
The diagnostic value of spectral CT plain scan after direct lymphangiography in chylothorax as an applicable tool
Introduction
Lymphatic circulation is essential for the maintenance of fluid balance and the formation of celiac disease through intestinal fat absorption, and lymphatic fluid flows into the veins through the various lymphatic systems located throughout the body. In normal circumstances, lymphatic fluid returns to the right and left venous horns through lymphatic vessels; however, a variety of factors can lead to rupture or blockage branches. Congenital disorders (e.g., lymphatic malformations), trauma, infections, tumors, and surgical disruption, can cause rupture or blockage of lymphatic vessels, leading to chyle leakage and accumulation in the chest, which is clinically referred to as chylothorax, with surgery for malignant tumors accounting for the majority of cases (1). Chylothorax is a clinical syndrome characterized by the accumulation of chyle within the pleural cavity. It is usually caused by the damage to the thoracic duct (TD) or its branches (2,3). Chylothorax can cause edema, cough and dyspnea, and can also be combined with chyle pericardium and chyle abdomen (4-7), so early diagnosis is crucial for the treatment and prognosis of the patients, and by early diagnosis, complications such as lung infections can be minimized, and the quality of survival of the patients can be improved. The key to determining whether the pleural effusion is chylothorax lies in analyzing the levels of cholesterol and triglycerides (8). A diagnosis can be confirmed when the triglyceride level exceeds 1.24 mmol/L (110 mg/dL) (9). Direct lymphangiography (DLG) is considered the gold standard for evaluating lymphatic flow disorders and provides information related to lymphatic leakage (10). Spectral computed tomography (CT), with its high resolution, enables accurate localization of lesions. A spectral CT plain scan is typically performed 20 minutes to 2 hours after DLG, which can clearly depict the abnormal distribution and deposition of the contrast agent within the body, allowing for more accurate observation of lymphatic abnormalities such as dilation and tortuosity of lymphatic vessels. Currently, research on chylothorax consists primarily of case reports, leading to limited available data. By retrospectively analyzing the clinical and imaging data of 59 patients with chylothorax in our hospital in the past five years, and observing the abnormal distribution of the contrast agent in the body, our study aims to investigate the value of the application of spectral CT plain scan after DLG in the diagnosis of chylothorax. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1472/rc).
Methods
The retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Ethics Committee of Beijing Shijitan Hospital (No. 6187022956). The requirement of informed consent was waived due to its retrospective nature of the study.
Study population
We retrieved data on a total of 1,526 patients with chylothorax who were clinically diagnosed at our hospital between January 2018 and December 2023, and performed a case review of all patients, excluded 1,461 patients with incomplete information. Inclusion criteria: (I) clinical manifestation of milky white or yellow pleural effusion, positive pleural effusion chyle test; (II) those patients with complete clinical and spectral CT after DLG data, exclusion criteria: (I) non-chylous pleural effusion; (II) those with incomplete clinical and spectral CT after DLG data. Of the 65 patients with chylothorax who had complete information, 6 patients had spectral CT with enhancement after DLG, and finally, a total of 59 patients were enrolled, as shown in Figure 1. Among these 59 patients with chylothorax, there were 33 cases of obstructed lymphatic reflux, 7 postoperative cases, 7 cases of lymphangioma, 4 cases of Gorham-Stout syndrome, and 8 cases of lymphangiectasia.
CT image acquisition
GE Innova 2000-IQ digital subtraction angiography (DSA) machine (Chicago, IL, USA) was used for DLG. The patient was instructed to lie supine, and 1–2 mL of a 1:1 mixture of methylene blue and 2% lidocaine was used subcutaneously and intradermally on the unilateral dorsum of the foot between the 1st–3rd toes to perform a puncture of the foot lymphatic vessels, then the skin was incised transversally in the mid dorsum of the foot under local anesthesia under the microscope, and one blue-stained superficial lymphatic vessel was found from the subcutaneous area, which was then punctured into the lumen with a puncture needle, and 10–15 mL ultra-liquid iodized oil (Lipiodol UF, Guerbet, France) was injected by the high-pressure syringe at a flow rate of 6–8 mL/h. lipiodol UF, Guerbet, France) 10–15 mL, and the passage of lipiodol pathway lymphatic trunk and lymphatic conduit was observed dynamically and intermittently under DSA for 1.5–4 h. The lymphatic trunk and lymphatic conduit were then removed from the skin.
Spectral CT plain scan was performed using a 512-slice Revolution CT instrument (GE Healthcare) 20 min–2 h after the end of DLG. Patients were asked to lie on their backs, raise their hands above their heads, and hold their breath at the end of deep inhalation to avoid respiratory motion artifacts. The SN line (sternal notch) was used as the baseline for localization, and the thoracic inlet to the bilateral lung bases were scanned with a continuous spiral scanning in a transverse plane with the following parameters: tube voltage of 80–120 kV, tube current of 250–300 mA, layer thickness of 5 mm, layer spacing of 5 mm, pitch of 1.00, and a matrix of 512×512. After scanning, the data were transferred to the post-processing workstation, and the acquired CT images were reconstructed in multi-planar reconstruction (MPR) as a lung window and a soft tissue window using a standard algorithm, with a reconstructed layer thickness of 2 mm and a layer spacing of 1.8 mm. The lung window had a window width of 1,600 Hounsfiled unit (HU) and a window position of −600 HU, and the mediastinal window had a window width of 450 HU and a window position of 45 HU, with a noise index of 10.0.
Radiological evaluation
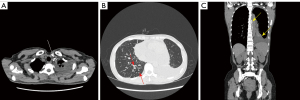
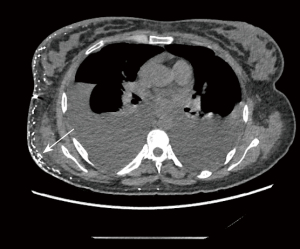
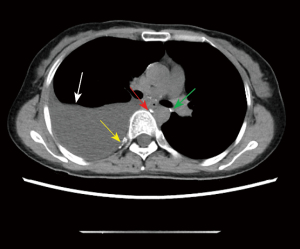
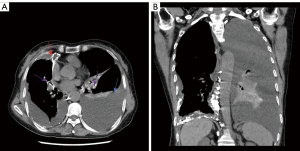
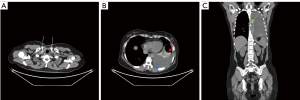
The results of spectral CT plain scan after DLG were evaluated by two experienced radiologists (they all have 14 years experience) who read the spectral CT plain scan images after DLG of 59 patients with chylothorax and recorded the evaluation results, and in case of disagreement, the 2 people discussed and agreed on the results. According to Sun et al. (11), when lymphatic reflux is normal, post-DLG lipiodol does not distribute to the lungs, pleura, chest wall, mediastinum, or pericardium, and if contrast does distribute to these areas, then lymphatic vessel abnormalities should be considered. Evaluate chest CT findings of lymphatic vessel abnormalities and other abnormalities, including abnormal lipiodol distribution, changes in the lungs, pleura, chest wall, and mediastinum. Evaluation includes abnormal deposition of lipiodol in the lymphatic vessels of the chest on spectral CT plain scan after DLG, as well as abnormalities on the chest plain scan CT (Figures 2-6).
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (IBM Corporation, Armonk, NY, USA). Qualitative data were expressed as percentages, and quantitative data were expressed as mean ± standard deviation (SD).
Results
Baseline characteristics
The patients’ clinical features are summarised in Table 1. A total of 59 patients with chylothorax were selected in our study, including 26 males and 33 females, with a mean age of 41.5±16.8 years. The duration of the disease ranges from 1 month to 12 years, with an average duration of 1 year and 4 months. Seven patients had post-surgical chylothorax, i.e., secondary chylothorax, and 52 patients had primary chylothorax, and the clinical accompanying symptoms of the patients were chest tightness in the most common cases, which were as follows: 2 fever (3.4%), 5 wheezing (8.5%), 17 breath-holding (28.8%), 15 shortness of breath (25.4%), 13 cough (22.0%), 4 coughing up sputum (6.8%), 1 runny nose (1.7%), 36 chest tightness (61.0%) and 1 chest pain (1.7%).
Table 1
| Variables | Value |
|---|---|
| Age (years) | 41.5±16.8 |
| Gender | |
| Male | 26 (44.1) |
| Female | 33 (55.9) |
| Clinical symptoms† | |
| Fever | 2 (3.4) |
| Wheezing | 5 (8.5) |
| Breath-holding | 17 (28.8) |
| Shortness of breath | 15 (25.4) |
| Cough | 13 (22.0) |
| Coughing up sputum | 4 (6.8) |
| Runny nose | 1 (1.7) |
| Chest tightness | 36 (61.0) |
| Chest pain | 1 (1.7) |
| Complication | |
| Chyle abdomen | 9 (15.3) |
| Chyle pericardium | 17 (28.8) |
Data are presented as mean ± standard deviation or n (%). †, a patient may exhibit multiple concomitant symptoms simultaneously. CT, computed tomography; DLG, direct lymphangiography.
Radiological features
The patients’ radiological features are summarised in Tables 2,3. Abnormal deposition of lipiodol was seen in all 59 patients with chylothorax on spectral CT plain scan after DLG, and could be seen simultaneously in different parts of the root of neck, the mediastinum, the lungs, the thoracic cavity, the pleural, the chest wall and the other sites, especially common in the jugular vein angle area (Table 2) (Figures 2-6). In addition, as shown in Table 3, chest CT plain scan in these patients also demonstrated the radiologic features of thickening of bronchial vascular bundles, thickening of the interlobular septum, thickening of the bronchial wall, ground glass opacities, consolidations, thickening of the pleural and pleural effusion, as well as mediastinal classically rounded/striated/sacral hypodensity shadows.
Table 2
| Sites of abnormal lipiodol deposition† | N (%) |
|---|---|
| Cervical root | |
| Left venous angle | 29 (49.2) |
| Right venous angle | 1 (1.7) |
| Bilateral venous angle | 7 (11.9) |
| Left supraclavicular | 2 (3.4) |
| Bilateral supraclavicular | 2 (3.4) |
| Left subclavian lymphatic vessel | 3 (5.1) |
| Bilateral subclavian lymphatic vessel | 1 (1.7) |
| Middle mediastinum | |
| Hilar | 6 (10.2) |
| Pericardium | 5 (8.5) |
| Posterior mediastinum | |
| Thoracic duct | 10 (16.9) |
| Para-thoracic aorta | 3 (5.1) |
| Posterior esophagus | 3 (5.1) |
| Thoracic spine | 3 (5.1) |
| Lung | |
| Left | 6 (10.2) |
| Right | 7 (11.9) |
| Bilateral | 5 (8.5) |
| Bronchial vascular bundles | |
| Parabronchial vascular bundles of both bronchioles | 2 (3.4) |
| Thoracic cavity | |
| Left | 4 (6.8) |
| Right | 4 (6.8) |
| Bilateral | 4 (6.8) |
| Pleural | |
| Right pleural | 6 (10.2) |
| Bilateral pleural | 9 (15.3) |
| Chest wall | |
| Left | 2 (3.4) |
| Right | 2 (3.4) |
| Other sites | |
| Axilla | 4 (6.8) |
†, a patient may exhibit abnormal lipiodol distribution at multiple sites. CT, computed tomography; DLG, direct lymphangiography.
Table 3
| Variables | Value |
|---|---|
| CT attenuation (HU) | 14.8±8.0 |
| CT findings† | |
| Affected lung | |
| Bronchial vascular bundle thickening | 17 (28.8) |
| Interlobular septal thickening | 11 (18.6) |
| Bronchial wall thickening | 5 (8.5) |
| Ground glass opacities | 17 (28.8) |
| Consolidations | 2 (3.4) |
| Affect pleura | |
| Pleural thickening | 3 (5.1) |
| Pleural effusion (chylothorax) | |
| Left | 15 (25.4) |
| Right | 19 (32.2) |
| Bilateral | 25 (42.4) |
| Affect mediastinum | |
| Mediastinal fat infiltration | 1 (1.7) |
| Mediastinal rounded/striated/cystic hypodense shadow | 4 (6.8) |
Data are presented as mean ± standard deviation or n (%). †, a patient may have 2 or more abnormal findings. CT, computed tomography; DLG, direct lymphangiography.
Pathological findings
Five patients underwent puncture biopsy of pleural effusion, and the results were lymphocytes, histiocytes and mesothelial cells, and 2 patients underwent pathologic examination of deep cervical lymph nodes, which resulted in a large number of dilated lymphatic vessels and smooth muscle hyperplasia seen within fibro-fatty connective tissue.
Discussion
The lymphatic system plays a crucial role in maintaining fluid balance (12). Lymphatic vessels are found throughout the body. The TD starts from the chyle pool and develops to the right descending aorta, then crosses the left vein and flows into the left venous angle (13). It is the largest lymphatic vessel in the body (14), with a length of 45 cm and a diameter of 2–5 mm, and it drains lymphatic fluids at the junction of the subclavian vein and jugular vein, if the integrity of the TD is disrupted, lipid-rich chyle leaks into the pleural cavity and chylothorax is formed (15,16). Chylothorax can lead to severe respiratory disease (17) and it is usually on the right side because most of the ducts are in the right hemithorax (18). However, the distribution patterns in our study contrast with previous reports. Of our 59 patients, 19 (32.2%) had right sided chylothorax, 15 (25.4%) had had left sided chylothorax, and 25 (42.4%) had bilateral chylothorax.
In recent years, radiology has played an increasingly important role in the diagnosis and treatment of diseases of the lymphatic system (14). Lymphoscintigraphy (LS) and single-photon emission computed tomography (SPECT)/CT have been used for lymphatic imaging, which does not clearly show lymphatic vessels. Non-contrast T2-weighted imaging (T2WI) magnetic resonance lymphangiography (MRL) can show lymphatic vessels, but its limitation lies in the fact that it does not distinguish the lymphatic vessels from the nearby aqueous structures. The complexity of the process of intranodal lymphangiography, which involves placing an intranodal needle outside the magnetic field under ultrasound guidance and then transferring the patient with an unstable needle to the magnetic resonance imaging (MRI) scanner, has hampered its clinical application (13).
Anger et al. (4) concluded that chylothorax can cause cough and dyspnea, and in our study, there were 13 cases of cough (22.0%), 4 cases of sputum (6.8%), 36 cases of chest tightness (61.0%), 1 case of chest pain (1.7%), 17 cases of breath-holding (28.8%), and 15 cases of shortness of breath (25.4%), which is in line with previous reports (5). According to the literature (19), patients with chylothorax can be combined with chyle abdomen and chyle pericardium, and in our study patients with chylothorax was accompanied by chyle abdomen in 9 cases (15.3%), and chyle pericardium in 17 cases (28.8%), which was consistent with previous studies.
Spectral CT plain scan after DLG provides clearer anatomical details of the lymphatic system (20), this examination is important for the exact localization of chyle leakage, showing the location, size, and distribution of the lesion (21), under normal circumstances. When the lipiodol is injected into the superficial lymphatic vessels of the foot, it will be absorbed by the deep lymphatic vessels of the ipsilateral lower limbs, and the lymphatic fluid of the deep lymphatic vessels of the lower limbs flows from the ipsilateral iliac lymphatic duct (ILD), the lumbar trunk through the chyle pool into the TD, and rises up along TD and finally collects in the venous system near the confluence of the left subclavian vein and the left jugular vein, and the appearance of the lipiodol at any other site in the course of the process is defined as an abnormal distribution, and this distribution indicates lymphatic dilatation or lymphatic leakage (21). Due to the high spatial resolution of CT, it is possible to accurately show the site of abnormal distribution of the lipiodol in the body (22). In our study, 59 patients with chylothorax were seen to have an abnormal distribution of lipiodol in the cervical root, the mediastinum, the lung, the thoracic cavity, the pleural, the chest wall and the other sites. In these areas, the jugular vein angle is the most common.
It has been reported in the literature (23) that CT can localize pleural effusion to aid drainage and show lung parenchymal abnormalities associated with lymphatic destruction, and it (23) is believed that patients with chylothorax can have abnormalities of the lung parenchyma and the mediastinum, common findings of the lung parenchymal abnormalities are thickening of the lobular septa as well as contrast infiltration into the pulmonary lymphatics leading to reverse flow of the lymphatic fluid. In our study, lobular septal thickening was observed in 11 patients (18.6%), bronchial vascular bundle thickening was observed in 17 patients (28.8%), ground glass shadow was observed in 17 patients (28.8%), and abnormal distribution of pulmonary lipiodol was observed in 18 patients (30.5%). Thickening of bronchial vascular bundle, thickening of interlobular septum, grinding glass shadow of both lungs and thickening of interstitium without fibrosis may be related to lymphatic hyperplasia and expansion (11,23). Another study (24) shows that chest CT of chylothorax patients can show lung consolidation and pleural thickening. In our study, 2 patients (3.4%) showed solid shadow, and 3 patients (5.1%) showed signs of pleural thickening.
It was reported that TD fistulae, lymphatic malformations and lymphatic dilatation can occur in the mediastinum of patients with chylothorax, and axial images of chest CT can show mediastinal fat infiltration and the hypodensity, which may be related to lymphatic dilatation (23). In our study, mediastinal fat infiltration could be seen in 1 patient (1.7%), and mediastinal rounded/striated/cystic hypodense shadow could be seen in 4 patients (6.8%).
The limitations of this study are mainly due to the single-center study and the limited pathology sampling, which is to be followed up with a multicenter to expand the sample size for the study, in addition, our study is not a randomized controlled study, so there may be a selection bias.
Conclusions
In conclusion, spectral CT plain scan after DLG can localize the site of lipiodol leakage, which is of great value in the clinical diagnosis and treatment of chylothorax.
Acknowledgments
We would like to thank all the medical staff involved in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1472/rc
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1472/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and was approved by the Ethics Committee of Beijing Shijitan Hospital (No. 6187022956). The requirement for informed consent was waived due to its retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yazicioglu A, Yazici U, Aydin E, Karaoglanoglu N. A strange bullet which caused chylomediastinum and chyloptysis. Thorac Cardiovasc Surg 2014;62:372-4. [PubMed]
- Agrawal A, Chaddha U, Kaul V, Desai A, Gillaspie E, Maldonado F. Multidisciplinary Management of Chylothorax. Chest 2022;162:1402-12. [Crossref] [PubMed]
- Lee IH, Kim SG, Park KS, Ahn DJ, Kim MK. Chylothorax associated with primary membranous nephropathy: a case report. Ann Palliat Med 2023;12:834-42. [Crossref] [PubMed]
- Anger M, Hofmann J, Ruf B, Steinborn M, Reber D, Warncke K, Rieber N. Cough-induced chylothorax in a two-year-old boy - case report and review of the literature. BMC Pediatr 2023;23:416. [Crossref] [PubMed]
- De Pauw V, Daelemans S, Depoorter L, Brussaard C, Smets D. Refractory chylothorax after severe vomiting and coughing in a 4-year-old child. J Surg Case Rep 2023;2023:rjad466. [Crossref] [PubMed]
- Handal-Orefice R, Midura D, Wu JK, Parravicini E, Miller RS, Shawber CJ. Propranolol Therapy for Congenital Chylothorax. Pediatrics 2023;151:e2022058555. [Crossref] [PubMed]
- Liu D, Xia W, Tang Q, Wang J, Wang M, Zhang C, Zhou W, Shi J, Zhou Q, Zhang H, Xie Y, Shao Y. Application of lymphography in the location and treatment decision of chyle leakage: an analysis of 177 cases. Zhonghua Wai Ke Za Zhi 2016;54:281-5. [PubMed]
- Braun CM, Ryu JH. Chylothorax and Pseudochylothorax. Clin Chest Med 2021;42:667-75. [Crossref] [PubMed]
- Hvass M, Fransen JL, Bruun JM. Chylothorax. Ugeskr Laeger 2017;179:V05170429. [PubMed]
- Schwartz FR, James O, Kuo PH, Witte MH, Koweek LM, Pabon-Ramos WM. Lymphatic Imaging: Current Noninvasive and Invasive Techniques. Semin Intervent Radiol 2020;37:237-49. [Crossref] [PubMed]
- Sun X, Shen W, Xia S, Wen T, Wang R. Diffuse Pulmonary Lymphangiomatosis: MDCT Findings After Direct Lymphangiography. AJR Am J Roentgenol 2017;208:300-5. [Crossref] [PubMed]
- Benjamin J, O'Leary C, Hur S, Gurevich A, Klein WM, Itkin M. Imaging and Interventions for Lymphatic and Lymphatic-related Disorders. Radiology 2023;307:e220231. [Crossref] [PubMed]
- Shima T, Hara T, Sato K, Kan N, Kinjo T. Fusion imaging of single-photon emission computed tomography and magnetic resonance lymphangiography for post-Fontan chylothorax. Radiol Case Rep 2023;18:1471-6. [Crossref] [PubMed]
- Lee E, Biko DM, Sherk W, Masch WR, Ladino-Torres M, Agarwal PP. Understanding Lymphatic Anatomy and Abnormalities at Imaging. Radiographics 2022;42:487-505. [Crossref] [PubMed]
- Bojanapu S, Khan YS. Thoracic Duct Leak. Treasure Island (FL): StatPearls Publishing; 2023.
- Petrini M, Colombi D, Bodini FC, Morelli N, Ciatti C, Quattrini F, Maniscalco P, Michieletti E. Thoracic duct leakage in a patient with Type B-Non-Hodgkin lymphoma treated with transvenous retrograde access embolization: a case report. Acta Biomed 2023;94:e2023043. [PubMed]
- Tutor JD. Chylothorax in infants and children. Pediatrics 2014;133:722-33. [Crossref] [PubMed]
- Braun CM, Ryu JH. Chylothorax and Pseudochylothorax. Clin Chest Med 2021;42:667-75. [Crossref] [PubMed]
- Liu D, Xia W, Tang Q, Wang J, Wang M, Zhang C, Zhou W, Shi J, Zhou Q, Zhang H, Xie Y, Shao Y. Application of lymphography in the location and treatment decision of chyle leakage: an analysis of 177 cases. Zhonghua Wai Ke Za Zhi 2016;54:281-5. [PubMed]
- Li L, Wu X, Liu D, Zhang W, Yang L, Pan F. Preliminary Exploration of Transpedal Lymphangiography With High-Dose Ethiodized Oil Application in the Treatment of Postoperative Chylothorax. Front Med (Lausanne) 2021;8:754781. [Crossref] [PubMed]
- Jin D, Sun X, Shen W, Zhao Q, Wang R. Diagnosis of Lymphangiomatosis: A Study Based on CT Lymphangiography. Acad Radiol 2020;27:219-26. [Crossref] [PubMed]
- Zhang Y, Sun X, Shen W, Hao K, Hao Q, Li X, Wang R. Systematic lymphatic abnormality-related osseous lesions: a study based on CT lymphangiography. Quant Imaging Med Surg 2022;12:4549-58. [Crossref] [PubMed]
- Sun JD, Shum T, Behzadi F, Hammer MM. Imaging Findings of Thoracic Lymphatic Abnormalities. Radiographics 2022;42:1265-82. [Crossref] [PubMed]
- Fukuda S, Reetz JA, Hamamoto K, Griffin L, Schaffer PA. Diagnostic imaging and histopathologic features of rounded atelectasis in four cats and one dog: A descriptive case series study. Vet Radiol Ultrasound 2023;64:411-9. [Crossref] [PubMed]



