm on 4-dimensional (4D) imaging to dynamically characterize tumoral heterogeneity, thereby predicting recurrence-free survival in breast cancer. However, this method is relatively complex. In this study, we designed 6 TAC curve features that comprehensively describe the entire TAC curve morphology while maintaining computational simplicity. These features encapsulate various aspects of tumor metabolic information.
Upon conducting an in-depth analysis of the 10 patients enrolled in the study, it was observed that the values tend to be lower in the OR group (mean =–0.02) compared to the non-OR group, which typically exhibited higher values (mean =0.18). The final patient, as depicted in Figure 5 and evaluated according to the iRECIST criteria for treatment response as PR, exhibited a value of 0.059. Further examination of this case revealed that the patient received an injection of 18F-FDG in the right dorsal hand vein. Due to the presence of a tumor thrombus in the veins of the right upper limb, the radiotracer activity lacked an initial rapid accumulation phase, thereby yielding a value that was higher than that of other patients in the OR group. Consequently, within a larger sample cohort, the mean value for the OR group might be even lower. This study identified 2 cutoff values for , namely 0.070 and –0.018. Based on the aforementioned analysis, the selection of –0.018 as the optimal cutoff value is more congruent with clinical reality when there is no involvement of the venous system in the injected upper limb.
This study is not without limitations. A notable limitation of this study is its relatively small sample size and single-center design. Future investigations would benefit from a multi-center approach with a larger patient cohort to enhance the generalizability of findings. However, the Mann-Whitney U test, which is suitable for small samples, was employed in this study to compare the differences in characteristic parameters between the two groups. The results obtained indicate that only the feature exhibits statistical differences, which to a certain extent reflects the advantage of this feature in predicting treatment outcomes. Moreover, among the conventional metabolic parameters with no statistical differences, MTV performs better than other parameters, which is consistent with the conclusions of most similar studies in the past (20,21). Secondly, the duration of DPI is longer than that of conventional PET scanning, which may pose a certain challenge for patients with poor basic conditions to endure or even cause movement artifacts (26,27). However, subsequent procedures can reduce the duration of patient scanning by limiting the imaging to a window of 10–30 minutes post-injection of the radiotracer. Thirdly, this study primarily focused on exploring early imaging biomarkers to evaluate treatment response rather than long-term survival outcomes. The prognostic value of these findings should be further explored with the inclusion of a larger patient cohort in future studies. Lastly, in order to explore clinically interpretable features that can be readily implemented in routine practice, our study did not incorporate machine learning methodologies (28), which could potentially provide a more comprehensive assessment of tumor metabolic heterogeneity.
Conclusions
Our study identified the feature (), a novel predictive biomarker derived from DPI with 18F-FDG, which outperforms conventional metabolic parameters in reflecting metabolic heterogeneity between tumors and predicting treatment outcomes in ES-SCLC. The values () were significantly lower in the OR group, indicating its potential as a robust indicator for treatment efficacy. Despite the small sample size and longer DPI duration, shows promise for facilitating precise, personalized treatment plans. Future studies should validate these findings and address practical challenges.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1687/rc
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1687/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Cancer Hospital & Shenzhen Hospital of Chinese Academy of Medical Sciences (approval No. KYLH2022-1). All patients signed a written informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin 2025;75:10-45. [Crossref] [PubMed]
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17:725-37. [Crossref] [PubMed]
- Lattuca-Truc M, Timsit JF, Levra MG, Ruckly S, Villa J, Dumas I, Pinsolle J, Ferrer L, Guillem P, Moro-Sibilot D, Toffart AC. Trends in response rate and survival in small-cell lung cancer patients between 1997 and 2017. Lung Cancer 2019;131:122-7. [Crossref] [PubMed]
- Zhu D, Wang Y, Wang L, Chen J, Byanju S, Zhang H, Liao M. Prognostic value of the maximum standardized uptake value of pre-treatment primary lesions in small-cell lung cancer on 18F-FDG PET/CT: a meta-analysis. Acta Radiol 2018;59:1082-90. [Crossref] [PubMed]
- Chang H, Lee SJ, Lim J, Lee JS, Kim YJ, Lee WW. Prognostic significance of metabolic parameters measured by (18)F-FDG PET/CT in limited-stage small-cell lung carcinoma. J Cancer Res Clin Oncol 2019;145:1361-7. [Crossref] [PubMed]
- Özdemir Ö, Batum Ö, Ermin S, Aksel N, Kömürcüoğlu B, Mertoğlu A, Deniz S, Balcı G, Koparal H, Özbilek E, Yılmaz U. Metabolic activity of primary tumour on PET/CT has a relationship with survival in stages I-III small-cell lung carcinoma. Clin Respir J 2020;14:695-702. [Crossref] [PubMed]
- Aktan M, Koc M, Kanyilmaz G, Yavuz BB. Prognostic value of pre-treatment (18)F-FDG-PET uptake in small-cell lung cancer. Ann Nucl Med 2017;31:462-8. [Crossref] [PubMed]
- Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016;164:681-94. [Crossref] [PubMed]
- Dimitrakopoulou-Strauss A, Pan L, Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: general considerations, current clinical applications, and future perspectives. Eur J Nucl Med Mol Imaging 2021;48:21-39. [Crossref] [PubMed]
- Mabrouk R, Dubeau F, Bentabet L. Dynamic cardiac PET imaging: extraction of time-activity curves using ICA and a generalized Gaussian distribution model. IEEE Trans Biomed Eng 2013;60:63-71. [Crossref] [PubMed]
- Chitalia R, Viswanath V, Pantel AR, Peterson LM, Gastounioti A, Cohen EA, Muzi M, Karp J, Mankoff DA, Kontos D. Functional 4-D clustering for characterizing intratumor heterogeneity in dynamic imaging: evaluation in FDG PET as a prognostic biomarker for breast cancer. Eur J Nucl Med Mol Imaging 2021;48:3990-4001. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983;3:1-7. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- Lee YJ, Cho A, Cho BC, Yun M, Kim SK, Chang J, Moon JW, Park IK, Choi HJ, Kim JH. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res 2009;15:2426-32. [Crossref] [PubMed]
- Kim SJ, Chang S. Limited Prognostic Value of SUV max Measured by F-18 FDG PET/CT in Newly Diagnosed Small Cell Lung Cancer Patients. Oncol Res Treat 2015;38:577-85. [Crossref] [PubMed]
- Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, Bom HS. Whole-body metabolic tumour volume of 18F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging 2012;39:925-35. [Crossref] [PubMed]
- Christensen TN, Andersen PK, Langer SW, Fischer BMB. Prognostic Value of (18)F-FDG-PET Parameters in Patients with Small Cell Lung Cancer: A Meta-Analysis and Review of Current Literature. Diagnostics (Basel) 2021;11:174. [Crossref] [PubMed]
- Hashimoto K, Kaira K, Imai H, Miura YU, Shiono A, Mouri A, Yamaguchi OU, Kobayashi K, Kagamu H, Kuji I. Metabolic Tumor Volume as Significant Predictor for Chemotherapy Containing PD-L1 Blocker in Extensive Stage Small Cell Lung Cancer. Anticancer Res 2024;44:1541-51. [Crossref] [PubMed]
- Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017;17:223-38. [Crossref] [PubMed]
- Bashir A, Brennum J, Broholm H, Law I. The diagnostic accuracy of detecting malignant transformation of low-grade glioma using O-(2-[18F]fluoroethyl)-l-tyrosine positron emission tomography: a retrospective study. J Neurosurg 2018;130:451-64. [Crossref] [PubMed]
- Röhrich M, Huang K, Schrimpf D, Albert NL, Hielscher T, von Deimling A, Schüller U, Dimitrakopoulou-Strauss A, Haberkorn U. Integrated analysis of dynamic FET PET/CT parameters, histology, and methylation profiling of 44 gliomas. Eur J Nucl Med Mol Imaging 2018;45:1573-84. [Crossref] [PubMed]
- Kunz M, Albert NL, Unterrainer M, la Fougere C, Egensperger R, Schüller U, Lutz J, Kreth S, Tonn JC, Kreth FW, Thon N. Dynamic 18F-FET PET is a powerful imaging biomarker in gadolinium-negative gliomas. Neuro Oncol 2019;21:274-84. [Crossref] [PubMed]
- Sun T, Wu Y, Wei W, Fu F, Meng N, Chen H, Li X, Bai Y, Wang Z, Ding J, Hu D, Chen C, Hu Z, Liang D, Liu X, Zheng H, Yang Y, Zhou Y, Wang M. Motion correction and its impact on quantification in dynamic total-body 18F-fluorodeoxyglucose PET. EJNMMI Phys 2022;9:62. [Crossref] [PubMed]
- Wu Y, Fu F, Meng N, Wang Z, Li X, Bai Y, Zhou Y, Liang D, Zheng H, Yang Y, Wang M, Sun T. The role of dynamic, static, and delayed total-body PET imaging in the detection and differential diagnosis of oncological lesions. Cancer Imaging 2024;24:2. [Crossref] [PubMed]
- Bianchetti G, Taralli S, Vaccaro M, Indovina L, Mattoli MV, Capotosti A, Scolozzi V, Calcagni ML, Giordano A, De Spirito M, Maulucci G. Automated detection and classification of tumor histotypes on dynamic PET imaging data through machine-learning driven voxel classification. Comput Biol Med 2022;145:105423. [Crossref] [PubMed]


